Abstract
Background:
Endometriosis-associated malignant transformation in abdominal surgical scar (EAMTAS) is a very rare and aggressive phenomenon. Our current article aims to provide a clinical overview, focusing on risk factors affecting survival.
Methods:
We performed a Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant systematic review based on prior reviews and case reports regarding the phenomenon published as abstracts in English, from January 1980 to November 2016. Overall, we identified 47 cases, and we included another case from our institution. We further contacted previous investigators to receive updated follow-up regarding their patients. We analyzed the data, focusing on risk factors that might affect overall survival.
Results:
All the patients reported in the literature had a uterine surgery, mainly caesarean section. The median time-lag from first surgery to the diagnosis of cancer was about 19 years. Clear-cell carcinoma (CCC) was the most prevalent histology (67%), followed by endometrioid adenocarcinoma (15%). Most of the patients were treated by extensive surgery and chemotherapy and/or radiation. Overall 5 years survival was about 40%. Median overall survival was 42 months (95% confidence interval of [18.7, 65.3]). Although our review is currently the largest in the literature, we cannot draw any statistical significant results due to the limited number of patients reported. According to univariate Cox-regression models, a tendency toward worse prognosis was shown for 3-year disease-free survival clear cell histologic-type (P = .169), and tumor diameter ≥8 cm in nonclear-cell histology, 18 months postdiagnosis (P = .06).
Conclusion:
EAMTAS is a rare and aggressive disease. It is mostly related to cesarean section scars and is diagnosed many years postsurgery. Clear-cell histology tends to endure from the worse prognosis. The treatment is mainly extensive surgery and adjuvant chemotherapy and/or radiotherapy.
Keywords: abdominal wall endometriosis, cesarean section, clear-cell carcinoma, malignant transformation
1. Introduction
Endometriosis is a common condition in women of reproductive age. It represents the presence of a functioning endometrial gland and stroma outside the uterus and was first described by Rokitansky in 1860. Abdominal wall endometriosis follows mostly obstetrical and gynecological procedures.
In 1903, Robert Meyer was the first to describe the presence of endometriosis in the postoperative scar. Scar endometriosis can be explained by iatrogenic transplantation of endometrial tissue to the wound edge during any surgical procedure. The typical manifestations of endometriosis in surgical scars are presence of a slowly developing immobile lump in the scar or near it, with swelling and pain during menstruation.[1] The incidence of endometriosis in abdominal surgical scar is 0.03% to 1.08% of women undergoing pelvic surgery,[1–3] and malignancy transformation is very rare.
Although endometriosis is considered a benign condition, malignant transformation is well documented. About 80% of endometriosis-associated malignancies have been found in the ovary, whereas 20% are localized in extra-gonadal sites like intestine, rectovaginal septum, abdominal wall, pleura, and others.[4]
In 1925, Sampson proposed 3 criteria for the diagnosis of malignancy arising in endometriosis as follows: demonstration of both benign and neoplastic endometrial tissues in the tumor, the histology being compatible with endometrial origin, and no other primary tumor sites being found.[5] Further, in 1953, Scott added a 4th criterion: the morphologic demonstration of benign endometriosis contiguous with the malignant tissue is a prerequisite for the adjudication of a malignancy originating in endometriosis.[6]
We conducted a systematic review of the published literature to identify all the cases regarding endometriosis-associated malignant transformation in abdominal surgical-scar (EAMTAS). The purpose of our updated review and analysis is to allow a better understanding of the pathogenesis, risk factors, and treatment options for this phenomenon, while adding one new case treated in our medical center.
2. Material and methods
The current systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[7] and includes existing reports with English abstract on malignancy arising from endometriosis in abdominal wall scar.
We searched PUBMED and Google Scholar using a combination of key words and text related to “carcinoma” (clear cell carcinoma, adenocarcinoma, and sarcoma), “malignancy,” “endometriosis” (endometrioma, endometriosis nodule), and “abdominal wall scar” (cesarean section, wall scar) from January 1980 to November 2016. We also consulted previously review articles published on this subject and their reference lists were further retrieved and analyzed. Overall, we identified 48 records which were case reports. Three reports were excluded based on the publication date, and 15 reports were excluded because of different origin or final histology of the tumor (appendix). All articles were included in the final analyses, with 1 additional case from our institution.
Moreover, we contacted the corresponding authors who provided an email address to try to obtain an updated report and more data on the patient presented in the identified case reports. We received 7 responses and the data were updated accordingly.[8–14]
Since this phenomenon is extremely rare, the quality of the case reports that were included was not evaluated.
