Abstract
Rationale:
Advanced ovarian cancer is usually associated with intra-abdominal metastases and while it commonly spreads directly to the omentum, intestine, liver, or other organs, it can also metastasize through the lymphatic channels and the hematogenous pathway. With an increasing number of invasive operations being performed with chemoradiotherapy, the incidence of extra-abdominal metastases has risen. Nevertheless, ovarian cancer with skin metastases is quite rare.
Patient concerns:
We report a case of ovarian cancer with two independent incidences of skin metastases in the umbilicus and abdominal wall.
Diagnoses:
The patient was a 67-year-old woman who was diagnosed with ovarian cancer stage IIIC and underwent cytoreductive surgery. A solitary brown cauliflower-like metastatic lesion, approximately 6 × 5 × 4 cm was identified in the umbilicus area two years after primary surgery. During tumorectomy, intraoperative exploration revealed that while the tumor was located close to the peritoneum, there was no penetration.
Interventions:
The patient recovered well and received multiple rounds of chemotherapy. Ten months later, the patient presented with skin lesions located on the abdominal wall that grew rapidly and spread from the lower abdomen wall to the bilateral waist and femoral skin. These lesions were multiple, ulcerated, rough heliotrope plaques that produced a foul-smelling faint yellow liquid. Biopsy analysis revealed skin metastasis of poorly differentiated serous adenocarcinoma.
Outcomes:
The patient was treated with chemotherapy but died 3 months after the skin metastasis occurred for the second time.
Lessons:
Ovarian cancer with skin metastasis is a rare condition with poor prognosis. Pathological diagnosis of early skin lesions is essential for ovarian cancer patients and that systemic and local disease should be treated with surgery or palliative therapy in order to provide patients with the best chances of survival. Tumorectomy is appropriate when lesions are isolated and when the patient's performance status is good. However, systemic therapy including chemotherapy and radiotherapy should be considered when skin lesions are associated with severe intro-abdominal disease.
Keywords: abdominal wall metastasis, extra-abdominal metastasis, ovarian cancer, skin metastases, umbilicus metastasis
1. Introduction
Most ovarian cancers are in their advanced stages when first diagnosed and accompanied by pelvic organs metastases, which is the most common route of ovarian cancer metastasis. Ovarian cancer can also metastasize through the lymphatic channels and the hematogenous pathway. The incidence of ovarian cancer associated with skin metastases is rare and ranges from 1.9% to 5.1%.[1–4] Several metastatic patterns are known to exist: isolated cutaneous nodule, multiple cutaneous nodules, inflammatory metastasis, and cicatricial plaques.[5] Typical umbilical metastatic nodules are known as Sister Joseph's nodule, which often represents advanced gastrointestinal tract and ovarian malignancy with a poor prognosis.[6] In this report, we described the case of a patient with ovarian cancer who suffered 2 independent incidences of skin metastases in the umbilicus and abdominal wall within an interval of 10 months.
2. Case presentation
Ethical approval was obtained from Ethics Committee of Qilu Hospital, Shandong University. Informed consent was obtained from the patient for publication of this case report and any accompany images.
The patient was a 67-year-old woman who complained of abdominal distension and was admitted to our hospital in July 2013. Examination revealed massive ascites, smears of which confirmed the presence of cancer cell. Serum carbohydrate antigen 125 (CA125) was 1527 U/mL. The patient's medical history and family history were of no significance. The patient received 2 cycles of neoadjuvant chemotherapy with paclitaxel (175 mg/m2), cisplatin (75 mg/m2), and interleukin-2 (3 MIU); each administered via a single intraperitoneal injection. When the patient's CA125 level fell to within the normal range (8th October 2013), we carried out an exploratory laparotomy, including complete hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendectomy, and cytoreductive surgery for pelvic metastases. Histopathological analysis revealed bilateral ovarian serous adenocarcinoma and omental tissue metastatic adenocarcinoma (Fig. 1A) of International Federation of Gynecology and Obstetrics stage IIIC. The patient refused to accept adjuvant therapy and an elevated CA125 level was detected 1 year after surgery. Consequently, the patient received chemotherapy with paclitaxel (175 mg/m2) and carboplatin with AUC (5 mg/mL/min) for 10 cycles.
