Abstract
Background
The association between circulating adiponectin levels and atrial fibrillation (AF) is uncertain. We, therefore, investigated whether an increased serum adiponectin level is implicated in the long-term recurrence of AF after ablation therapy.
Methods
Our study included 100 consecutive patients (88 men; median age, 57.9±10.9 years) who underwent catheter ablation for AF at our hospital between 2011 and 2013. The adiponectin and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were measured before ablation and compared between those in whom AF recurred and those in whom AF did not recur.
Results
Elevation in adiponectin levels was significantly associated with female sex, non-paroxysmal AF, heart failure, higher NT-proBNP and matrix metallo-proteinase-2 levels, and lower body mass index. After a stepwise adjustment for any potential confounding variables, the adiponectin levels remained significantly associated with female sex (beta=0.2601, P=0.0041), non-paroxysmal AF (beta=0.2708, P=0.0080), and higher NT-proBNP levels (beta=0.2536, P= 0.0138). During the median follow-up period of 26.2 months, AF recurred in 48 of the 100 patients. Stepwise multivariate adjustment showed that an increased log-transformed NT-proBNP (Hazard ratio [HR], 2.18; 95% confidence interval [CI] 1.25–4.00; P=0.0055), longer duration of AF (HR, 1.87; 95%CI 1.01–3.76; P=0.0465), and decreased left ventricular ejection fraction (HR, 0.96; 95%CI 0.93–0.99; P=0.0391) were independent predictors of recurrent AF after catheter ablation, but adiponectin was not.
Conclusions
Our data indicated that adiponectin was partially responsible for progression of AF, but the correlation between adiponectin levels and AF recurrence was not significant.
Keywords: Atrial fibrillation, Adiponectin, NT-proBNP, Ablation
1. Introduction
Adiponectin is a peptide hormone with insulin-sensitizing, anti-inflammatory, and anti-atherosclerotic effects. Generally, low levels of circulating adiponectin are considered detrimental to the cardiovascular metabolism, contributing to the development of atherosclerosis. However, some studies have shown a link between relatively high levels of plasma adiponectin and heart failure, coronary heart disease, and even mortality [1], [2]. In addition, Macheret et al. have shown a significant association between elevated adiponectin levels and increased incidence of atrial fibrillation (AF) among older adults [3].
Over the past decade, catheter-based pulmonary vein isolation (PVI) has become widely accepted as a therapeutic option for AF. However, recurrence of AF after ablation occurs in approximately 20–30% of patients with paroxysmal AF; this proportion is even higher in patients with non-paroxysmal AF. The major contributors to AF recurrence are PV reconnections [4], [5] and progressive atrial remodeling, accompanied by an increase in atrial interstitial fibrosis, hypertrophy, or left atrial dilation [6], [7], [8]. We previously described the importance of the biomarkers of inflammation and the extracellular matrix turnover as predictors of both progressive atrial remodeling and recurrence of AF after catheter ablation [7], [8]. Relatively high plasma adiponectin levels are associated with persistent AF, which is accompanied by an increased serum carboxy-terminal telopeptide level [9] as one of the collagen turnover-related biomarkers. In light of these findings, we hypothesized that circulating adiponectin levels could reflect the atrial remodeling related to the recurrence of AF after ablation. We, therefore, assessed the effect of adiponectin on atrial remodeling and also whether a high adiponectin level was associated with the recurrence of AF after ablation.
