Abstract
Abdominal pain is one of the key symptoms of irritable bowel syndrome (IBS). Studies have indicated an increase in the incidence of IBS in Asia. However, yet the pathophysiology of this disease remains unknown. Women are more likely to develop the condition than men, especially the constipation-predominant type. Essential fatty acid (EFA) malnutrition is one of several theories discussing the mechanism of IBS.
The authors hypothesized that significant EFA deficiency may cause abdominal pain in patients with IBS. However, because patterns in the oral intake of EFAs differ between cultures, the authors narrowed this study to examine the nutritional status of Asian female patients with IBS
The authors investigated Asian female patients with IBS and compared them with a group of healthy controls. Thirty patients with IBS and 39 healthy individuals were included in this study. The participants’ age, height, weight, and waist size were recorded. The 24-item Hamilton Depression Rating Scale was documented. Both erythrocyte and plasma fatty acid content were analyzed through gas–liquid chromatography.
The authors found that patients with IBS exhibited significantly higher scores for depression, higher proportions of plasma saturated fatty acids and monounsaturated fatty acids, and lower proportions of docosahexaenoic acid and total omega-3 polyunsaturated fatty acids in plasma are associated with IBS in Asian female patients. Further study is indicated to confirm the causality of this association.
Keywords: docosahexaenoic acid, eicosapentaenoic acid, essential fatty acid, irritable bowel syndrome
1. Introduction
Irritable bowel syndrome (IBS) is a disorder that presents with normal gross appearance of the gastrointestinal tract but irritating symptoms such as abdominal pain and bowel habit changes. Abdominal pain is a particularly key characteristic of IBS,[1,2] yet the underlying mechanism of IBS remains poorly understood. Several mechanisms have been proposed to explain the cause of IBS; one of them is essential fatty acid (EFA) malnutrition,[3] which can cause low-grade inflammation.[4]
Fatty acids are divided into saturated fatty acids (SFAs) and unsaturated fatty acids, with the latter being further divided into monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). Omega-3 and omega-6 fatty are the 2 most crucial components of PUFAs. Alpha-linolenic acid (ALA, C18:3n3) and linoleic acid (LA, C18:2n6) are EFAs.[5] Both ALA and LA must be obtained through the diet because they cannot be synthesized by the body. Long-chain PUFAs, such as eicosapentaenoic acid (EPA, C20:5n3), docosahexaenoic acid (DHA, C22:6n3), and arachidonic acid (AA, C20:4, n6), are acquired from food or synthesized from EFAs.[5] A crucial function of EFAs, omega-3 fatty acids, and omega-6 fatty acids is the regulation of chronic inflammation,[4,6] because long-chain PUFAs work as precursors to generate lipoxins and resolvins, which exert strong anti-inflammatory activity.[5] Both ALA and LA also have a role in cell membrane signaling and function.[7]
Siguel and Lerman noted PUFA deficiency in patients with chronic gastrointestinal disorder.[8] Malnutrition with EFA insufficiency was also observed in diseases of the bowel, with poor EFA absorption noted in patients with celiac disease.[9] In research on IBS, Solakivi et al found intestinal PUFA malabsortion to be the main characteristic of IBS.[3] Sun et al showed that DHA (22:6n3) in erythrocytes and plasma provided the strongest correlations with EFAs intake.[10] Erythrocyte fatty acid content represents the long-term intake of EFAs, whereas plasma fatty acid content indicates more recent dietary intake of EFAs.[11]
A meta-analysis by Lovell and Ford showed that the incidence of IBS is moderately higher among women than men. The constipation-predominant type of IBS is also more common in women.[12]
The authors hypothesized that patients with IBS might have a unique fatty acid deficiency that may produce low-grade inflammation, thereby causing abdominal pain. Different culture and gender may have difference sense and reaction to pain sensation. Study found that Eastern women have better pain tolerance than Western people.[13,14] Different cultures have different food intake patterns, and the presentation of stool patterns differs between men and women[12]; therefore, to avoid bias, the authors analyzed the erythrocyte and plasma fatty acid content of Asian female patients with IBS to investigate the role of fatty acid nutrition in IBS development.
2. Methods
This study was conducted at Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan. The Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital approved this study (20120813R), and the study was registered at ClinicalTrials.gov (NCT02179905).
2.1. Patients
This study included 30 patients with IBS and 39 healthy individuals from the Division of Gastroenterology at the study hospital. The period of recruitment is from January 2013 to December 2013. All participants were Asian women with ages ranging from 20 and 50 years. The Rome III Diagnostic Criteria for IBS were used as the inclusion criteria to confirm the IBS diagnosis. Patients were excluded if they had colon and small intestinal disease, psychiatric disease, or diabetes, or if they had received major surgery in the past 5 years or if they had a metal implant.
