Abstract
Syncope is a clinical syndrome defined as a relatively brief self-limited transient loss of consciousness (TLOC) caused by a period of inadequate cerebral nutrient flow. Most often the trigger is an abrupt drop of systemic blood pressure. True syncope must be distinguished from other common non-syncope conditions in which real or apparent TLOC may occur such as seizures, concussions, or accidental falls. The causes of syncope are diverse, but in most instances, are relatively benign (e.g., reflex and orthostatic faints) with the main risks being accidents and/or injury. However, in some instances, syncope may be due to more worrisome conditions (particularly those associated with cardiac structural disease or channelopathies); in such circumstances, syncope may be an indicator of increased morbidity and mortality risk, including sudden cardiac death (SCD). Establishing an accurate basis for the etiology of syncope is crucial in order to initiate effective therapy. In this review, we focus primarily on the causes of syncope that are associated with increased SCD risk (i.e., sudden arrhythmic cardiac death), and the management of these patients. In addition, we discuss the limitations of our understanding of SCD in relation to syncope, and propose future studies that may ultimately address how to improve outcomes of syncope patients and reduce SCD risk.
Keywords: Syncope, Sudden cardiac death, Risk assessment
1. Introduction
Syncope is a clinical syndrome defined as a relatively brief and self-limited transient loss of consciousness (TLOC) caused by a period of inadequate cerebral nutrient flow. Most often the trigger is an abrupt drop of systemic blood pressure. True syncope must be distinguished from other common non-syncope conditions in which real TLOC may have occurred such as seizures or concussions, or in which TLOC may seem to have occurred such as with accidental falls or psychogenic pseudosyncope.
The causes of syncope are diverse, but in most cases the cause itself is relatively benign (e.g., reflex or orthostatic faints) with the main risks being the consequences of loss of postural tone, such as falls, leading to accidents and injury. However, in some instances, syncope may be due to more worrisome conditions (particularly those associated with cardiac structural disease or channelopathies), and, in such circumstances, syncope may be an indicator of increased morbidity and mortality risk, including sudden cardiac death (SCD). Further, the likelihood of a cardiac origin for syncope, and the consequent increased risk of serious adverse events, are greater in older patients than in the young, paralleling the inevitable development of underlying serious structural heart disease with advancing age.
Overall, morbidity and mortality in syncope patients is low, but 1-year mortality can reach 33% in certain subgroups of patients having a cardiac etiology of syncope. Consequently, establishing an accurate diagnosis and instituting effective preventive measure is essential [1]. Unfortunately, however, rates of unexplained syncope remain high, emphasizing the importance of both developing more effective diagnostic strategies and promoting their acceptance by clinicians [2].
2. Sudden cardiac death: definition, etiology, and pathophysiology
SCD is a term used to refer to a mode of cardiac death, and is frequently used as an outcome of interest in research and epidemiological studies. While there has been some debate, the definition of SCD by Myerburg and Castellanos is widely accepted: “A natural death due to cardiac causes, heralded by abrupt loss of consciousness, within 1 hour after the onset of acute symptoms or an unwitnessed, unexpected death of someone seen in a stable medical condition less than 24 hours previously with no evidence of a non-cardiac cause.” [3], [4]. In these cases, it is assumed that there is a sudden cessation of organized cardiac electrical activity, leading to hemodynamic collapse. If circulation is restored, either spontaneously or through intervention (e.g., defibrillation, anti-arrhythmic drugs), the event is referred to as either a sudden cardiac arrest (SCA) or SCD, but, if fatality occurs, it can only be termed as SCD. If, on the other hand, resuscitation is initially effective, but the patient dies somewhat later, then it is classified as a ‘non-sudden’ cardiac death.