2. Material and methods
2.1. Study patients
One hundred consecutive patients (88 men, 12 women; median age, 57.9±10.9 years; median duration of AF, 48.0 months) who underwent catheter ablation for drug-refractory AF between July 2011 and March 2013 were enrolled in our study. All patients were treated at our hospital, and no patients referred for repeat ablation were included in the study. The group comprised 55 patients with paroxysmal AF (spontaneous termination of AF within 7 days) and 45 patients with non-paroxysmal AF (AF lasting over 8 days). The study protocol was approved by the institutional review board of Nihon University Itabashi Hospital (Date of IRB approval; February 20, 2012; Approval number, RK-120210-4). All patients provided written informed consent for the electrophysiologic study, ablation procedure, and use of their anonymized data in this study. Adequate oral anticoagulation was administered for at least one month prior to the ablation, and all antiarrhythmic drugs were stopped for at least 5 half-lives prior to the ablation. Before the ablation procedure, transesophageal and transthoracic echocardiograms were obtained for each patient, and the standard echocardiographic measurements, i.e., left atrial dimension (LAD) at end systole in the parasternal long-axis view and left ventricular ejection fraction (LVEF) by the Teichholz method, were calculated. In addition, all patients underwent 3-dimensional computed tomography (CT) (320-row detector, dynamic volume CT scanner; Aquilion ONE, Toshiba Medical Systems, Tokyo, Japan) for visualization of the left atrium (LA) and pulmonary veins (PVs). After ablation, all patients were followed up regularly at 1 month, 3 months, 6 months and, subsequently, every 6 months at the outpatient clinic. Routine electrocardiograms (ECGs) were obtained at each visit, and 24-hour Holter monitoring was scheduled to follow the 3-, 6- and 12- month follow-up visits. A blanking period of 2 months was established, and recurrence of AF was defined as any ECG recording of AF or any Holter recording of AF lasting more than 30 seconds.
2.2. Ablation procedure
Ablation was performed under sedation, which was achieved with an intravenous infusion of dexmedetomidine and fentanyl [7], [8]. In brief, after vascular access was obtained, a single trans-septal puncture was performed; this was followed by an extensive ipsilateral PVI, guided by 2 Lasso catheters and a 3-dimensional geometric map generated using a NavX (St. Jude Medical, St. Paul, MN, USA) or CARTO (Biosense Webster, Inc., Diamond Bar, CA, USA) mapping system. A 3.5-mm irrigated-tip catheter (NAVISTAR THERMOCOOL; Biosense Webster) or 4-mm irrigated-tip catheter (Safire BLU Duo; St. Jude Medical, Minneapolis, MN, USA) was used for ablation. Radiofrequency energy was delivered at a maximum power output of 20–30 W, and the upper temperature limit was set at 41 °C, with a saline irrigation rate of 17–30 mL/min (COOLFLOW Pump; Biosense Webster) or 13–20 mL/min (Cool Point Irrigation Pump, St. Jude Medical). The endpoint of the PVI was the demonstration of complete entrance and exit block. In patients in whom AF was not terminated by the PVI or in whom sustained AF was inducible after the PVI, linear ablation at the LA roof, LA floor along the coronary sinus, LA appendage ridge, and LA septum (indicated by LA ablation) were performed. The step-by-step ablation procedure was stopped once AF termination was achieved or once all ablation had been performed in patients in whom the AF did not terminate.
2.3. Blood sampling and measurement of the biomarkers of inflammation and extracellular matrix turnover
In the electrophysiology laboratory, just before the ablation, blood samples were drawn from the jugular vein of each patient via a sheath placed for the coronary sinus catheter. The serum high-sensitivity CRP (hs-CRP) was measured using particle-enhanced immunonephelometry (the BN II System; Siemens Healthcare Diagnostics Inc., Marburg, Germany); the serum N-terminal pro-brain natriuretic peptide (NT-proBNP) level was determined using a chemiluminescent enzyme immunoassay (Elecsys proBNP sandwich immunoassay; Roche Diagnostics, Mannheim, Germany); the serum matrix metallo-proteinase-2 (MMP-2) level was measured using a 1-step sandwich enzyme immunoassay (antihuman MMP-2 monoclonal antibody; Daiichi Fine Chemical Co., Ltd., Toyama, Japan); and the plasma adiponectin level was measured using a latex particle-enhanced turbidimetric assay (Human Adiponectin Assay Kit; Mitsubishi Kagaku Iatron Inc., Chiba, Japan).