Since this study was a meta-analysis of published studies, and we added 1 case, according to our institutional review board ethical approval was not required.
2.1. Data analysis
The main objective of the study was to try to obtain data on survival and prognosis of patients with EAMTAS and to identify the risk factors and an effective treatment regimen for improved survival.
Statistical analysis was performed using SPSS 23.0. Relevant summary descriptive statistics data are presented. Comparison background covariates between 2 groups was done using unpaired t test for continuous variables and chi-squared test of independence or Fisher exact test (when appropriate) for categorical variables. Survival analysis was done using the Kaplan–Meier estimates. The effects of categorical explanatory variables were evaluated using the log-rank test. Univariate and multivariable Cox proportional hazards models for overall survival and disease-free survival were constructed. P < .05 was considered significant.
3. Results
We included 48 patients in our systematic review analysis – 47 patients identified from review of the literature[15–50] and 1 new case from our institution, all reporting endometriosis associated malignant transformation in surgical scar.
The patients’ characteristics are shown in Table 1, the mean age of patients at the time of diagnosis was 46 years (range 37–60 years). The delay period from the 1st surgery to time of diagnosis was reported in 37 cases and is on average 17 years (standard deviation – 8 years), median 19 years (range 5–41 years).
Table 1.
Patient's characteristics.
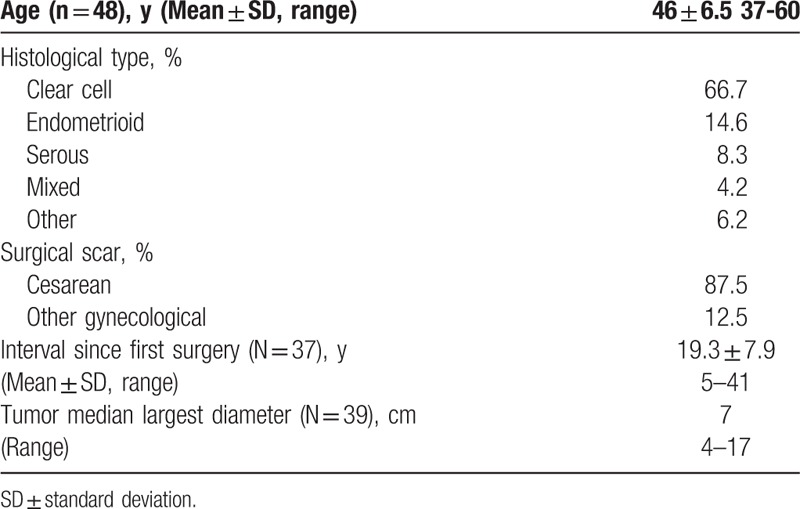
EAMTAS was related to uterine surgery, mainly cesarean section. A total of 42 patients (87.5%) had history of at least 1 cesarean section, while 17 patients had 2 CS[13,36,41] and 3 patients had 3 CS.[12,42,49] Six patients (12.5%) had other gynecological surgery usually laparotomy for uterine surgery.
Previous history of endometriosis was reported only in 22 patients[13,22,31] (56% of the patients with available information). Seventeen patients did not complain of any endometriosis related symptoms before diagnosis. However, all the patients reported seeking consult for pain or swelling in the surgical abdominal scar.
From the 22 patients with history of endometriosis, 10 (41.7%) had at least 1 additional surgery related to endometriosis, including the removal of endometriosis nodules from the site of the surgical scar.[13,27,31,44]
Women were usually diagnosed with surgical scar masses that reached very large dimensions up to 20 cm – Table 1.
The presurgery work-up reported, included imaging (ie, pelvic and abdominal ultrasound and/or computerized tomography and/or MRI), CA-125 levels, and biopsy of the tumor.
The presurgical CA-125 was reported in 21 cases. In 12 cases it was in the normal range (ie, up to 35 U/mL), while in 9 cases it was above the normal range, with the highest level reported as 243 U/mL.[43]
The most common histological type was clear-cell carcinoma (CCC), which was present in 32 patients (66.7%) followed by endometrioid carcinoma (7 patients, 14.6%). Other histologic types included serous papillary carcinoma, adenocarcinoma, sarcoma, and mixed types – Table 1.