Figure 1.
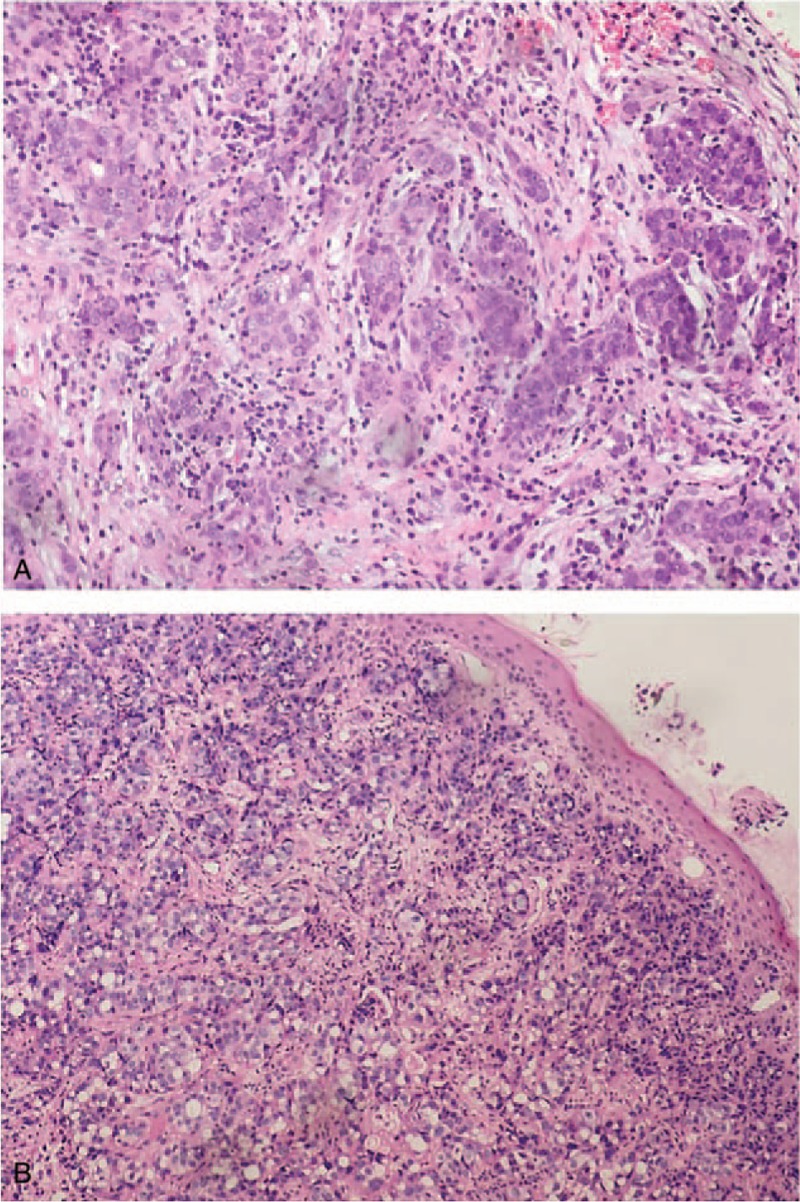
Images of pathological tissue slices (original magnification, ×40). A, Histopathology shows ovarian serous adenocarcinoma after primary surgery. B, A biopsy shows skin metastasis with poorly differentiated serous adenocarcinoma.
In October 2015, a solitary brown cauliflower-like excrescence was found in the umbilicus area. That was 6 × 4 × 5 cm in size. The patient's serum CA125 was 928.3 U/mL and there was no evidence to suggest metastasis in the peritoneum or elsewhere. Tumorectomy was performed on 23rd October 2015. Intraoperative exploration revealed that the tumor was located close to the peritoneum but did not penetrate. The bowel surface was smooth, and biopsy of the parietal peritoneum did not show any obvious metastasis in the abdominal cavity. Histopathological analysis showed that the umbilicus area was associated with subcutaneous metastatic serous adenocarcinoma. The patient received multiple rounds of intravenous chemotherapy after tumorectomy, including cyclophosphamide, pharmorubicin, and cisplatin (1 cycle), ifosfamide and epirubicin (5 cycles), and docetaxel and capecitabine (1 cycle).