Abnormal electrical activity associated with SCD may be broadly classified as being tachyarrhythmias and non-tachyarrhythmias. The former includes ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT), which most often arise from cardiac causes; only rarely are they due to non-cardiac causes (e.g., pulmonary embolism). In any case, these life-threatening arrhythmias require urgent therapy, specifically direct current (DC) shock and often other interventional cardiac procedures [5], [6]. The non-tachyarrhythmia group includes pulseless electrical activity (PEA), asystole, and extreme bradycardia. While commonly associated with non-cardiac factors (e.g., major pulmonary embolism), PEA may have a primary cardiac cause, including severe pump failure and acute coronary syndrome. Secondary cardiac causes of PEA include those that occur following spontaneous or electrical termination of VT or VF [7].
In industrialized countries and, most likely as well, in developing economies, the most common cardiac cause of SCD is myocardial ischemia due to atherosclerotic coronary artery disease (CAD) [4], [8]. However, SCD may also be a complication of a wide variety of other cardiac conditions (Table 1) [4], [9]. Finally, there are non-arrhythmic forms of SCD, such as aortic dissection, massive pulmonary embolism, cardiac tamponade, and atrial myxoma. In this report, we use “cardiac syncope” to refer to syncope secondary to a cardiac arrhythmia. Non-arrhythmic forms are reasonably categorized separately as of ‘structural cardiovascular origin.’.
Table 1.
SCD causes and contributing factors other than coronary atherosclerosis.
| Bradyarrhythmias (e.g. complete heart block) |
| Cardiomyopathies |
| - Alcohol |
| - Chagas disease |
| - Dilated – idiopathic |
| - Hereditary |
| - Hypertrophic |
| - Hypertensive |
| - Infiltrative (e.g., amyloid, sarcoid, etc) |
| - Cellular conduction disturbances (e.g., ARVC) |
| - Peripartum |
| - Takotsubo (Stress-induced) |
| Channelopathies |
| -Brugada syndrome |
| - Catecholaminergic polymorphic VT |
| - Early repolarization |
| - Long and Short QT syndrome |
| Conduction system abnormalities (Wolf-Parkinson-White) |
| Congenital coronary-artery anomalies |
| Congenital heart diseases |
| Coronary artery abnormalities (e.g., spasm, dissection, embolism) |
| Laminopathies |
| Left ventricular noncompaction |
| Mechanical interference of venous return (e.g., pulmonary embolism, tamponade) |
| Neuromuscular diseases (e.g., muscular and myotonic dystrophies) |
| Pulmonary hypertension (primary and secondary causes) |
| Myocarditis |
| Valvular disease (mitral valve prolapse, aortic stenosis) |
ARVC represents arrhythmogenic right ventricular cardiomyopathy; SCD, sudden cardiac death; VT, ventricular tachycardia
Table limited to arrhythmogenic causes of SCD that are cardiac in origin (e.g., excludes pulmonary embolism, which can occasionally cause ventricular arrhythmias)
3. Syncope and its prognosis
The mortality associated with syncope, including SCD risk, is greatest in those cases in which syncope is of a cardiac cause. Mortality rates of 18% to 30% at 1 year, as compared to only 6% in adult patients with syncope of unknown origin (the majority of which are probably reflex or orthostatic), have been reported [10], [11]. Pre-syncope, in at least one study, has been shown to be as important as true syncope from a prognostic perspective, and, therefore, is similarly managed [12].
The report by Soteriades et al., derived from Framingham Heart Study data, despite being limited by incomplete diagnostic testing and the uncertain ‘neurological syncope’ category, was among the first to highlight the importance of ‘cardiac cause’ as a major mortality risk factor in syncope patients [11]. Of community-dwelling participants in Framingham, the incidence of a first report of syncope was 6.2 per 1000 person-years. Of those, 21.2% were believed to be vasovagal syncope, 9.5% cardiac syncope, 9.4% orthostatic, and 36.6% were of unknown cause. Multivariable-adjusted hazard ratios among individuals with presumed cardiac syncope were 2.01 (95%CI 1.48 to 2.73) for death from any cause, 2.66 (95%CI 1.69–4.19) for myocardial infarction or death from coronary heart disease (including sudden and non-sudden death), and 2.01 (95%CI 1.06 to 3.80) for fatal or nonfatal stroke. There was no increased risk of cardiovascular morbidity or mortality associated with a presumptive diagnosis of vasovagal syncope. In brief, although the Framingham report did not assess SCD risk per se, it showed a concerning association between cardiac syncope and increased mortality risk.
