Abstract
Background:
Methimazole is an antithyroid drug that is widely used for the treatment of hyperthyroidism. As an inhibitor of the enzyme thyroperoxidase, methimazole is generally well-tolerated. However, there have been increasing reports of methimazole-induced liver damage, although this effect of methimazole has been limited by the absence of objective diagnosis of the liver condition or the inappropriate use of the Naranjo scale. We present the case of an elderly man with hyperthyroidism, gastritis, and epilepsy who developed liver damage after administration of multiple drugs.
Key points from the case:
Considering the low sensitivity of the Naranjo scale in detecting rare reactions associated with liver damage, we used the Roussel-Uclaf Causality Assessment Method scale, with a finding of cholestatic jaundice hepatitis induced by methimazole. The patient's liver enzyme levels improved after discontinuation of methimazole.
Main lessons learned:
Our case underlines the possible hepatoxicity associated with the use of methimazole. A review of the literature confirmed a selective hepatoxicity risk in individuals of Asian ethnicity, which has not been identified in Caucasian or Black populations. Physicians should be aware of the risk of hepatoxicity when prescribing oral methimazole to patients of Asian ethnicity.
Keywords: cholestatic jaundice, CIOM/RUCAM scale, hyperthyroidism, methimazole
1. Introduction
Methimazole is a widely prescribed thyrotoxic drug for the treatment of hyperthyroidism. Common adverse effects of methimazole, including rash, indigestion, and vomiting, are generally well-tolerated.[1] However, methimazole-induced cholestatic hepatitis is a rare but serious adverse reaction that may be misdiagnosed using the Naranjo scale, [2] and that has often been reported from observation only in the literature.[3–6] A reliable assessment is required to identify possible methimazole-induced hepatic damage in patients using multiple drugs, with potential liver toxicity. In this report, we describe a rare case of cholestatic liver injury in a 69-year-old man with hyperthyroidism, which was identified as being specifically associated with the use of oral methimazole using the Roussel-Uclaf Causality Assessment Method (RUCAM) scale, instead of the Naranjo scale.
2. Case report
2.1. Presenting concerns and relevant demographic information
A 69-year-old Asian man was admitted to the affiliated Yancheng Hospital of Southeast University Medical College with complaints of pruritus and lack of appetite. He had developed jaundice 1 week before admission. The patient had no history of liver disease and did not report any prior allergy to medications, with no history of alcohol use, smoking, or the use of illicit drugs. The patient was being treated for epilepsy and gastroesophageal reflux, which included the use of carbamazepine (CBZ; 200 mg/bid) and omeprazole (20 mg/qd). At the time of admission, he had been taking CBZ for 10 years, with monthly monitoring to ensure stable plasma levels of CBZ and normal liver function tests. Moreover, he had been taking omeprazole for >3 months, without any obvious adverse effects. The patient's surgical history included radical surgery for esophageal carcinoma performed 5 years prior. He had been diagnosed with hyperthyroidism, with methimazole treatment (10 mg/tid) initiated 4 weeks before admission. Relevant laboratory findings at 4 weeks before admission are reported in Table 1.
Table 1.
The laboratory values of the patient 4 weeks ago.

2.2. Clinical findings
On admission, the patient was awake and alert, with no fever or severe icterus in the sclera, a blood pressure of 133/81 mm Hg, respiratory rate of 19 breaths/min, and heart rate of 71 beats/min. Physical examination findings of the abdomen, heart, liver, and spleen were unremarkable. However, the patient was thin.
2.3. Diagnostic assessments
Liver function tests revealed slightly increased levels of aspartate aminotransferase (AST; 88.2 U/L) and alanine aminotransferase (ALT; 59.2 U/L), with remarkably high levels of alkaline phosphatase (ALP; 631 U/L) and total bilirubin (TB; 370.3 μmol/L), and direct bilirubin and indirect bilirubin levels of 295.6 μmol/L (normal range 0–5 μmol/L) and 74.7.0 μmol/L (normal range 0–19 μmol/L), respectively. Results of serologic tests for infectious mononucleosis and acute viral hepatitis A, B, and C were negative. Results for antinuclear antibody titer, antismooth muscle antibody, ceruloplasmin, alpha fetoprotein, carcinoembryonic antigen, and tumor marker 125 and 199 were normal as well.