In August 2016, 10 months after the skin tumorectomy, the patient again presented with skin lesions located on the abdominal wall that grew rapidly and spread from the lower abdomen wall to the bilateral waist and femoral skin. These lesions were multiple, ulcerated, rough heliotrope plaques up to 30 × 10 cm in size and released a foul-smelling faint yellow liquid. There were multiple, nodular, erythematous lesions, up to 2 cm in size, located on the right breast skin. The patient complained of pain, exudation, and itching (Fig. 2). The biopsy revealed skin metastasis of poorly differentiated serous adenocarcinoma (Fig. 1B) and the CA125 level was 1099 U/mL. Magnetic resonance imaging of the abdomen and pelvis showed multiple metastases in the abdominal wall and bilateral femoral skin, right costicartilage, gluteus muscle, and pelvic cavity (Fig. 3). Because of severe exudation, we were forced to change dressings every other day. The patient received chemotherapy with carboplatin and pemetrexed disodium for 1 cycle. Unfortunately, on 23rd November 2016, 3 months after the second skin metastases, the patient died of multiple organ failure.
Figure 2.
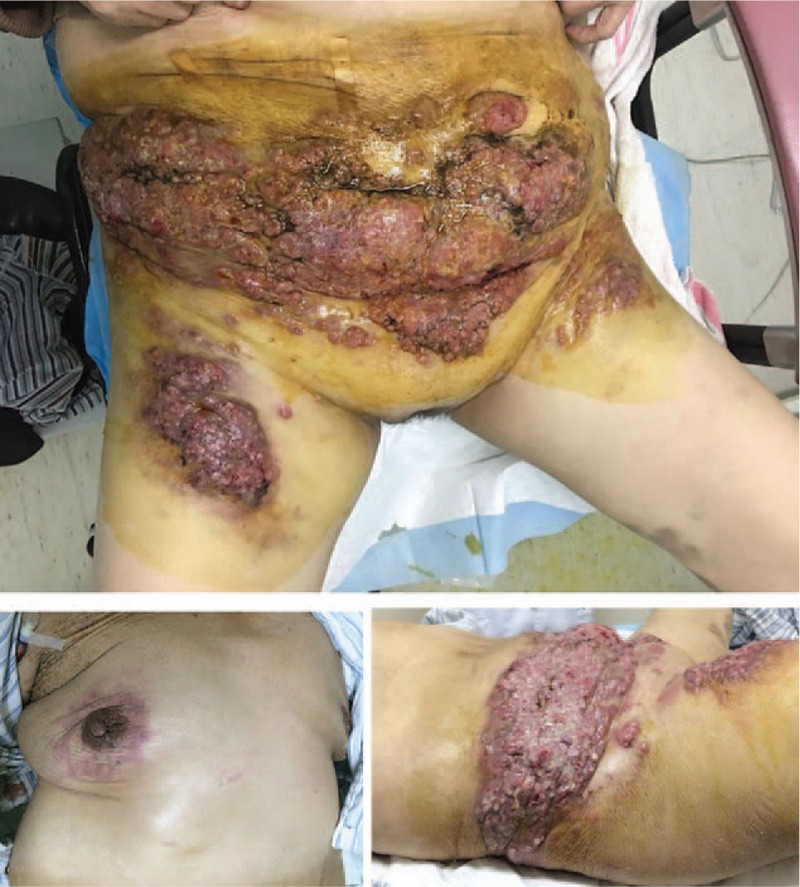
Skin lesions located on the abdominal wall that grew rapidly and spread from the lower abdomen wall to the bilateral waist and femoral skin, and were multiple, ulcerated, rough heliotrope plaques up to 30 × 10 cm in size releasing a foul-smelling faint yellow liquid. There were multiple, nodular, erythematous lesions, up to 2 cm in size located on the right breast.