Surgery was a major part of the treatment in all patients. It was the primary treatment in 46 patients (95.8%), including our case. In 5 cases, surgery followed neoadjuvant chemotherapy (mainly platinum based).[11,13,32,37,38] In 2 cases surgery followed hormonal treatment with progestative agents.[25,37]
The primary surgical treatment was based on wide local excision of the tumor with removal of extensive abdominal tissue. Due to the extent of the fascial defect, in 20 patients (41.7%) mesh was used for the reconstruction of the abdominal wall[9,11,12,14,21,23,25–27,31–35,38–41,49] including our case.
Other common components of the surgery included hysterectomy (23 cases, 47.9%) and/or bilateral salpingo-oophorectomy (23 cases, 47.9%) and omentectomy (12 cases 25%), mostly as part of the primary or secondary surgery.
More extensive surgery was reported in 6 cases, including resection of mons pubis,[31] cystectomy,[29,36,44] and colectomy.[33,43]
Eighteen patients needed at least 1 more surgery[12,24,26,27,29–31,33–35,38,39,41,43,45,46,48,49] while 4 patients needed more than 2 surgeries.[37–39,42]
Adjuvant treatment was mainly based on chemotherapy, usually platinum-based treatment. Twenty-nine patients (60.4%) received between 1 and 6 courses of intravenous adjuvant chemotherapy. The treatment was interrupted due to patient's poor compliance, adverse effects, or partial response to treatment.
Twenty-one patients were offered radiotherapy after the surgery and chemotherapy. Only 19 patients (39.6%) accepted radiation, and 2 patients refused further treatment at that point.[27,30]
The long-term outcome was recorded for 35 patients (our patient is still under treatment and was not included) and the date was further updated for 7 patients, based on the response of the authors.[8–14] The mean follow-up period was 30 months (range 6–168 months). Fourteen patients died of the disease between 6 and 132 months following the diagnosis, despite extensive treatment. High mortality rate was found to be up to 50 months following diagnosis, as shown on the Kaplan–Meier survival curve (Fig. 1), with median survival time of 42 months after the diagnosis.
Figure 1.
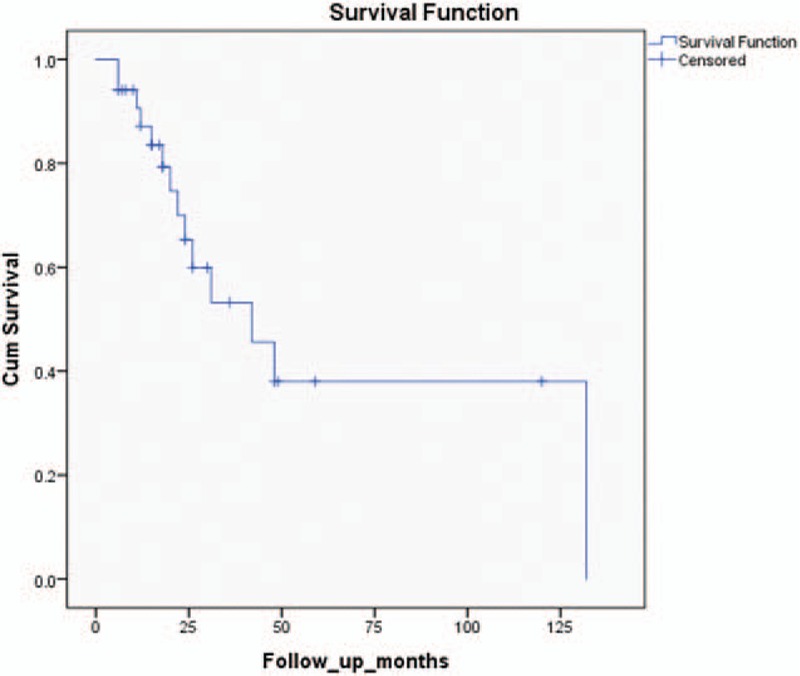
Kaplan–Meier curve for survival in patients with endometriosis associated malignant transformation in abdominal surgical-scar showing high mortality up to 50 months following diagnosis.
Relapse of the disease was reported in 16 patients.[9,12,20,22,24,25,27,29–31,33,34,39,41,48,49] The recurrent sites were: local (4 cases),[29,31,48,49] lymphatic (7 cases, all inguinal),[12,27,30,33,34,39,41] and distant metastases – usually hepatic,[22,25] pulmonary,[9,20,22,24] brain,[17] and bone.[9,20]
In the statistical analysis evaluating the risk factors regarding survival, no statistical significant findings were found regarding surgical treatment (local excision with or without hysterectomy and/or bilateral salpingo-oophoretomy), hormonal treatment (with vs without), and age at diagnosis (less vs above 45 years old on diagnosis).