2.4. Division of patients into groups and the comparative study
In addition to the serum biomarkers of inflammation and the extracellular matrix turnover, the following variables and outcomes were recorded: basic patient clinical characteristics, such as the age and sex, body mass index (BMI), type of AF, concomitant medical conditions, medications used, echocardiographic measurements, follow-up time, and recurrence of AF after ablation. The patients were then divided between those in whom AF recurred after ablation and those in whom it did not, and the study variables were compared between the 2 groups.
2.5. Statistical analysis
Continuous variables are expressed as the mean±SD or median and interquartile range. The between-group differences in the continuous variables were analyzed using a two-tailed t test or Mann–Whitney U test. The between-group differences in categorical variables were analyzed using the chi-square test. The correlation between the adiponectin levels and other clinical variables was analyzed using a simple linear regression analysis and Spearman's rank correlation coefficient. To determine the optimal cutoff value of the adiponectin and NT-proBNP levels for recurrence of AF, receiver-operating characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated. The differences between the two AUCs were compared using the z test. In the multiple regression analysis or Cox hazard model, a log transformation was performed for the NT-proBNP levels and AF duration, which were skewed. All variables with a p-value ≤0.1 were included in a multiple regression analysis and multivariate Cox proportional hazard model, which was constructed using a stepwise backward elimination method. A Cox proportional hazard model was used to investigate the impact of variables on the recurrence of AF after ablation, and hazard ratios (HRs) with 95% confidence intervals (CIs) were obtained. A p-value <0.05 was considered significant for all analyses, and all analyses were performed using JMP software version 11.0.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Relation between adiponectin and the covariates of interest
Adiponectin levels were significantly higher in female patients than in male patients (15.0±5.7 vs. 8.7±4.1 µg/mL, P<0.0001), in patients with non-paroxysmal AF than in those with paroxysmal AF (11.7±5.6 vs. 7.7±2.9 µg/mL, P<0.0001), and in patients with heart failure than in those without heart failure (11.9±5.7 vs. 9.0±4.4 µg/mL, P=0.0238). There were no differences in the adiponectin levels between patients with and without hypertension (10.2±4.9 vs. 8.5±4.4 µg/mL, P=0.0648), diabetes mellitus (7.9±5.3 vs. 9.6±4.7 µg/mL, P=0.3214), dyslipidemia (8.0±3.6 vs. 9.9±5.0 µg/mL, P=0.0756), ischemic heart disease (5.2±1.1 vs. 9.7±4.8 µg/mL, P=0.0668), and LA ablation in whom AF was sustained even after the PVI (10.1±5.0 vs. 9.0±4.6 µg/mL, P=0.2603). The correlations between the adiponectin levels and clinical continuous variables, biomarker levels, and echocardiographic variables are shown in Table 1. A weak to moderate correlation was found between the serum adiponectin levels and BMI (r=−0.2921, P=0.0032), adiponectin and NT-proBNP (r=0.4158, P<0.0001), and serum adiponectin level and MMP-2 (r=0.2025, P=0.0433). No correlation was found between the serum adiponectin level and any other variables examined. After adjustment by a stepwise multiple regression analysis for the confounding variables, adiponectin remained significantly related to female sex (beta=0.2601, P=0.0041), non-paroxysmal AF (beta=0.2708, P=0.0080), non-ischemic heart disease (beta=0.1980, P=0.0189), and NT-proBNP level (beta=0.2536, P=0.0138).
Table 1.
Correlation of the adiponectin levels and continuous variables.
| r | P-value | |
|---|---|---|
| Age, years | 0.1653 | 0.1002 |
| AF duration, month | 0.0268 | 0.7913 |
| Body mass index, kg/m2 | −0.2921 | 0.0032 |
| NT-proBNP, pg/mL | 0.4158 | <0.0001 |
| Hs-CRP, ng/mL | −0.2381 | 0.0171 |
| MMP-2, ng/mL | 0.2025 | 0.0433 |
| LA diameter, mm | −0.0613 | 0.5443 |
| LVEF, % | −0.0711 | 0.4823 |
AF, atrial fibrillation; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high-sensitivity CRP; MMP-2, matrix metallo-proteinase-2; LA, left atrial; LVEF, left ventricular ejection fraction.
