Abstract
Rationale:
Clostridium difficile infection (CDI) is a symptomatic infection due to the spore-forming bacterium, C. difficile. Asymptomatic C. difficile colonization is the stage in absence of symptoms, with a prevalence of 1.4% to 21% on hospital admission. Proton-pump inhibitors (PPIs) was implicated as a novel potential contributor to CDI. PPIs injection could make asymptomatic C. difficile colonization progress to C. difficile associated diarrhea (CDAD).
Patient concerns:
A postoperative colon cancer patient, who had been taking omeprazole for 4 years after operation, got asymptomatic C. difficile colonization. When he developed clinical symptoms of digestive tract, tumor recurrence was first suspected and intravenous omeprazole was prescribed, which ultimately led to progression to symptomatic CDI. In this report, we tell the confusing differential diagnosis of cancer-associated diseases and CDAD, and discuss the possibility of solving the PPIs overuse problem by making clinical pathway of PPIs use in Chinese hospitals.
Diagnoses:
CDAD, incomplete intestinal obstruction, postoperation of colon cancer.
Intervention:
Electrolyte replacement and rehydration. Parenteral nutrition support. Omeprazole was prescribed but withdrawn later, and oral vancomycin was given at a dose of 0.25 g 4 times per day for 10 days.
Outcomes:
Diarrhea was resolved, so long as the acid reflux and vomiting.
Lessons:
We have 2 lessons here: Be aware of PPIs induced CDI, especially the asymptomatic C. difficile colonization. Making clinical pathway specified on PPIs use by pharmacists could be a practical way to solve the problem of PPIs overuse.
Keywords: asymptomatic Clostridium difficile colonization, clinical pathway specified on PPIs use, Clostridium difficile infection, omeprazole, postoperation of colon cancer
1. Introduction
Clostridium difficile is a ubiquitous spore-forming gram-positive anaerobic bacillus, first described by Hall and O’Toole in 1935.[1] Morbidity is caused by toxin-mediated disruption of cytoskeletal elements leading to inflammation and cell death, mainly toxins A and B, produced by this pathogen. Symptoms include watery stool, abdominal pain, fever, sometimes nausea and vomiting, or even more serious intestinal conditions. Clostridium difficile is the leading cause of health care associated diarrhea [2,3] and can result in asymptomatic carriage.[4] Rates of asymptomatic C. difficile colonization on hospital admission range from 1.4% to 21%.[5] Leekha et al reported that asymptomatic C. difficile colonization at hospital admission was detected in nearly 1 of 10 patients in a tertiary care hospital in Minnesota.[6]
Proton-pump inhibitors (PPIs) are a group of drugs whose main action is long-lasting reduction of gastric acid production. They are being widely used in the treatment of gastrointestinal diseases, such as dyspepsia peptic ulcer disease, gastroesophageal reflux disease, Barrett esophagus, stress gastritis and ulcer prevention, and so on. Recent years, PPIs have been implicated as a novel potential contributor to Clostridium difficile infection (CDI).[7,8] Cadle et al reported that patients receiving PPIs were 4.17 times more likely to have C. difficile associated diarrhea (CDAD) as compared with their counterparts.[9] A multilevel model case–control study among 64 US academic medical centers in 2014 indicated that PPIs was one of the several medications that were associated with the risk of patients developing healthcare-associated CDI.[10]
Here, we report a postoperative colon cancer patient, who developed asymptomatic C. difficile colonization by taking oral omeprazole for 4 years after operation and progressed to CDI in hospital by intravenous injection of omeprazole.
2. Case report
An 84-year-old man was admitted to our hospital complaining of nausea, acid reflux, anorexia, and abdominal distention for the last 8 months and symptoms increased for recent 1 month. Four years ago, he was diagnosed as colon cancer and received right hemicolectomy. He recovered well after the operation and had been taking oral omeprazole 20 mg twice daily since then till now.