Regarding the histologic type, univariate Cox-regression model found a tendency (P = .169) toward less favorable prognosis for patients with CCC versus patients with non-CCC in the first 3 years, as shown on the Kaplan–Meier curve (Fig. 2).
Figure 2.
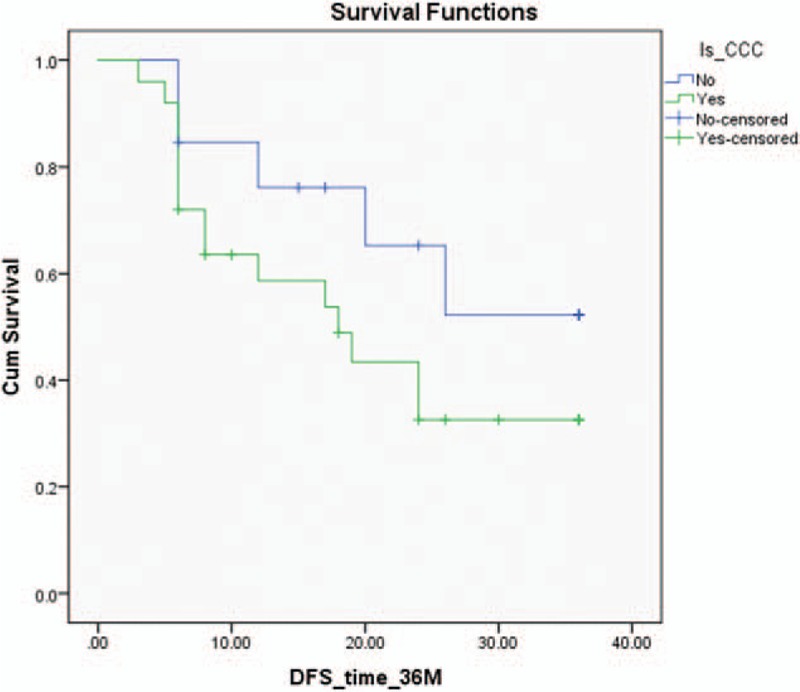
Kaplan–Meier curve for 3-year disease-free survival comparison between clear cell carcinoma (CCC) and non-CCC showing greater mortality for the CCC cases (the findings are not supported statistically).
We calculated that at least 152 patients (101 with CCC and 51 patients with non-CCC) are needed to reach statistical significance regarding this analysis if the same tendency will be kept.
Regarding the size of the tumor at the time of diagnosis, we compared the patients with tumor size of up to 7 cm, and 8 cm or more and we found that patients with CCC did not show any statistical difference regarding survival. Patients with non-CCC and tumor size ≥8 cm showed a tendency for decreased survival, starting 18 months postdiagnosis (P = .06).
As for the additional case from our institution, at diagnosis the patient was 47 years old, with a history of emergency cesarean section 22 years ago. Since the surgery complained of pain in the area of surgical scar and was treated for years with NSAIDS. In the last year she noticed that the area in the surgical scar was swollen so she was referred to a general surgeon for evaluation. The physical exam revealed a solid, fixed mass about 6 cm in size in the lower abdomen. She was referred to do a vaginal and abdominal ultrasound. The abdominal ultrasound revealed a 5.4 × 11.2 × 11 cm solid, heterogenic mass close to the previous CS scar. The CT revealed the uterus of normal size, a multilocular mass in the abdominal wall on its exterior part, partially involving the abdominal muscles (Fig. 3). CA 125 level before the surgery was 96. The biopsy from the mass revealed primary abdominal wall CCC from scar endometriosis. We performed a radical resection of the tumor, TAH, BSO and closure of the abdominal wall with mesh. The treatment was continued with 9 courses of platinum-based chemotherapy with PET-CT and CA 125 monitoring. Due to positive inguinal lymph nodes on PET-CT and stationary levels of CA 125 above normal, an interdisciplinary meeting took place, including oncology, surgery, gynecology, and radiology and was decided to continue with radiotherapy treatment.
Figure 3.