Figure 3.
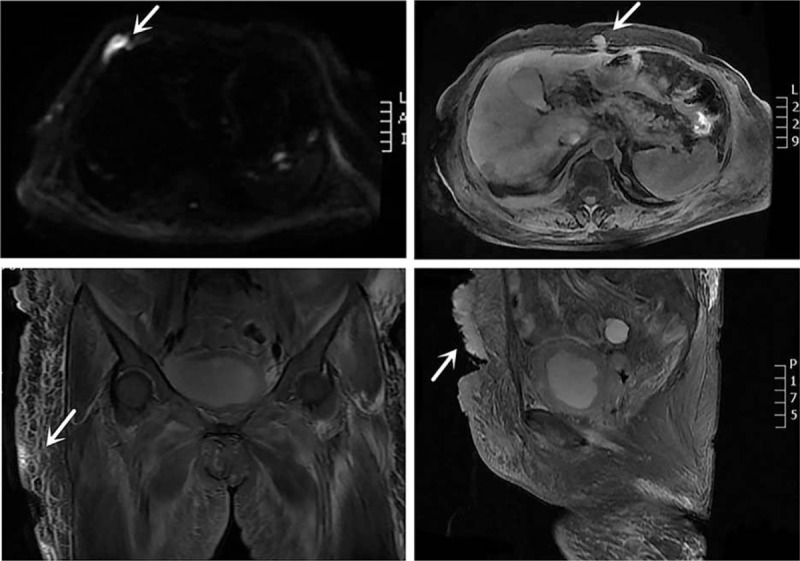
Magnetic resonance imaging (MRI) scan of the abdominal and pelvis shows multiple metastases in the abdominal wall and bilateral thigh skin, right costicartilage, and pelvic cavity (arrows). Lesions showed hyperintensity on diffusion-weighted imaging (DWI).
3. Discussion
Ovarian cancer with extra-abdominal metastasis is rare and accounting for approximately 3% of metastatic site of ovarian cancer. The skin is the fourth common site of extra-abdominal metastases in ovarian cancer; other sites include the pleura, lungs, brain, lymph nodes, and bone.[4,7] Several theories have been put forward to explain the occurrence of skin metastases in ovarian cancer. These include direct invasion from underlying growth, accidental implantation of tumor cells during surgical procedures (iatrogenic), and the contiguous extension of tumor cells throughout the lymphatic root.[8] In some cases, direct invasion of ovarian cancer can occur through surgical scars. Literature also shows that cancer can also progress to the skin and metastasize through the hematogenous and lymphatic pathway.[5,8–22] We present an overview of the current literature relating to ovarian cancer with skin metastasis in Table 1. The most common metastatic site of ovarian cancer is the umbilicus area. It also called Sister Joseph's nodule .[6] Yoon et al.[9] reported a case of mucinous cystadenocarcinoma ovarian cancer that metastasized to the left shoulder and scalp in Korea. In another study, Matsui et al[16] presented a Japanese patient with serous adenocarcinoma ovarian cancer who grew a soft mass, 5 cm in diameter, in the right occipital region of the scalp 29 months after the original diagnosis of ovarian cancer. Nasal metastasis as a first symptom of ovarian cancer has also been reported.[22] In addition, ovarian cancer can metastasize to the skin of the chest and breast, back, vulvovaginal area, arms, and thighs.