The patient had a soft abdomen. The body temperature was 36.5°C. The white blood cell (WBC) count was 3.96 × 109/L, and the neutrocyteproportion (N%) was 51.7%. The procalcitonin was normal (0.07 ng/mL). Infection with common pathogenic bacteria was first excluded. The abdominal computed tomography showed that his right colon presented postoperative view; other than that, no obvious abnormality was observed. The positron emission tomography/computed tomography (PET/CT) revealed dilatation of part of small intestine and incomplete intestinal obstruction, with no signs of tumor recurrence (Fig. 1). In combination of the level of tumor markers, which were normal, tumor recurrence was then excluded. The standing abdominal plain film showed several air-fluid levels (Fig. 2A). The upper gastrointestinal radiography showed accumulation of gas in distal jejunal and dilatation of it (Fig. 2B). On the basis of all above, he was diagnosed as incomplete intestinal obstruction and postoperation of colon cancer. The treatment included electrolyte replacement and rehydration, parenteral nutrition support, and omeprazole 40 mg intravenously daily to suppress acid.
Figure 1.
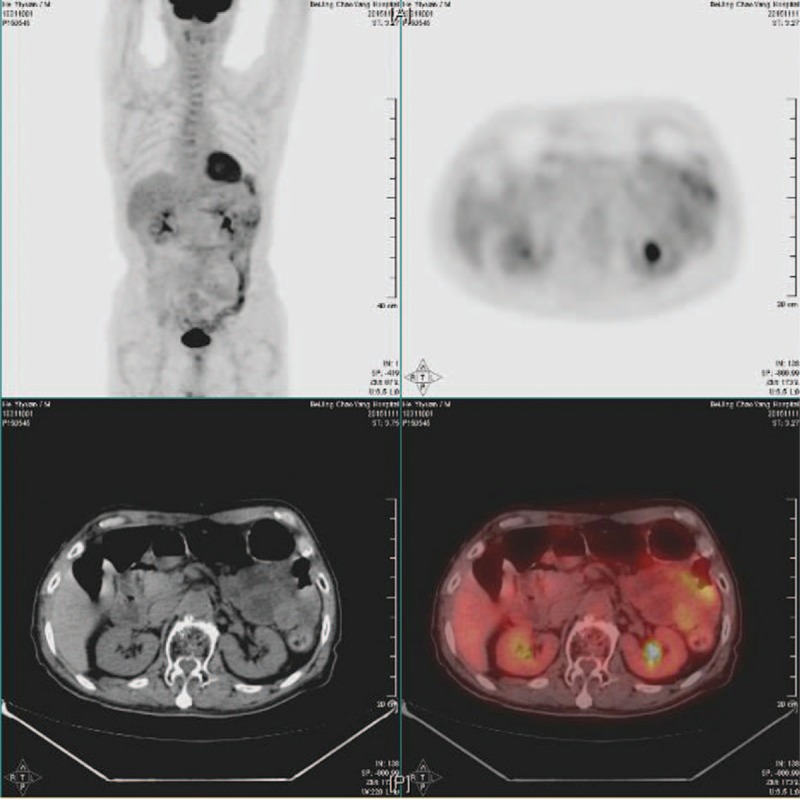
The positron emission tomography/computed tomography (PET/CT). Signals were normal except for local high metabolism in the colon, which was most probably attributed to contents in it.
Figure 2.