Demographic data including the participants’ age, height, weight, and waist size were recorded. All patients received a psychometric interview. The 24-item Hamilton Depression Rating Scale was used for the depression evaluations, respectively.[15]
2.2. Fatty acid analysis
Serum samples were taken after overnight fasting and were stored at −80°C until use. Gas–liquid chromatography was employed to explore the serum fatty acid and erythrocyte fatty acid content.[16]
The authors extracted the lipids in plasma and erythrocytes into methanol and chloroform. The chloroform phase was dehydrated under N2. The lipids were then hydrolyzed and transesterified. After water was added, fatty acid methyl esters were evaporated and redissolved in heptadecanoic acid and then measured through gas–liquid chromatography. Individual peaks were identified by comparing them with standards.[16] The amounts of each fatty acid are stated as a percentage of total fatty acids.
2.3. Statistical analysis
All of the results are presented as the mean. The Student t test was used to analyze the difference between the controls and patients with IBS.
3. Results
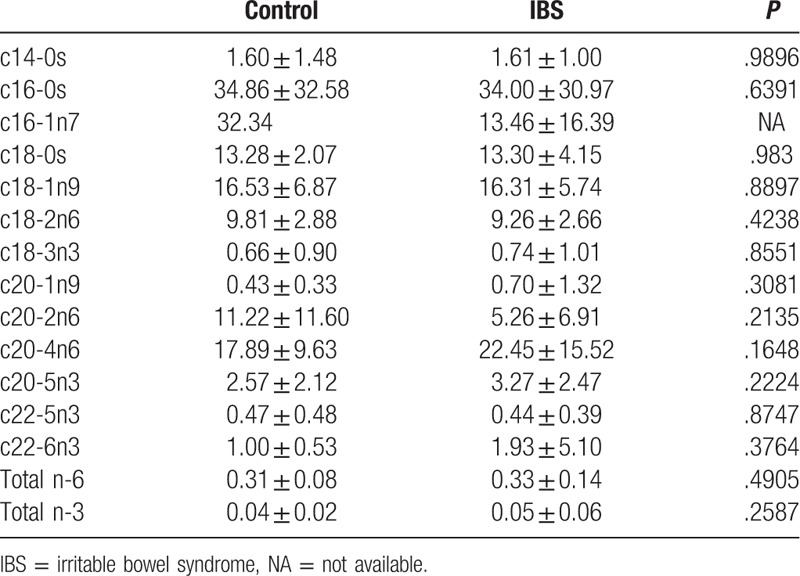
Compared with the controls, the patients in the IBS group were on average slightly younger (36.2 ± 7.2 vs 37.2 ± 8.3 years), although the difference was nonsignificant (P > .05), and they attained significantly higher depression scores (24.47 ± 15.94 vs 12.33 ± 9.47; P < .05) (Table 1). In the patients with IBS, the authors found that plasma palmitic acid (c16-0) was significantly reduced, plasma oleic acid (c18-1n9) was increased, plasma DHA (c22-6n3) was reduced, and total plasma omega-3 in IBS patients was lower compared the controls (Table 2). However, no significant difference was observed in the erythrocytes fatty acid levels between the IBS group and controls (Table 3).
Table 1.
Patients’ characters.

Table 2.
Plasma fatty acid between IBS and control individuals.
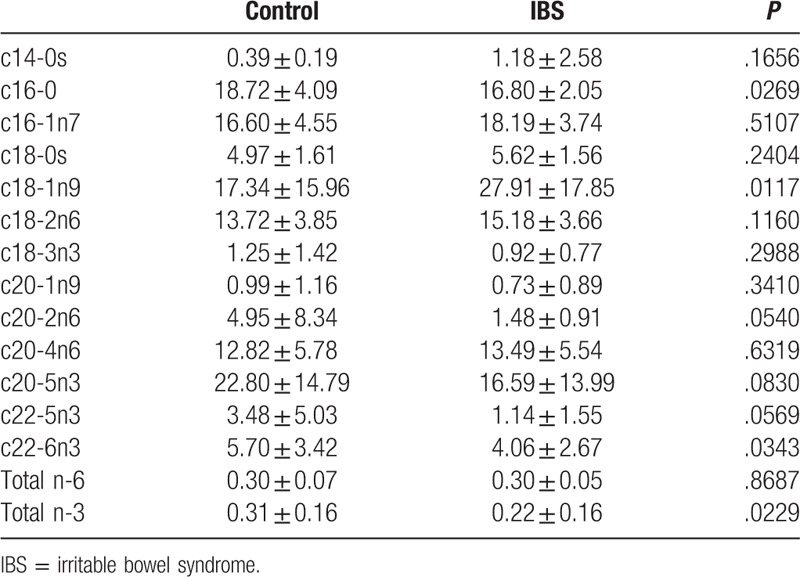
Table 3.