3.2. Patient characteristics and ablation outcomes
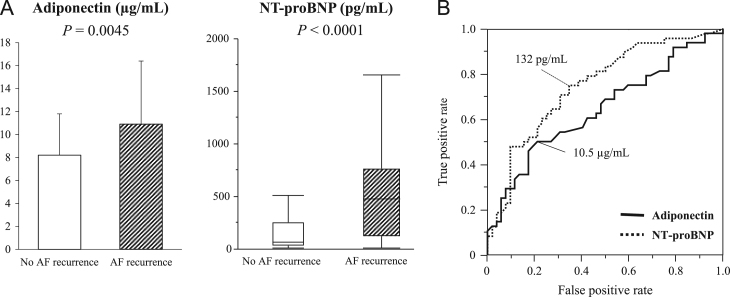
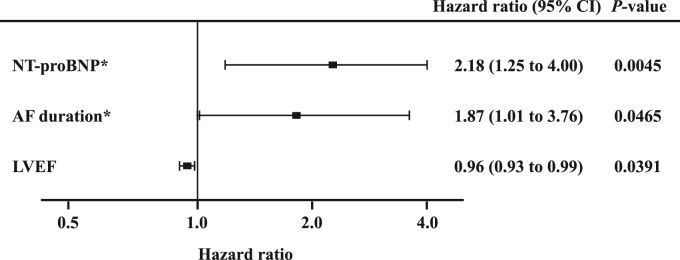
AF recurred in 48 (48.0%) of the 100 patients during a median follow-up period of 26.2 (range, 4.3 – 45.8) months. The clinical characteristics, medications, biomarker levels, and echocardiographic variables are shown for the total patients and for the patients in each group, in Table 2. AF recurrence was significantly associated with older age, longer duration of AF, non-paroxysmal AF, and the LA diameter (P<0.05 for all). Patients in whom AF recurred had significantly higher adiponectin (10.9±5.5 vs. 8.2±3.6 µg/mL, P=0.0045) and NT-proBNP levels (481 [121–765] vs. 67 [30–255] pg/mL, P<0.0001) (Fig. 1A). When the patients were divided into paroxysmal AF and non-paroxysmal AF groups, no association between adiponectin levels and recurrence of AF was observed in the paroxysmal AF group (8.1±3.1 vs. 7.5±2.8 µg/mL, P=0.4801), and the association was marginal in the non-paroxysmal AF group (12.7±5.9 vs. 9.8±4.8 µg/mL, P=0.0999); however, the association of AF recurrence with the NT-proBNP levels remained significant for both groups (paroxysmal AF: 133 [59–451] vs. 47 [23–98] pg/mL, P=0.0138; non-paroxysmal AF: 635 [356–977] vs. 250 [107–515] pg/mL, P=0.0177). ROC curves for serum adiponectin levels to differentiate recurrence of AF had an AUC of 0.64 (95% CI 0.54– 0.75; P=0.0039), identifying an adiponectin level of ≥10.5 µg/mL as the most predictive cutoff value (sensitivity 50.0%, specificity 78.9%) (Fig. 1B). The ROC curves revealed a better prognostic performance for the NT-proBNP (AUC: 0.75, 95% CI 0.65–0.85; P<0.0001) (P=0.0075 vs. AUC for adiponectin levels by z test). The best cutoff value of the NT-pro BNP level was ≥132 pg/mL in order to achieve a sensitivity of 75.0% and specificity of 65.4% (Fig. 1B). A stepwise multivariate Cox proportional hazards regression analysis showed that the log-transformed NT-proBNP elevation (HR, 2.18; 95% CI 1.25–4.00; P=0.0055) and the log-transformed AF duration (HR, 1.87; 95% CI 1.01–3.76; P=0.0465), and LVEF (HR, 0.96; 95% CI 0.93–0.99; P=0.0391) were significant predictors of recurrence of AF (Fig. 2); however, no association of adiponectin and the other variables with AF recurrence was observed.
Table 2.