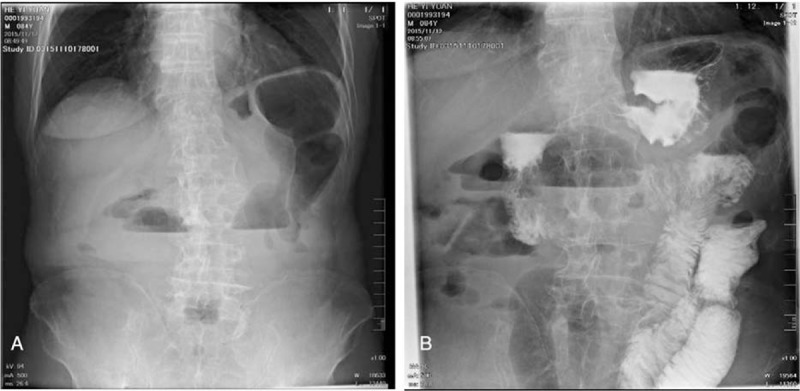
The standing abdominal plain film (A) and the upper gastrointestinal radiography (B). The standing abdominal plain film showed several air-fluid levels (A). The upper gastrointestinal radiography showed accumulation of gas in distal jejunal and dilatation of it (B).
However, after 10 days’ treatment, he developed watery stool, 10 times a day at most. Leucocytes in stool were 6/HP and the occult blood test was positive. Montmorillonite powder was given, but diarrhea did not get any better. No fungal spores or candida were found in stool sample. CDAD was seriously suspected. The stool sample was sent immediately for inspection for C. difficile toxins and turned out to be positive. Omeprazole was withdrawn immediately and treatment with oral vancomycin 0.25 g 4 times per day was started. After 10 days’ treatment of vancomycin, diarrhea was resolved, so long as the acid reflux and vomiting. Another stool culture was sent and C. difficile toxins turned negative. No leucocytes were found anymore in stool and the fecal occult blood test turned negative. The patient discharged then.
3. Discussion
The diagnosis was confusing at first. With no vigilance of his history of 4 years intake of omeprazole, CDI was ignored. The patient had been taking omeprazole for the last 4 years before this admission to the hospital. His gastric acid secretion was under suppression for quite a long time. The increased gastric pH greatly weakened the defense mechanism for suppressing C. difficile or its spores, which made him much more vulnerable to the C. difficile colonization. C. difficile colonized patients could be asymptomatic, which was more prevalent than symptomatic CDI among hospital patients.[11] So, he could probably have C. difficile colonization before his admission. Asymptomatic C. difficile colonized patients could potentially act as an infection reservoir and might progress to symptomatic CDI.[4,12] Treatment of intravenous omeprazole in the hospital might trigger the progression to symptomatic CDI.
Nowadays, PPIs are commonly prescribed in the outpatient and inpatient settings worldwide, even more prevalent in Asia. Lu et al[13] surveyed the Swedish Prescribed Drug Register and found sales of PPIs increased by over 50% from 2000 to 2008. Unnecessary use and overuse of PPIs are the main problems now. Unnecessary use occurs when acid suppression therapy is prescribed without an appropriate indication. Overuse occurs when the dosing and the course of treatment exceeds what is really needed. Chia et al[14] reported the overuse of PPIs in Asian populations. Many patients once started on a PPI in a hospital would frequently continue on for an indefinite period of time even after discharge. Sir William Osler, the founder father of Johns Hopkins Hospital said that, one of the first duties of the physician was to educate the masses not to take medicine. What we have done is obeying this discipline. The expected course of acid suppression should be seriously considered when initiating therapy[15] and clearly told to the patients.
Then how to solve the PPIs overuse problem? In our opinion, making clinical pathways specified on PPIs use could be an effective measure. Clinical pathways, also known as care pathways, critical pathways, integrated care pathways or care maps, are one of the main tools used to manage the quality in health care concerning the standardization of care processes. It has been shown that their implementation reduces the variability in clinical practice and improves outcomes.[16,17] Generally, clinical pathways refer to medical guidelines, while we could also make a single pathway specified on PPIs use, to promote rational use and efficient patient care based on evidence-based medicine. We are now working on this specified clinical pathway in 8 hospitals in Beijing, China. The effect is being expected.