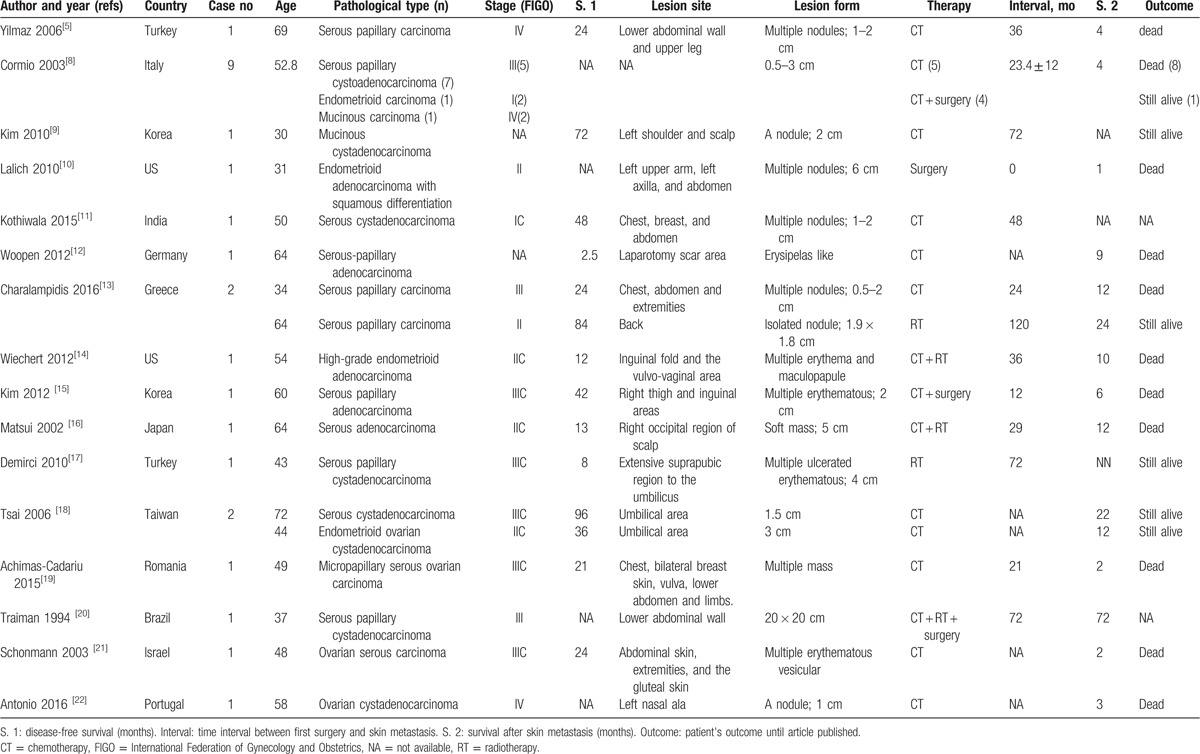
Table 1.
Review of the literature of ovarian carcinoma with skin metastasis.
Skin lesion can be isolated or multiple and skin metastases can occur coincident with the diagnosis of advanced ovarian cancer, or occur during the postoperative period as the disease progression. Several metastatic patterns have been identified: isolated cutaneous nodule, multiple cutaneous nodules, inflammatory metastases, and cicatricial plaques.[5] Cormio et al[4] conducted a research studying involving 162 patients with epithelial ovarian cancer and concluded that the significant risk factors for the development of skin metastases are tumor stage, grade, and lymph node involvement; stages III and IV ovarian cancer are more likely to develop skin metastasis.
Cheng et al[7] performed a retrospective study involving 20 cases of ovarian cancer patients with extra-abdominal metastases and 645 cases of ovarian cancer patients without extra-abdominal metastases and found that some risk factors may be associated with extra-abdominal metastases. The important prognostic factors associated with survival are Karnofsky Performance Status, sensitivity of primary chemotherapy, metastatic site, and systemic therapy after the diagnosis of extra-abdominal metastases.