Red blood cell fatty acid between controls and IBS individuals.
4. Discussion
The authors analyzed both erythrocytes and plasma fatty acids. Notably, the authors found that only plasma fatty acids differed between the patients with IBS and controls; the difference in erythrocyte fatty acids was nonsignificant. Katan et al showed that serum cholesteryl esters were more sensitive to recent diet, because the half-life of EPA in the serum is 4.8 days, whereas that of erythrocytes is 120 days, and the incorporation half-life of EPA in erythrocytes is 4 weeks.[11] They concluded that erythrocyte fatty acids reflect oral intake over recent months, whereas serum fatty acids reflect oral intake over recent weeks. This may explain why our patients were deficient in fatty acids.
Compared with the controls, our patients with IBS had higher proportions of SFAs and MUFAs and lower DHA and total n-3 in plasma. The results are similar to those of Solakivi et al.[3] The relatively low PUFA levels in plasma may have enhanced the endogenous synthesis of SFAs and MUFAs. This phenomenon may reflect the increase in oleic acid desaturase activity and augmented lipid peroxidation, and could be an early marker of protein-calorie malnutrition in patients with IBS.[17]
Palmitic acid is converted from carbohydrates. Gupta et al found that palmitic acid might engender the release of tumor necrosis factor alpha and interleukin 6 from cultured astrocytes and may be related to inflammatory processes, whereas DHA inhibits palmitic acid activity in inflammatory signaling in astrocytes.[18] Mazidi et al study showed subclinical inflammation marker, high sensitivity C-reactive protein, is possible modulated by dietary fatty acid intake.[19] In this study, palmitic acid was higher and the DHA level was lower in the IBS group than in the controls, and this may be related to the inflammatory process in patients with IBS.
No significant difference was observed in plasma AA between the IBS group and controls. This result is in contrast to the findings of Clarke et al,[4] who found that AA and total n-3 PUFA levels were higher in patients with IBS than in the controls, despite both the present study and the study by Clarke et al investigating IBS in women.
In this study, the authors found that depression was more prevalent among the patients with IBS, and they also exhibited a lower percentage of DHA and total n-3 series compared with the controls. Previous studies have shown that the incidence rates of depression and anxiety are higher in patients with IBS and that depression and anxiety can also increase the risk of developing IBS.[20,21] PUFA plays a key role in neurotransmission and immunological regulation in the brain cell membrane.[7] Many studies have shown that omega-3 deficiency is related to the symptoms of depression. This study results are similar to those reported by Lin et al, in which patients with depression were shown to have lower DHA and total n-3 PUFA levels but not lower EPA levels.[22] However, Peet and Horrobin reported that EPA, but not DHA, inhibits phospholipase A2 and plays a critical role in depression.[23] In a double-blind, placebo-controlled trial, interventional study by Su et al, supplementation of omega-3 PUFAs was found to ameliorate major depressive disorders.[24] The evidence from these studies suggests that omega-3 fatty acids play a key part in the regulation of depression.
Testing for long-chain PUFA deficiency is suitable for screening for malnutrition. A previous study revealed that malabsorption and inadequate intake are related to PUFA deficiency in patients with chronic intestinal disorders.[8] However, in this study, although the authors did not record the dietary intake of the participants, the results are still useful for predicting the type of fat in the diet,[25] because the production of EFAs and DHA from ALA is only 5% and <0.5%, respectively, and ALA must be taken orally.[26] In general, the proportion of long-chain PUFAs received from oral intake is higher.
In this study, depression scores were higher among the patients with IBS than the controls. Furthermore, PUFA deficiency was predominant in the IBS group. Kilkens et al[27] reported that both affective regulation and IBS share the same condition of omega-3 PUFA deficiency. PUFAs play an important role in signaling in the brain cell membrane. This may be evidence that both diseases share part of the same mechanism. Antidepressive treatment and omega-3 were demonstrated to alleviate depression.[24] Antidepressive agents may also be effective in the treatment of IBS,[28] possibly indicating that PUFA supplements may be effective in treating IBS patients with abdominal pain; however, further interventional research must be conducted to test this hypothesis.
The authors confined this study to Asian female IBS patient to avoid bias of different culture, gender, and food cultures in IBS analysis. Studies showed that different cultures have different actions to pain sensation.[13] Western people have poorer toleration than Eastern population, and men are less tolerant of pain than are women. People searching medical care and treatment from overs in Asia is now a trend.[29] Different cultures may have medical variability. The nutrition status should take into consideration in patient with different countries and races.