In young people, syncope is common but mostly benign in terms of mortality risk; however, unexpected deaths may occur, and, in the case of young athletes, unexpected deaths have become a highly visible public health concern. In a cohort of 7568 young athletes (age 16.2±2.4 years) undergoing pre-participation screening, a syncopal episode was reported in 474 (6.2%) over the previous five years [13]. Syncope was unrelated with exercise in most cases (411 athletes, 86.7%), was post-exertional in 57 (12.0%), and was exertional in 6 (1.3%). These last six individuals underwent further testing: one had hypertrophic cardiomyopathy, one right ventricular outflow tract tachycardia, the remaining had positive responses to tilt-testing, but none were associated with adverse outcomes. Remarkably, all episodes of non-exertional or post-exertional syncope had typical features of reflex faints. These latter observations highlight the importance of taking and documenting a detailed, careful history of events. In particular, there is a considerable prognostic difference between syncope during ‘full flight’, which has a very worrisome mortality risk (i.e., true exertional syncope), compared to the lesser mortality concern associated with events occurring either after completion of exertion (i.e., post-exertional) or at rest (i.e., non-exertional but excluding ‘supine syncope’, discussed later).
Syncope carries a poor prognosis in patients with pre-existing substantial cardiovascular morbidity. In a post-hoc analysis from the SCD-HeFT randomized controlled implantable cardioverter-defibrillator (ICD) trial, [14] syncope after ICD implantation occurred in 14% of subjects and was associated with an increase in all-cause mortality, cardiovascular mortality, and SCD, despite randomization to an ICD.
Finally, in an unselected and nationwide Danish registry of SCD in the young (ages 1–35 years), among 89 individuals with SCD, negative toxicology, and no prior drug abuse, 19% were identified as having a history of prior lifetime syncope or presyncope. Other important warning symptoms included dyspnea (15%), chest pain (9%), aborted SCD (2%), and palpitations (2%). In a group of 74 controls, who died by accidental causes, none had prior syncope [15].
4. Patient evaluation
As a rule, when patients present for evaluation of a presumed TLOC event, they use non-specific terms to describe their symptom experience; common descriptors in North America are ‘collapse’ or ‘blackouts’ or falls. However, the clinician should not assume that these episodes were ‘true syncope,’ because that might lead to overlooking other potential causes of real or apparent TLOC (e.g., seizures, accidents, drug abuse, psychogenic pseudosyncope/seizure). Consequently, the first hurdle faced by the clinician is determining whether the episode(s) was due to syncope, or of some other cause of real or apparent TLOC. For purposes of this communication, we assume that the basis for collapse was ‘syncope’ as defined earlier, and consequently the next hurdle is ascertaining the underlying cause.
4.1. Medical history
The foundation for defining the etiologic basis for syncope is a comprehensive medical history taken by an experienced clinician. Importantly, a detailed account should be obtained from the patient and, when possible, witnesses. The initial assessment should include careful documentation of several symptomatic episodes, looking for similarities suggesting a causal diagnosis. Finally, it is crucial to document pre-existing medical conditions, ongoing and newly introduced drug therapy, and family history.
In general, a thorough detailed clinical history taken by an experienced practitioner will be sufficient to differentiate syncope from non-syncope in most cases, and even provide a reasonable explanation for the collapse in 40 to 70% of cases [16]. However, in many instances distinguishing true syncope from non-syncope collapse may be challenging and/or a plausible etiologic diagnosis may not be evident.
Once it is clear that syncope has occurred, differentiating benign causes from those that could be life-threatening is essential. From a mortality and SCD perspective, it is essential to identify ‘cardiac’ causes of syncope (i.e., cardiac structural causes as well as channelopathies), because these comprise the highest mortality risk cases.