The prognosis of ovarian cancer with skin metastasis remains poor. Dauplat et al[1] reported that the median time interval between the diagnosis of ovarian cancer and skin metastasis was 12 months (range, 1–41 months). However, the overall survival time after the diagnosis of skin metastasis from ovarian cancer was 4 months (range, 2–65 months) in Cormio et al's case series.[8] The most important prognostic factor associated with survival is the interval time between the diagnosis of ovarian cancer and skin metastases. Data showed a better survival (mean, 9.7 months) in patients who had an umbilical metastasis detected before definitive treatment of the primary tumor,[23] compared with those who had an umbilical metastasis detected after definitive treatment of the primary tumor. Biopsy pathology provides strong evidence to diagnose skin metastasis associated with ovarian cancer. It is worth mentioning that skin metastasis of ovarian cancer should be included in the differential diagnosis of pilomatrical carcinoma. Immunohistochemistry can reveal expression of estrogen receptor, progesterone receptor, keratin 7, and CA125 in the gland-forming elements of tumors.[10] PAX8 expression may be a particularly useful marker to discriminate metastatic ovarian cancers from other primary adnexal cancers and help provide guidance for treatment and prognosis.[24]
There is no treatment criterion for ovarian cancer with skin metastases. The option of therapy depends on the patient's general condition, the location of the metastases, and the history of previous treatments. Control of the abdominal disease remains a major problem in the management of patients with ovarian cancer.[11] It is appropriate to perform surgical resection when cutaneous metastasis is isolated, and the patient's general condition and life expectancy should be evaluated. For extensive cutaneous metastases, palliative therapy is usually the most appropriate because the skin lesions are typically associated with intra-abdominal lesions.[15] It has been reported that electrocoagulation or external radiotherapy may be effective for local control with less pain, hemorrhage, and infection, but these approaches are also limited in the treatment of systemic disease.[17,25]
Existing literature shows that the median interval after skin metastasis was 13.7 months (range, 1–72 months) in the Table 1. The longer the interval between first surgery and skin metastasis, the longer the patient survives. Our patient suffered 2 independent incidences of metastases in the umbilicus and abdominal wall within an interval of 10 months. The first skin lesion was isolated to the cutaneous nodule and did not penetrate the peritoneum. Tumorectomy has proved to be an effective treatment for this kind of lesion. To our knowledge, the second incidence of metastasis in this case was the first to present with such extensive metastatic lesions with such severe intra-abdominal metastases. However, the patient ultimately died from the progression of ovarian cancer.
4. Conclusion
In conclusion, ovarian cancer with skin metastasis is a rare condition with poor prognosis. The learning we can draw from this case is that the pathological diagnosis of early skin lesions is essential for patients with ovarian cancer and that systemic and local disease should be treated with surgery or palliative therapy to provide patients with the best chances of survival. Tumorectomy is appropriate when lesions are isolated and when the patient's performance status is good. However, systemic therapy including chemotherapy and radiotherapy should be considered when skin lesions are associated with severe intra-abdominal disease.
Footnotes
Abbreviations: CA125 = carbohydrate antigen 125, DWI = diffusion-weighted imaging, ER = estrogen receptor, FIGO = International Federation of Gynecology and Obstetrics, KPS = Karnofsky Performance Status, MRI = magnetic resonance imaging.
This work was supported by key research and development project of Shandong Province Science and Technology Development (2015GSF118068).
The authors have no conflicts of interest to disclose.