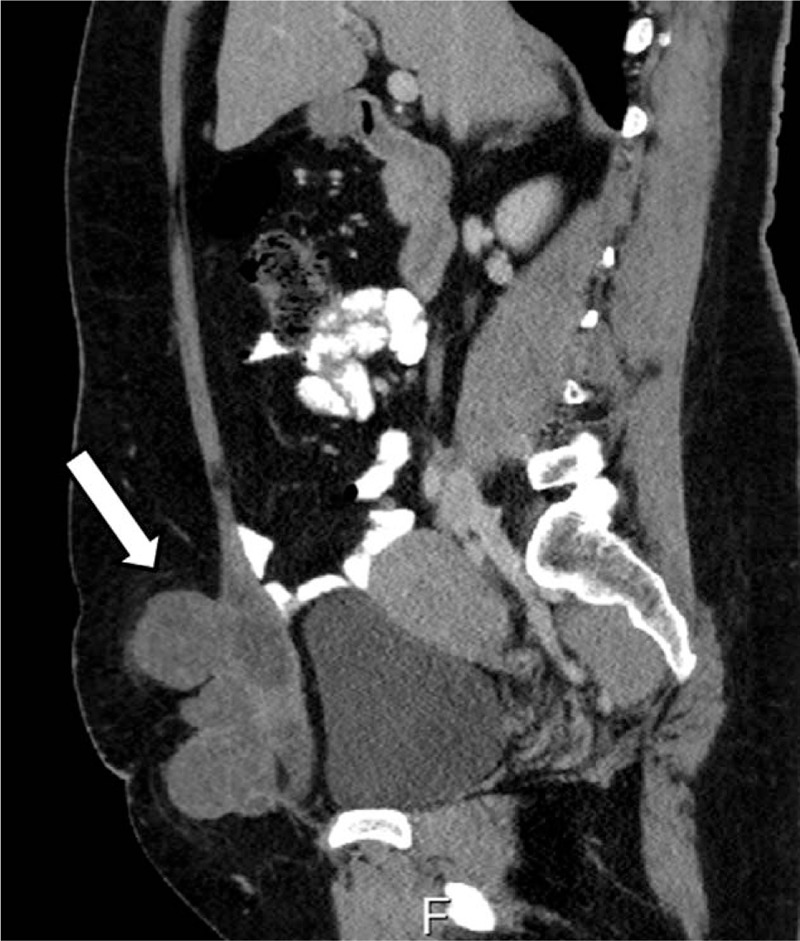
Computed tomography (CT) sagittal view showing the tumor involving the abdominal wall.
4. Discussion
Although endometriosis is known as a relatively benign disease, epidemiologic, histopathologic, and molecular data suggest that endometriosis has malignant potential and is associated with various malignancies, with the best evidence for ovarian cancer.[4] Although the definite pathogenesis of endometriosis is still unknown, many theories have been proposed, including retrograde menstruation, coelomic metaplasia, embryonic cell rest, lympho-vascular metastasis, and stem cell.
The most common cancerous histological types associated with endometriosis are CCC, followed by endometroid carcinoma. This is also true regarding our findings in EAMTAS and is in accordance with other reviews.[9,13,27,30,38,39,43,48]
Unfortunately, even with thorough and meticulous review of the literature and the use of multiple statistical models, we cannot draw any statistical significant findings.
However, we can suggest some general directions for future therapeutic approach and research, while emphasizing few findings that seem to emerge from our data:
-
(1)
EAMTAS, as an entity, appears in relative young women and is an aggressive disease with poor prognosis and a 5 years survival of about 40%.
-
(2)
It is an iatrogenic disease that emerges in an abdominal wall scar after gynecological surgeries, cesarean sections being the most common.
-
(3)
It evolves slowly and it is diagnosed between 4and 41 years after surgery. In this circumstances, it is frequently recurs from endometriosis benign nodules, in some cases despite repetitive surgical treatment.
-
(4)
Our analysis indicates that preoperative diagnosis is difficult and many times incorrect. Routine imaging is not helpful in detecting malignancy and there is no specific marker for the malignant transformation.
-
(5)
Usually at time of diagnosis the tumor size is large, necessitating extensive surgery with repair of abdominal wall defect.
-
(6)
Epidemiological studies have indicated a correlation of endometriosis specifically with 2 histological types of ovarian cancer: CCC followed by endometrioid type.
-
(7)
In non-CCC, tumors with larger diameter (≥8 cm) tend to have poorer prognosis 18 months after diagnosis.
-
(8)
Regarding 3-years disease-free survival, there is a trend for worse outcomes in CCC versus non-CCC.
-
(9)
The main adjuvant treatment reported in the literature is platinum-based chemotherapy with/without local radiation.
-
(10)
Hormonal treatment with progestins or GNRH before the surgical treatment was proposed, but the benefit is unclear.