Abdominal ultrasonography performed on postadmission day 2 revealed thickening of the gallbladder, with a moderate amount of debris, with no evidence of stones in the common bile duct or gallbladder. Hepatobiliary iminodiacetic acid scan revealed delayed gallbladder visualization, which is consistent with chronic cholecystitis. Magnetic resonance cholangiopancreaticography excluded the presence of any stone, stricture, or mass in the common bile duct, allowing us to exclude the diagnosis of obstructive jaundice.
The plasma concentration of CBZ was slightly elevated (13.57 mg/L), and the dose was adjusted to 200 mg/100 mg/d, with therapeutic drug monitoring (TDM) of CBZ on day 3, indicating a normal level of 11.02 mg/L. This change in CBZ level did not influence liver enzyme levels. The patient's TDM for concentration of CBZ, for the 15-day period of hospitalization, is reported in Fig. 1.
Figure 1.
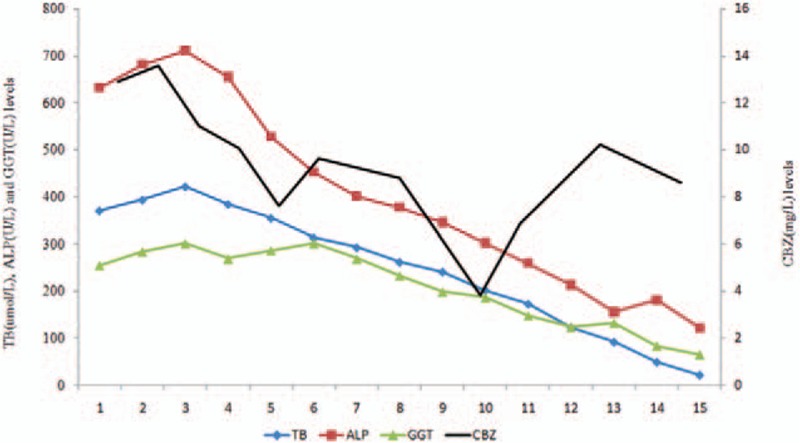
The patient′s liver enzyme levels are shown; only TB, ALP, and GGT included which were most relevant to cholestatic jaundice on the left and CBZ levels on the right over the course of his hospitalization (days 1–15). Methimazole was discontinued on day 3 by RUCAM scale; the doses of CBZ were adjusted by TDM on days 2 and 10, respectively. ALP = alkaline phosphatase, CBZ = carbamazepine, GGT = gamma-glutamyl transferase, TB = total bilirubin, TDM = therapeutic drug monitoring.
Based on an assumption of drug-induced liver injury, we used the Naranjo scale, with a score of 4 calculated for CBZ and 3 for methimazole, with an adverse drug reaction (ADR) score of 1 to 4 possible. Using the RUCAM scale, the ADR score for CBZ remained at 4, with the score for methimazole increasing to 7. Based on the RUCAM scale, methimazole was likely to be the cause of cholestatic jaundice.
2.4. Therapeutic interventions
Institutional review board approval is not required for routine treatment. After weighed against the risk of drug-induced liver injury (DILI), written consent was obtained from the patient. Based on the RUCAM score, methimazole was discontinued on day 3.
Further, considering the possible contribution of CBZ to liver toxicity, we maintained the adjusted dose of CBZ by TDM, omeprazole treatment was continued. Liver enzyme levels decreased rapidly over the next 10 days, as follows: ALP 122 U/L, gamma-glutamyl transferase 64.9 U/L, and TB 21.3 μmol/L, with normal levels of AST and ALT. Liver enzyme levels throughout the 15-day period of hospitalization are shown in Fig. 1. Of note, the plasma concentration of CBZ fluctuated, but was maintained within the allowable range.
2.5. Follow-up and outcomes
The patient was discharged on postadmission day 15, with omeprazole 20 mg/d and CBZ 200 mg/bid continued at home. With regard to the hyperthyroidism, the patient would be a candidate for radioactive iodine ablation of the thyroid gland.
3. Discussion
According to the guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists, methimazole is recommended for patients who are thyrotoxic.[7] However, there have been previous reports of hepatoxicity complications with the use of methimazole.[4–6] A review of the 30 previously reported cases of methimazole-induced hepatoxicity indicated that women are at a higher risk than men for methimazole-induced cholestasis.[2,6] In fact, excluding our case, only 2 previous cases of hepatic toxicity, due to thiamazole treatment, have been reported in men since the first case of methimazole-induced hepatotoxicity reported by Hung et al[8,9] in 1999. Of note, cases of hepatoxicity in males have only been reported in individuals of Asian ethnicity; however, whether Asian males are at higher risk for methimazole-induced toxicity remains to be clarified.