Characteristics of the total study patients and of the patients in each group.
| Variable | Total patients (n=100) | No AF recurrence (n=52) | AF recurrence (n=48) | P value |
|---|---|---|---|---|
| Age, years | 57.9±10.9 | 55.8±11.2 | 60.3±10.2 | 0.0399 |
| Male sex | 88 (88%) | 48 (92%) | 40 (83%) | 0.1677 |
| AF duration, month | 48 (18–83) | 36 (15–65) | 57 (25–97) | 0.0600 |
| Non-paroxysmal AF | 45 (45%) | 16 (31%) | 29 (60%) | 0.0029 |
| Body mass index, kg/m2 | 24.1±3.6 | 23.9±3.8 | 24.2±3.3 | 0.6512 |
| Hypertension | 57 (57%) | 25 (48%) | 32 (67%) | 0.0607 |
| Diabetes mellitus | 8 (8%) | 4 (8%) | 4 (8%) | 0.9060 |
| Dyslipidemia | 24 (24%) | 13 (25%) | 11 (23%) | 0.8075 |
| Ischemic heart disease | 4 (4%) | 3 (6%) | 1 (2%) | 0.3474 |
| Heart failure | 16 (16%) | 8 (15%) | 8 (17%) | 0.8613 |
| Class I antiarrhythmic drugs | 47 (47%) | 21 (40%) | 26 (54%) | 0.1677 |
| Class III antiarrhythmic drugs | 30 (30%) | 13 (25%) | 17 (35%) | 0.2561 |
| NT-proBNP, pg/mL | 144 (49–621) | 67 (30–255) | 481 (121–765) | <0.0001 |
| Hs-CRP, ng/mL | 930 (385–1905) | 845 (348–1760) | 1025 (483–2400) | 0.2927 |
| MMP-2, ng/mL | 743±153 | 716±140 | 772±162 | 0.0641 |
| Adiponectin, µg/mL | 9.5±4.8 | 8.2±3.6 | 10.9±5.5 | 0.0045 |
| LA diameter, mm | 38.7±6.5 | 36.7±4.8 | 40.7±7.5 | 0.0019 |
| LVEF, % | 66.1±8.7 | 67.7±8.1 | 64.4±9.1 | 0.0569 |
| LA ablation | 42 (39%) | 18 (35%) | 24 (50%) | 0.1194 |
Values are the median (interquartile range), or n (%). P-values were obtained by a two-tailed t test, Mann–Whitney U test, or chi-square test.
AF, atrial fibrillation; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high-sensitivity CRP; MMP-2, matrix metallo-proteinase-2; LA, left atrial; LVEF, left ventricular ejection fraction.
Fig. 1.
Adiponectin and NT-proBNP levels between patients with and without AF recurrence (A) and receiver-operating characteristic (ROC) curves of the adiponectin and NT-proBNP levels for differentiating AF recurrence (B). Magnitude of the bar graph and error bar indicate the mean±SD of the adiponectin levels and the median and interquartile ranges of the NT-proBNP levels. Values between the 2 groups were compared using a two-tailed t test or Mann–Whitney U test. AF, atrial fibrillation; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Fig. 2.
A forest plot showing the hazard ratio and 95% confidence intervals (CIs) for the significant variables after an adjustment by stepwise multivariate Cox proportional hazards regression analysis. *Log-transformed values. AF, atrial fibrillation; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction.
4. Discussion
4.1. Main findings
Our main findings were as follows: (1) elevated adiponectin levels were independently associated with female sex, non-paroxysmal AF, non-ischemic heart disease, and increased levels of NT-proBNP; (2) significant factors related to AF recurrence by a univariate analysis were old age, long duration of AF, non-paroxysmal AF, increased LA diameter, and high NT-proBNP and adiponectin levels; (3) a high NT-proBNP level, long duration of AF, and decreased LVEF, but not adiponectin levels, were shown to be independent predictors of recurrence of AF after ablation after an adjustment for the co-variables related to recurrence of AF.