4. Conclusion
PPIs are among the most widely sold drugs in the world, while asymptomatic C. difficile colonization induced by PPIs was easily neglected by surgeons, which might delay the patients’ treatment or even lead to serious medical outcomes. It deserved more attention. On the contrary, overuse and unnecessary use of PPIs are a worldwide problem now. We strongly agree and recommend that the expected course of acid suppression should be seriously considered by doctors when initiating therapy. The clinical pathway specified on PPIs use could be a practical way to solve this problem and promote efficient patient care.
Footnotes
Abbreviations: CDAD = Clostridium difficile associated diarrhea, CDI = Clostridium difficile infection, PET/CT = positron emission tomography/computed tomography, PPIs= proton pump inhibitors.
Written informed consent was obtained from the patient to participate to this case report and any accompanying images. This material is readily available should the Editor request them.
The authors report no conflicts of interest.
References
- [1].McFarland LV, Mulligan ME, Kwok RY, et al. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989;320:204–10. [DOI] [PubMed] [Google Scholar]
- [2].Barbut F, Jones G, Eckert C. Epidemiology and control of Clostridium difficile infections in healthcare settings: an update. Curr Opin Infect Dis 2011;24:370–6. [DOI] [PubMed] [Google Scholar]
- [3].Hubner C, Hubner NO, Muhr M, et al. Cost analysis of hospitalized Clostridium difficile-associated diarrhea (CDAD). GMS Hyg Infect Control 2015;10:Doc13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Furuya-Kanamori L, Marquess J, Yakob L, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis 2015;15:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kong LY, Dendukuri N, Schiller I, et al. Predictors of asymptomatic Clostridium difficile colonization on hospital admission. Am J Infect Control 2015;43:248–53. [DOI] [PubMed] [Google Scholar]
- [6].Leekha S, Aronhalt KC, Sloan LM, et al. Asymptomatic Clostridium difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control 2013;41:390–3. [DOI] [PubMed] [Google Scholar]
- [7].Biswal S. Proton pump inhibitors and risk for Clostridium difficile associated diarrhea. Biomed J 2014;37:178–83. [DOI] [PubMed] [Google Scholar]
- [8].Croft L, Ladd J, Doll M, et al. Inappropriate antibiotic use and gastric acid suppression preceding Clostridium difficile infection. Infect Control Hosp Epidemiol 2016;37:494–5. [DOI] [PubMed] [Google Scholar]
- [9].Cadle RM, Mansouri MD, Logan N, et al. Association of proton-pump inhibitors with outcomes in Clostridium difficile colitis. Am J Health Syst Pharm 2007;64:2359–63. [DOI] [PubMed] [Google Scholar]
- [10].Pakyz AL, Jawahar R, Wang Q, et al. Medication risk factors associated with healthcare-associated Clostridium difficile infection: a multilevel model case-control study among 64 US academic medical centres. J Antimicrob Chemother 2014;69:1127–31. [DOI] [PubMed] [Google Scholar]
- [11].Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011;365:1693–703. [DOI] [PubMed] [Google Scholar]
- [12].Riggs MM, Sethi AK, Zabarsky TF, et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007;45:992–8. [DOI] [PubMed] [Google Scholar]
- [13].Lu Y, Sverden E, Ljung R, et al. Use of non-steroidal anti-inflammatory drugs and proton pump inhibitors in correlation with incidence, recurrence and death of peptic ulcer bleeding: an ecological study. BMJ Open 2013;3:pii: e002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chia CT, Lim WP, Vu CK. Inappropriate use of proton pump inhibitors in a local setting. Singapore Med J 2014;55:363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mezoff EA, Cohen MB. Acid suppression and the risk of Clostridium difficile infection. J Pediatr 2013;163:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care 2003;15:509–21. [DOI] [PubMed] [Google Scholar]
- [17].Song XP, Tian JH, Cui Q, et al. Could clinical pathways improve the quality of care in patients with gastrointestinal cancer? A meta-analysis. Asian Pac J Cancer Prev 2014;15:8361–6. [DOI] [PubMed] [Google Scholar]