Certain clinical symptoms and features have been associated with cardiac syncope and are summarized in Table 2 [16], [17], [18], [19], [20]. Several patient characteristics (e.g., age or the presence of structural heart disease) drastically increase the pre-test probability of cardiac syncope. For example, Alboni et al. found that underlying heart disease was an independent predictor (sensitivity of 95% and specificity of 45%) of a cardiac cause of syncope, and the absence of heart disease excluded a cardiac cause of syncope in 97% of patients [16]. However, an important caveat with respect to the latter findings [16] is that, at the time of the study, the importance of certain channelopathies was not yet fully appreciated. Age is another important factor. In this regard, Del Rosso et al. compared findings in patients above and below age 65; they reported that the diagnosis of the cause of syncope was possible by history alone in 26% of younger and only 5% of older patients [21]. Finally, the value of medical history often becomes less reliable as patients age. For instance, it is now recognized that older patients with syncope may have a period of retrograde amnesia that undermines their recollection of preceding events. In fact, they often insist that they never lost consciousness. Only with the aid of reliable witnesses can the story become clear [22].
Table 2.
Common clinical predictors in syncope subtypes.
| Clinical features that suggest a diagnosis on initial evaluation |
|---|
| Neurally mediated syncope: |
| - Absence of heart disease |
| - Absence of trauma |
| - After exertion |
| - After sudden exposure to pain or an unpleasant sight, sound, emotion, or smell |
| - Long history of recurrent syncope or long duration between episodes (e.g., >4 years) |
| - Nausea, vomiting, or abdominal pain associated with syncope |
| - Occurs with head rotation or pressure on carotid sinus (e.g., neck tie, tumors, collars) |
| - Prolonged sitting or standing, especially in crowded or hot places |
| - Prandial or post-prandial |
| Syncope due to orthostatic hypotension |
| - After standing up |
| - Associated with vasodepressive medications |
| 0 Presence of autonomic neuropathy or Parkinsonism |
| - Prolonged standing, particularly in crowded, hot places |
| - Standing after exertion |
| Cardiac syncope |
| - Abnormal ECG |
| - During effort or while supine |
| - Family history of unexplained sudden death or an inherited condition |
| - History of structural heart disease |
| - Palpitations followed by syncope |
| ECG, electrocardiogram |
4.2. Risk stratification schemes
In addition to the above noted clinical findings that help unmask cardiac causes of syncope, and thus identify patients at higher SCD risk, there are several ‘risk stratification’ schemes proposed to aid in clinical decision-making [18], [23], [24], [25], [26], [27], [28], [29], [30], [31]. Most of these schemes have focused on Emergency Department triage with regard to the need for immediate hospitalization; however, as a rule these schemes only offer short-term risk assessment (typically 1 week to 1 month [Table 3]). The ACC/AHA/HRS 2017 practice guidelines [32] offer a detailed assessment of the available risk stratification tools.
Table 3.
Principal published syncope risk scores.
| Examples of syncope risk scores | ||||
|---|---|---|---|---|
| Study/Author | Sample Size | Outcome Definition | Predictors | Adverse events in lowest risk subgroup |
| Osservatorio Epidemiologico sulla Sincope nel Lazio (OESIL) [23] | 270 | 1-year death | Age >65; CVD in clinical history; No prodrome; Abnormal ECG; | 0% (0% in the validation cohort, n=328) |
| San Francisco Syncope Rule [24], [31] | 684 | 7-day serious eventsa | Abnormal ECG; CHF; Shortness of breath; Hematocrit <30%; SBP <90 mmHg; | 0.8% [0.3% in the validation cohort, n=371] |
| Boston Syncope Rule [25] | 293 | 30-day serious eventsb | ACS signs/symptoms; signs of conduction disease; worrisome cardiac history; valvular heart disease; family history of sudden death; abnormal vital signs; volume depletion; primary CNS event | 1.4% |