4.2. Increase in adiponectin levels and AF
Relatively high plasma adiponectin levels have been shown to be associated with AF [3], [6], [7]. Shimano et al. reported the association between high plasma adiponectin levels and persistent AF, which is accompanied by an increased serum level of carboxy-terminal telepeptide of type 1 collagen [9]. Macheret et al. reported an independent association between high levels of adiponectin and an increased risk of AF in older adults, despite the documented cardiometabolic benefits of adiponectin [3]. In addition, adiponectin concentration was shown to be higher in Whites than in Blacks, and a high concentration was shown to be independently associated with a greater risk of an incident AF [10]. Choi et al. reported a significant association between a relatively low plasma adiponectin concentration and paroxysmal AF [11]. However, Ermakov et al. showed that among women, elevated levels of serum resistin were significantly associated with high rates of an incident AF, and that it partially mediated the association between the BMI and AF, but that leptin and adiponectin elevations were not significantly associated with AF [12]. In a longitudinal community-based study, no statistically significant association was found between adiponectin and incident AF [13]. Therefore, race, sex, and pre- or postmenopausal status should be considered for interpretation of serum adiponectin levels. In our study, adiponectin levels were high in patients with non-paroxysmal AF, and they were significantly associated with a higher NT-proBNP level. MMP-2 is one of the key biomarkers associated with extracellular matrix remodeling in AF [4], [5], and the MMP-2 level also tended to be associated with an increased adiponectin level. In light of these findings, the serum adiponectin levels may reflect atrial structural remodeling and latent left ventricular dysfunction. One theory that a paradox increases in patients with AF is that adiponectin is driven by opposing factors, leading to a favorable association in a healthy population vs. an unfavorable association in patients with chronic disease. In a healthy condition, adiponectin is a marker for reduced adiposity levels and may have beneficial effects on insulin resistance and inflammation. In chronic diseases, adiponectin levels may be reactive and are increased owing to involuntary weight loss, sarcopenia, renal dysfunction, and an elevation in the natriuretic peptides. In patients with chronic diseases, the disease process may outweigh the beneficial effects of adiponectin, leading to its association with a worse clinical outcome [14].
4.2.1. Clinical implications
Our data indicated that increased adiponectin levels were strongly related to non-paroxysmal AF, female sex, and higher NT-proBNP and MMP-2 levels, and were inversely correlated to the BMI. Importantly, they were not associated with the LA volume or LVEF. LA dilatation and metabolic syndrome/obesity are well-known causes of AF and consequently result in structural remodeling [4], [5], [15], [16], [17]. Compared to men, women typically have a lower BMI and smaller LA volume; however, adiponectin levels are also associated with the LA substrate, evidenced by a low-voltage zone or an MRI-derived scar [18], [19]. We failed to obtain a statistical significance of adiponectin as a predictor of post-ablation recurrence of AF after multiple adjustments; however, the NT-proBNP levels remained significant, even after dividing the study patients into those with paroxysmal AF and non-paroxysmal AF. In addition, the prognostic performance of adiponectin was weak, as indicated by an AUC of 0.64. Therefore, the clinical utility of adiponectin for the ablation outcome may not overwhelm the NT-proBNP. The factors associated with recurrence of AF after ablation are complicated. One of the major mechanisms of AF recurrence is PV reconnections owing to the failure to create transmural lesions, or gaps in the PV encircling ablation lines [4], [5]. Another mechanism is structural remodeling, which is clinically manifested by atrial dilatation [6], [7], [8] or a low-voltage zone/scar in the atrium [18], [19]. Further, it is also related to the ablation methods, such as whether an intensive LA ablation was performed. The maintenance of AF stems from the balance between the incidence of PV triggers and the extent of progressive structural remodeling. Therefore, the multiple underlying factors may have weakened the prognostic performance of the adiponectin levels in the long-term outcome after ablation in our patients. Nonetheless, our data implicated that adiponectin might be one of the markers for predicting a progressive LA substrate in patients with a small LA diameter, characterized by female sex or a low BMI, which is mostly associated with latent left ventricular diastolic dysfunction and/or LA fibrosis.