-
(11)
Typical outcomes are the high recurrence rate and complications related to the repair of the abdominal defect.
Our systematic review shows that the number of cases reported increased over time, especially in the last years, probably due to increase in the number of cesarean sections and other uterine surgeries, but also due to higher attention of physicians from different specialties to this entity.
There is a need of increased awareness to the possibility of EAMTAS in the evaluation of a patient with a mass located near the surgical scar, especially with a history of pain or swelling of the area during menstruation.
The limitation of this systematic review is the rarity of the disease, leading to data collection based merely on case reports, with heterogenic information from different specialties. This caused lack of helpful data that limits the statistical analyses. Although we performed numerous statistical analyses, we could not achieve clear results, and the Kaplan–Meir analysis had limited statistical value. We calculated that in order to have a statistical relevance regarding the overall survival of CCC versus non-CCC, we need 152 cases – 3 times the number of cases reported in our review.
The strength of our review is being the largest and most through systematic review on this subject. The results of this analysis can guide toward achieving a better understanding of risk factors and optimal treatment, but should be interpreted within the limitation of our data.
5. Conclusion
EAMTAS is a rare and aggressive disease. It is mostly related to cesarean section scars and is diagnosed many years postsurgery. Clear-cell histology tends to endure worse prognosis. The treatment is mainly extensive surgery and adjuvant chemotherapy and/or radiotherapy. For earlier diagnosis, clinicians should have high susceptibility level. Additional studies need to be conducted to develop better screening and treatment approaches for malignant transformation of endometriosis.
Acknowledgments
The authors thank the corresponding author for providing an updated status of the patient published in this case report.
Footnotes
Abbreviations: CCC = clear-cell carcinoma, EAMTAS = endometriosis-associated malignant transformation in abdominal surgical-scar.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Horak S, Olejek A. Chaudhury K, Chaudhury B. Endometrial tumors in postoperative scars-pathogenesis, diagnostics and treatment, Cardiovascular pharmacology. Endometriosis – Basic Concepts and Current Research Trends. Poland: InTech; 2012. 85–100. [Google Scholar]
- [2].Van Gorp T, Amant F, Neven P, et al. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol 2004;18:349–71. [DOI] [PubMed] [Google Scholar]
- [3].Teng CC, Yang HM, Chen KF, et al. Abdominal wall endometriosis: an overlooked but possibly preventable complication. Taiwan J Obstet Gynecol 2008;47:42–8. [DOI] [PubMed] [Google Scholar]
- [4].Krawczyk N, Banys-Paluchowski M, Schmidt D, et al. Endometriosis-associated malignancy. Geburtshilfe Frauenheilkd 2016;76:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sampson JA. Endometrial carcinoma of ovary rising in endometrial tissue in that organ. Arch Surg 1925;10:1–72. [Google Scholar]
- [6].Scott RB. Malignant changes in endometriosis. Obstet Gynecol 1953;2:283–9. [PubMed] [Google Scholar]
- [7].Moher D, Liberati A, Tetzlaff J, et al. PRISMAGroup Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller DM, Schouls JJ, Ehlen TG. Clear cell carcinoma arising in extragonadal endometriosis in a caesarean section scar during pregnancy. Gynecol Oncol 1998;70:127–30. [DOI] [PubMed] [Google Scholar]
- [9].Matter M, Schneider N, McKee T. Cystadenocarcinoma of the abdominal wall following caesarean section: case report and review of the literature. Gynecol Oncol 2003;91:438–43. [DOI] [PubMed] [Google Scholar]
- [10].Li J-Y, Chen Y-J, Wu Y-C, et al. Two- and three-dimensional Doppler ultrasound analysis of abdominal wall clear cell carcinoma. Ultrasound Obstet Gynecol 2003;22:94–100. [DOI] [PubMed] [Google Scholar]