5. Conclusions
Short-term lower level of higher proportions of plasma SFAs and MUFAs, and lower proportions of DHA and total omega-3 PUFAs in plasma is with the unique symptoms of IBS. However, the causality of this association needs to be confirmed in further study.
Acknowledgment
The authors thank our participants for their participation in this study.
Footnotes
Abbreviations: AA = arachidonic acid, ALA = alpha-linolenic acid, DHA = docosahexaenoic acid, EFA = essential fatty acid, EPA = eicosapentaenoic acid, IBS = irritable bowel syndrome, LA = linoleic acid, MUFA = monounsaturated fatty acid, PUFA = polyunsaturated fatty acid, SFA = saturated fatty acid.
H-WC and J-LH have contributed equally to this work.
Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-102-DR-14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- [2].Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol 2010;7:565–81. [DOI] [PubMed] [Google Scholar]
- [3].Solakivi T, Kaukinen K, Kunnas T, et al. Serum fatty acid profile in subjects with irritable bowel syndrome. Scand J Gastroenterol 2011;46:299–303. [DOI] [PubMed] [Google Scholar]
- [4].Clarke G, Fitzgerald P, Hennessy AA, et al. Marked elevations in pro-inflammatory polyunsaturated fatty acid metabolites in females with irritable bowel syndrome. J Lipid Res 2010;51:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 2006;1:420–39. [DOI] [PubMed] [Google Scholar]
- [6].Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol Aspects Med 2012;33:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Delion S, Chalon S, Herault J, et al. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr 1994;124:2466–76. [DOI] [PubMed] [Google Scholar]
- [8].Siguel EN, Lerman RH. Prevalence of essential fatty acid deficiency in patients with chronic gastrointestinal disorders. Metabolism 1996;45:12–23. [DOI] [PubMed] [Google Scholar]
- [9].Solakivi T, Kaukinen K, Kunnas T, et al. Serum fatty acid profile in celiac disease patients before and after a gluten-free diet. Scand J Gastroenterol 2009;44:826–30. [DOI] [PubMed] [Google Scholar]
- [10].Sun Q, Ma J, Campos H, et al. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81. [DOI] [PubMed] [Google Scholar]
- [11].Katan MB, Deslypere JP, van Birgelen AP, et al. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–22. [PubMed] [Google Scholar]
- [12].Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 2012;107:991–1000. [DOI] [PubMed] [Google Scholar]
- [13].Belfer I. Nature and nurture of human pain. Scientifica (Cairo) 2013;2013:415279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hobara M. Beliefs about appropriate pain behavior: cross-cultural and sex differences between Japanese and Euro-Americans. Eur J Pain 2005;9:389–93. [DOI] [PubMed] [Google Scholar]
- [15].Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol 2003;13:99–103. [DOI] [PubMed] [Google Scholar]
- [17].Squali Houssaini FZ, Foulon T, Payen N, et al. Plasma fatty acid status in Moroccan children: increased lipid peroxidation and impaired polyunsaturated fatty acid metabolism in protein-calorie malnutrition. Biomed Pharmacother 2001;55:155–62. [DOI] [PubMed] [Google Scholar]
- [18].Gupta S, Knight AG, Gupta S, et al. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem 2012;120:1060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mazidi M, Gao HK, Vatanparast H, et al. Impact of the dietary fatty acid intake on C-reactive protein levels in US adults. Medicine 2017;96:e5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanuri N, Cassell B, Bruce SE, et al. The impact of abuse and mood on bowel symptoms and health-related quality of life in irritable bowel syndrome (IBS). Neurogastroenterol Motil 2016;28:1508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med 2016;46:3065–80. [DOI] [PubMed] [Google Scholar]
- [22].Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010;68:140–7. [DOI] [PubMed] [Google Scholar]
- [23].Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59:913–9. [DOI] [PubMed] [Google Scholar]
- [24].Su KP, Huang SY, Chiu CC, et al. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003;13:267–71. [DOI] [PubMed] [Google Scholar]
- [25].Nikkari T, Luukkainen P, Pietinen P, et al. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med 1995;27:491–8. [DOI] [PubMed] [Google Scholar]
- [26].Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 2007;32:619–34. [DOI] [PubMed] [Google Scholar]
- [27].Kilkens TO, Honig A, Maes M, et al. Fatty acid profile and affective dysregulation in irritable bowel syndrome. Lipids 2004;39:425–31. [DOI] [PubMed] [Google Scholar]
- [28].Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS ONE 2015;10:e0127815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yu JY, Ko TG. A cross-cultural study of perceptions of medical tourism among Chinese, Japanese and Korean tourists in Korea. Tourism Manage 2012;33:80–8. [Google Scholar]