4.2.2. Limitations
Our findings should be interpreted in light of the fact that this study was conducted at a single center and was limited to 100 patients who underwent catheter ablation of AF. The study was also limited by the fact that we did not evaluate any changes in adiponectin levels, which might have occurred after ablation. Finally, we did not directly evaluate the characteristics of the LA tissue using 3-dimensional electroanatomic maps, delayed enhancement MR images, or pathological examinations.
5. Conclusions
We found that increased serum adiponectin levels were strongly related to female sex, non-paroxysmal AF, and increased serum NT-proBNP levels, suggestive of atrial remodeling. However, when using multiple biomarkers related to atrial remodeling, the serum adiponectin levels failed to offer any advantages over serum NT-proBNP levels for predicting the long-term outcome after AF ablation therapy. This indicates a limitation for the prognostic performance of serum adiponectin levels in the field of AF ablation.
Conflict of interests
All authors declare no conflict of interest related to this study.
Acknowledgments
The authors thank Ms. Wendy Alexander-Adams and Mr. John Martin for their encouragement and assistance with the reporting of our findings in English.
References
- 1.Lindberg S., Pedersen S.H., Mogelvang R. Usefulness of adiponectin as a predictor of all cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:492–496. doi: 10.1016/j.amjcard.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Karas M.G., Benkeser D., Arnold A.M. Relations of plasma total and high-molecular-weight adiponectin to new-onset heart failure in adults ≥65 years of age (from the Cardiovascular Health Study) Am J Cardiol. 2014;113:328–334. doi: 10.1016/j.amjcard.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macheret F., Bartz T.M., Djousse L. Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart. 2015;101:1368–1374. doi: 10.1136/heartjnl-2014-307015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstenfeld E.P., Callans D.J., Dixit S. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol. 2003;14:685–690. doi: 10.1046/j.1540-8167.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang F., Antz M., Ernst S. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 6.Allessie M., Ausma J., Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [Review] [DOI] [PubMed] [Google Scholar]
- 7.Okumura Y., Watanabe I., Nakai T. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovas Electrophysiol. 2011;22:987–993. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki N., Okumura Y., Watanabe I. Increased levels of inflammatory and extracellular matrix turnover biomarkers persist despite reverse atrial structural remodeling during the first year after atrial fibrillation ablation. J Interv Card Electrophysiol. 2014;39:241–249. doi: 10.1007/s10840-013-9867-6. [DOI] [PubMed] [Google Scholar]
- 9.Shimano M., Shibata R., Tsuji Y. Circulating adiponection level in patients with atrial fibrillation. Circ J. 2008;72:1120–1124. doi: 10.1253/circj.72.1120. [DOI] [PubMed] [Google Scholar]
- 10.Dewland T.A., Vittinghoff E., Harris T.B. Inflammation as a mediator of the association between race and atrial fibrillation: results from the health, aging, and body composition study. JACC Clin Electrophysiol. 2015;1:248–255. doi: 10.1016/j.jacep.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi B.J., Heo J.H., Choi I.S. Hypoadiponectinemia in patients with paroxysmal atrial fibrillation. Korean Circ J. 2012;42:668–673. doi: 10.4070/kcj.2012.42.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermakov S., Azabal F., Stefanick M.L. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102:1354–1362. doi: 10.1136/heartjnl-2015-308927. [DOI] [PubMed] [Google Scholar]
- 13.Rienstra M., Sun J.X., Lubitz S.A. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: the Framingham Offspring Study. Am Heart J. 2012;162:119–124. doi: 10.1016/j.ahj.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett A.S., Picchini J.P., Sr. Adiponectin: an accurate biomarker for patients with at risk for atrial fibrillation? Heart. 2015;101:1351–1352. doi: 10.1136/heartjnl-2015-307816. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin E.J., Levy D., Vaziri S.M. Independent risk factors for atrial fibrillation in a population-based cohort. Fram Heart Study JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 16.Wang T.J., Parise H., Levy D. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H., Tanabe N., Watanabe T. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuppahally S.S., Akoum N., Badger T.J. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–884. doi: 10.1016/j.ahj.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolf S., Kircher S., Arya A. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]