- [11].Stevens EE1, Pradhan TS, Chak Y, et al. Malignant transformation of endometriosis in a cesarean section abdominal wall scar: a case report. J Reprod Med 2013;58:264–6. [PubMed] [Google Scholar]
- [12].Sosa-Durán EE, Aboharp-Hasan Z, Mendoza-Morales RC, et al. Clear cell adenocarcinoma arising from abdominal wall endometriosis. Cir Cir 2016;84:245–9. Spanish. [DOI] [PubMed] [Google Scholar]
- [13].Taburiaux L, Pluchino N, Petignat P, et al. Endometriosis-associated abdominal wall cancer: a poor prognosis? Int J Gynecol Cancer 2015;25:1633–8. [DOI] [PubMed] [Google Scholar]
- [14].Al Dhafiri S, Bosc R, Skalli D, et al. Reconstruction of anterior abdominal wall defect by artificial dermal mesh: after a wide excision of clear cells adenocarcinoma arising from ectopic endometriosis. Sch J Appl Med Sci 2016;4:909–13. [Google Scholar]
- [15].Schnieber D, Agner-Kolb D. Malignant transformation of extragenital endometriosis. Geburtshilfe Frauenheilkd 1986;46:658–9. [DOI] [PubMed] [Google Scholar]
- [16].Hitti IF, Glasberg SS, Lubicz S. Clear cell carcinoma arising in extraovarian endometriosis: report of three cases and review of the literature. Gynecol Oncol 1990;39:314–20. [DOI] [PubMed] [Google Scholar]
- [17].Markopoulos C, Gogas H, Eleftheriou G, et al. Endometrioid carcinoma arising in a scar of caesarean section. Case report. Eur J Gynaecol Oncol 1996;17:520–1. [PubMed] [Google Scholar]
- [18].Gucer F, Reich O, Kometter R, et al. Endometroid carcinoma arising with a scar endometriosis. Eur J Gynaecol Oncol 1997;18:42–3. [PubMed] [Google Scholar]
- [19].Park SW, Hong SM, Wu HG, et al. Clear cell carcinoma arising in a cesarean section scar endometriosis: a case report. J Korean Med Sci 1999;14:217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ishida GM, Motoyama T, Watanabe T, et al. Clear cell carcinoma arising in a cesarean section scar. Report of a case with fine needle aspiration cytology. Acta Cytol 2003;47:1095–8. [DOI] [PubMed] [Google Scholar]
- [21].Olejek A, Bichalski W, Rembielak-Stawecka B, et al. Adenocarcinoma arising from endometriosis in scar from a cesarean section treated with the use of plastic mesh. Ginekol Pol 2004;75:797–801. [PubMed] [Google Scholar]
- [22].Sergent F, Baron M, Le Cornec JB, et al. Malignant transformation of abdominal wall endometriosis: a new case report. J Gynecol Obstet Biol Reprod (Paris) 2006;35:186–90. [DOI] [PubMed] [Google Scholar]
- [23].Alberto VO, Lynch M, Labbei FN, et al. Primary abdominal wall clear cell carcinoma arising in a caesarean section scar endometriosis. Ir J Med Sci 2006;175:69–71. [DOI] [PubMed] [Google Scholar]
- [24].Leng J, Lang J, Guo L, et al. Carcinosarcoma arising from atypical endometriosis in a cesarean section scar. Int J Gynecol Cancer 2006;16:432–5. [DOI] [PubMed] [Google Scholar]
- [25].Razzouk K, Roman H, Chanavaz-Lacheray I, et al. Mixed clear cell and endometrioid carcinoma arising in parietal endometriosis. Gynecol Obstet Invest 2007;63:140–2. [DOI] [PubMed] [Google Scholar]
- [26].Harry VN, Shanbhag S, Lyall M, et al. Isolated clear cell adenocarcinoma in scar endometriosis mimicking an incisional hernia. Obstet Gynecol 2007;110:469–71. [DOI] [PubMed] [Google Scholar]
- [27].Bats AS, Zafrani Y, Pautier P, et al. Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: case report and review of the literature. Fertil Steril 2008;90: 1197.e13-6. [DOI] [PubMed] [Google Scholar]
- [28].Rust MM, Susa J, Naylor R, et al. Clear cell carcinoma in a background of endometriosis. Case report of a finding in a midline abdominal scar 5 years after a total abdominal hysterectomy. Acta Cytol 2008;52:475–80. [DOI] [PubMed] [Google Scholar]
- [29].Achach T, Rammeh S, Trabelsi A, et al. Clear cell adenocarcinoma arising from abdominal wall endometriosis. J Oncol 2008;2008:478325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Williams C, Petignat P, Belisle A, et al. Primary abdominal wall clear cell carcinoma: case report and review of literature. Anticancer Res 2009;29:1591–4. [PubMed] [Google Scholar]
- [31].Matsuo K, Alonsozana EL, Eno ML, et al. Primary peritoneal clear cell adenocarcinoma arising in previous abdominal scar for endometriosis surgery. Arch Gynecol Obstetrics 2009;280:637–41. [DOI] [PubMed] [Google Scholar]