Although previous studies have used the Naranjo scale to estimate methimazole-induced cholestatic jaundice,[2] this scale does not have sufficient sensitivity to establish causality in cases of suspected or rare DILI. Specifically, the Naranjo scale uses drug concentrations and monitoring, and, therefore, dose effects, including effects of a decreasing dose, are not considered in the determination of DILI. As such, the Naranjo scale does not estimate the contribution of dose-dependent effects on ADRs. In contrast, the RUCAM scale provides a liver-specific method for a more accurate assessment of DILI than the Naranjo scale.[10] Accordingly, we selected to use the RUCAM scale to evaluate the causality between the methimazole dose and cholestasis, with the score decreasing significantly after methimazole discontinuation, and confirmed that the dose adjusted of CBZ was not a contributing factor. It is known that an excessive serum concentration of CBZ can lead to hepatotoxicity.[11] However, in our case, liver enzyme levels remained high even after CBZ levels were adjusted by TDM, on postadmission day 2. It is possible that cholestasis impaired excretion of CBZ, contributing to the increased liver enzyme levels. This potential interaction between cholestasis and CBZ on liver enzyme levels remains to be evaluated in future studies. We recognize that assessment of liver pathology by biopsy is an important component of the validated RUCAM scale in cases of ADR. However, we deemed that an invasive procedure would result in discomfort in our elderly patient. Moreover, drug-drug interactions between CBZ and omeprazole had not been previously reported in this patient.
4. Conclusions
Based on our case and previous cases of male-specific methimazole-induced hepatotoxicity, it is possible that Asian men may be at specific risk, and, therefore, physicians and clinical pharmacists should be aware of this potential risk when prescribing oral methimazole in Asian men. DILI is difficult to assess in patients using 2 or more drugs with potential hepatotoxicity. Compared with the Naranjo scale, the RUCAM scale provides a better definition of factors to be considered when evaluating DILI, and a more accurate measurement of these factors in the determination of DILI, including methimazole-induced hepatotoxicity and should be used in practice.
Footnotes
Abbreviations: ADR = adverse drug reaction, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CBZ = carbamazepine, DILI = drug-induced liver injury, RUCAM = Roussel-Uclaf Causality Assessment Method, TB = total bilirubin, TDM = therapeutic drug monitoring.
Funding: This work was supported by grant from Jiangsu Provincial Commission of Health and Family Planning, China (QNRC2016465).
The authors have no conflicts of interest to declare.
References
- [1].Cooper DS. Drug therapy: antithyroid drugs. New Engl J Med 2005;352:905–17. [DOI] [PubMed] [Google Scholar]
- [2].Ramos-Bonner LS, Goldberg TH, Moyer S, et al. Methimazole-induced cholestatic jaundice in anelderly hyperthyroid patient. Am J Geriatr Pharmacother 2007;5:236–40. [DOI] [PubMed] [Google Scholar]
- [3].Zhang M, Zhou H, He R, et al. Steroids for the treatment of methimazole-induced severecholestatic jaundice in a 74-year-old woman with type 2 diabetes. Endocrine 2010;37:241–3. [DOI] [PubMed] [Google Scholar]
- [4].Sein-Anand J, Chodorowski Z. Drug-induced liver failurecaused by thiamazole and methimazole–a case report. Przegl Lek 2007;64(4/5):320–1. [Polish]. [PubMed] [Google Scholar]
- [5].Yang J, Zhong J, Zhou LZ, et al. Sudden onset agranulocytosis and hepatotoxicityafter taking methimazole. Intern Med 2012;51:2189–92. [DOI] [PubMed] [Google Scholar]
- [6].Zou H, Jin L, Wang LR, et al. Methimazole-induced cholestatic hepatitis: two cases report and literature review. Oncotarget 2016;7:5088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leo SD, Sun YL, Braverman LE. Hyperthyroidism. Lancet 2016;388:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hung YT, Yu WK, Chow E. Delayed cholestatic hepatitis due to methimazole. Hong Kong Med J 1999;5:200–1. [PubMed] [Google Scholar]
- [9].Shen C, Zhao CY, Liu F, et al. Acute-on-chronic liver failure due to thiamazole in a patient with hyperthyroidism and trilogy of Fallot: case report. BMC Gastroenterol 2010;10:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2016;17:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kalapos MP. Carbamazepine-provoked hepatotoxicity and possible aetiopathological role of glutathione in the events. Retrospective review of old data and call for new investigation. Adverse Drug React Toxicol Rev 2002;21:123–41. [DOI] [PubMed] [Google Scholar]