References
- [1].Dauplat J, Hacker NF, Nieberg RK, et al. Distant metastases in epithelial ovarian carcinoma. Cancer 1987;60:1561–6. [DOI] [PubMed] [Google Scholar]
- [2].Tharakaram S. Metastases to the Skin. Int J Dermatol 1988;27:240–2. [DOI] [PubMed] [Google Scholar]
- [3].Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis 1987;39:119–21. [PubMed] [Google Scholar]
- [4].Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer 2003;13:125–9. [DOI] [PubMed] [Google Scholar]
- [5].Yilmaz Z, Bese T, Demirkiran F, et al. Skin metastasis in ovarian carcinoma. Int J Gynecol Cancer 2006;16(suppl 1 (S1)):414–8. [DOI] [PubMed] [Google Scholar]
- [6].Albano EA, Kanter J. Images in clinical medicine. Sister Mary Joseph's nodule. N Engl J Med 2005;352:1913. [DOI] [PubMed] [Google Scholar]
- [7].Cheng B, Lu W, Xiaoyun W, et al. Extra-abdominal metastases from epithelial ovarian carcinoma: an analysis of 20 cases. Int J Gynecol Cancer 2009;19:611–4. [DOI] [PubMed] [Google Scholar]
- [8].Cormio G, Capotorto M, Vagno GD, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol 2003;90:682–5. [DOI] [PubMed] [Google Scholar]
- [9].Kim YJ, Kim SK, Kim HD, et al. A case of skin metastasis of ovarian cancer (mucinous cystadenocarcinoma). Korean J Dermatol 2010;48:342–5. [Google Scholar]
- [10].Lalich D, Tawfik O, Chapman J, et al. Cutaneous metastasis of ovarian carcinoma with shadow cells mimicking a primary pilomatrical neoplasm. Am J Dermatopathol 2010;32:500–4. [DOI] [PubMed] [Google Scholar]
- [11].Kothiwala SK, Meena M, Maheshwari A, et al. Extensive inflammatory skin metastasis from ovarian carcinoma: carcinoma erysipeloides. Indian J Dermatol Venereolo Leprol 2015;81:425–7. [DOI] [PubMed] [Google Scholar]
- [12].Woopen H, Pietzner K, Darb-Esfahani S, et al. Extraperitoneal response to intraperitoneal immunotherapy with catumaxomab in a patient with cutaneous lymphangiosis carcinomatosa from ovarian cancer: a case report and review of the literature. Med Oncol 2012;29:3416–20. [DOI] [PubMed] [Google Scholar]
- [13].Charalampidis C, Lampaki S, Zarogoulidis P, et al. Fine-needle aspiration of skin metastasis in ovarian cancer-report of two cases and review of the literature. Ann Transl Med 2016;4:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wiechert AC, Garrett LA, Lin G, et al. Management of a skin metastasis in a patient with advanced ovarian cancer. Gynecol Oncol Case Rep 2012;2:124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat 2012;44:142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matsui H, Suzuka K, Yamazawa K, et al. Scalp metastasis of a serous ovarian cancer. Acta Obst Gynecol Scand 2002;81:577–8. [DOI] [PubMed] [Google Scholar]
- [17].Demirci S, Yavas F, Ozsaran Z, et al. Palliative radiotherapy for the skin metastasis of ovarian cancer: a case report and review of the literature. Med Oncol 2010;27:628–31. [DOI] [PubMed] [Google Scholar]
- [18].Tsai HW, Yuan CC, Wang PH. Umbilicus as the only site of metastasis in recurrent ovarian cancer. J Chin Med Assoc 2006;69:233–5. [DOI] [PubMed] [Google Scholar]
- [19].Achimas-Cadariu P, Vlad C, Fetica B, et al. Unusual skin metastasis in a patient with recurrent micropapillary serous ovarian carcinoma—a case report and review of the literature. Clujul Med 2015;88:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Traiman P, De Luca LA, Bacchi CE. An extremely large, cauliflower-type, cutaneous metastasis of ovarian cancer associated with good prognosis. Gynecol Oncol 1994;53:239–41. [DOI] [PubMed] [Google Scholar]
- [21].Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol 2003;90:670–2. [DOI] [PubMed] [Google Scholar]
- [22].Antonio AM, Alves JV, Goulao J, et al. Ovarian carcinoma presenting as cutaneous nasal metastasis. An Bras Dermatol 2016;91(5 suppl 1):101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ching AS, Lai CW. Sonography of umbilical metastasis (Sister Mary Joseph's nodule): from embryology to imaging. Abdom Imaging 2002;27:746–9. [DOI] [PubMed] [Google Scholar]
- [24].Fujiwara M, Taube J, Sharma M, et al. PAX8 discriminates ovarian metastases from adnexal tumors and other cutaneous metastases. J Cutan Pathol 2010;37:938–43. [DOI] [PubMed] [Google Scholar]
- [25].Reinhold RB, Lokich JJ. Electrocoagulation: palliative surgery to control metastatic cutaneous malignancy. J Surg Oncol 1979;11:207–12. [DOI] [PubMed] [Google Scholar]