- [32].Omranipour R, Najafi M. Papillary serous carcinoma arising in abdominal wall endometriosis treated with neoadjuvant chemotherapy and surgery. Fertil Steril 2010;93:e1347–8. [DOI] [PubMed] [Google Scholar]
- [33].Bourdel N, Durand M, Gimbergues P, et al. Exclusive nodal recurrence after treatment of degenerated parietal endometriosis. Fertil Steril 2010;93: 2074.e1-6. [DOI] [PubMed] [Google Scholar]
- [34].Drukała Z, Ciborowska-Zielińska B, Kubrak J, et al. Outcome of a multimodal therapy of a recurrent adenocarcinoma arising from Caesarean section scar endometriosis – a case report. Rep Pract Oncol Radiother 2010;15:75–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Da Ines D, Bourdel N, Charpy C, et al. Mixed endometrioid and serous carcinoma developing in abdominal wall endometriosis following cesarean section. Acta Radiol 2011;52:587–90. [DOI] [PubMed] [Google Scholar]
- [36].Sawazaki H, Goto H, Takao N, et al. Clear cell adenocarcinoma arising from abdominal wall endometriosis mimicking urachal tumor. Urology 2012;79:e84–5. [DOI] [PubMed] [Google Scholar]
- [37].Yan Y, Li L, Guo J, et al. Malignant transformation of an endometriotic lesion derived from an abdominal wall scar. Int J Gynaecol Obstet 2011;115:202–3. [DOI] [PubMed] [Google Scholar]
- [38].Mert I, Semaan A, Kim S, et al. Clear cell carcinoma arising in the abdominal wall: two case reports and literature review. Am J Obstet Gynecol 2012;207:e7–9. [DOI] [PubMed] [Google Scholar]
- [39].Shalin SC, Haws AL, Carter DG, et al. Clear cell adenocarcinoma arising from endometriosis in abdominal wall cesarean section scar: a case report and review of the literature. J Cutan Pathol 2012;39:1035–41. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Yang J, Cao D, et al. Clear-cell carcinoma of the abdominal wall after cesarean delivery. Obstet Gynecol 2012;120:445–8. [DOI] [PubMed] [Google Scholar]
- [41].Gundogdu B, Ureyen I, Kimyon G, et al. Primary abdominal wall clear cell carcinoma arising from incisional endometriosis. Asian Pac J Reprod 2013;2:244–7. [Google Scholar]
- [42].Heller DS, Houck K, Lee ES, et al. Clear cell adenocarcinoma of the abdominal wall: a case report. J Reprod Med 2014;59:330–2. [PubMed] [Google Scholar]
- [43].Fargas Fabregas F, Cusido Guimferrer M, Tresserra Casas F, et al. Malignant transformation of abdominal wall endometriosis with lymph node metastasis: case report and review of literature. Gynecol Oncol Case Rep 2014;8:10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu H, Leng J, Lang J, et al. Clear cell carcinoma arising from abdominal wall endometriosis: a unique case with bladder and lymph node metastasis. World J Surg Oncol 2014;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dobrosz Z, Paleń P, Stojko R, et al. Clear cell carcinoma derived from an endometriosis focus in a scar after a caesarean section – case report and literature review. Ginekol Pol 2014;85:792–5. [PubMed] [Google Scholar]
- [46].Aust S, Tiringer D, Grimm C, et al. Therapy of a clear cell adenocarcinoma of unknown primary arising in the abdominal wall after cesarean section and after hysterectomy. Wien Klin Wochenschr 2015;127:62–4. [DOI] [PubMed] [Google Scholar]
- [47].Usta TA, Sonmez SE, Oztarhan A, et al. Endometrial stromal sarcoma in the abdominal wall arising from scar endometriosis. J Obstet Gynaecol 2014;34:514–42. [DOI] [PubMed] [Google Scholar]
- [48].Ijichi S, Mori T, Suganuma I, et al. Clear cell carcinoma arising from cesarean section scar endometriosis: case report and review of the literature. Case Rep Obstet Gynecol 2014;2014:642483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ruiz MP, Wallace DL, Connell MT. Transformation of abdominal wall endometriosis to clear cell carcinoma. Case Rep Obstet Gynecol 2015;2015:123740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jiang M, Chen P, Sun L, et al. 18F-FDG PET/CT findings of a recurrent adenocarcinoma arising from malignant transformation of abdominal wall endometriosis. Clin Nucl Med 2015;40:184–5. [DOI] [PubMed] [Google Scholar]