| EGSYS score [18] | 260 | Mortality at mean (SD) follow up of 614 (73) days | Palpitations; Exertional; Supine; | 3% [2% in the validation cohort, n=256) |
| Abnormal ECG and/or CVD in clinical history; | ||||
| Autonomic prodrome (negative predictor); | ||||
| Predisposing and/or precipitating factors (negative predictor) | ||||
| Short-Term Prognosis of Syncope Study (STePS) [26] | 676 | 10-day serious eventsc | Abnormal ECG; Trauma; No prodrome; Male | NA |
| Syncope Risk Score [27] | 2584 | 30-day serious eventsd | Abnormal ECG; Age>90; Male; Positive troponin; History of arrhythmia; SBP>160; Near-syncope (negative predictor) | 2.5% |
| Risk Stratification of Syncope in the ED [28] | 550 | 30-day serious eventse or all cause death | BNP ≥ 300 pg/ml; Fecal occult blood; Hemoglobin≤9; O2Sat≤94; | 0.8% [1.5% in the validation cohort, n=538] |
| Abnormal ECG (presence of Q wave) | ||||
| Canadian Syncope Risk Score [29] | 4030 | 30-day serious adverse outcomesf | Predisposition to VVS; heart disease; any SBP in the ED < 90 or > 180 mmHg; troponin elevation; abnormal QRS axis; QRS>130 ms; QTc interval >480 ms; ED diagnosis of cardiac syncope; ED diagnosis of VVS | 0.4% |
| IC-FUC [30] | 393 | 30-day death or unplanned ED/hospital visit | History of heart disease; abnormal ECG; history of syncope | 18.6% |
ACS, acute coronary syndrome; BNP, brain natriuretic peptide; CHF, congestive heart failure; CVD, cardiovascular disease; ECG, electrocardiogram; ED, emergency department; SD, standard deviation; SBP, systolic blood pressure; VVS, vasovagal syncope
Events: death, major therapeutic procedure, myocardial infarction, arrhythmia, pulmonary embolism, stroke, sepsis, hemorrhage, life threatening sequelae of syncope.
Events: death, myocardial infarction, arrhythmia, pulmonary embolism, stroke, hemorrhage, re-admission.
Events: death, major therapeutic procedure, re-admission.
Events: death, arrhythmia, myocardial infarction, new diagnosis of severe structural heart disease, pulmonary embolism, aortic dissection, stroke/TIA, cerebral hemorrhage, significant anemia requiring blood transfusion.
Events: acute myocardial infarction, life-threatening arrhythmia, pacemaker or defibrillator implantation within 1 month of syncope, pulmonary embolus, cerebrovascular accident, intracranial hemorrhage, or subarachnoid hemorrhage, hemorrhage requiring transfusion, or acute surgical procedure or endoscopic intervention.
Events: arrhythmia, myocardial infarction, serious structural heart disease, aortic dissection, pulmonary embolism, severe pulmonary hypertension, severe hemorrhage, subarachnoid hemorrhage, any other serious condition causing syncope and procedural interventions for the treatment of syncope.
Apart from their short time horizon, caution should be exercised in using these risk stratification tools given the differences in study populations and study designs from which they were derived, and several were not subjected to validation re-testing in a separate patient validation population. Furthermore, it has been suggested that clinical judgment by experienced practitioners performed as well as the prediction tools in an individual patient data meta-analysis identifying serious outcomes at 10 and 30 days after an Emergency Department stay for syncope [33]. In brief, there is no current consensus on which of the risk stratification tools is the most effective; although an ongoing NIH supported study is attempting to address this issue [2].
4.3. Role of ambulatory ECG monitoring
An ambulatory ECG (AECG) is an invaluable tool to diagnose suspected arrhythmias in syncope patients, and the type of AECG chosen depends on the expected frequency of symptomatic episodes [17]. Due to the infrequent nature of symptoms in most patients with recurrent syncope, long-term miniaturized insertable cardiac monitors (ICM) have become widely accepted as a valuable diagnostic tool, especially if an initial wearable AECG monitoring device (e.g., event recorder or Mobile Cardiac Outpatient Telemetry [MCOT]) has been non-diagnostic. The estimated diagnostic yield in syncope is 1–5% for a 24–48 h standard Holter monitor; 5–10% for a 3–7 day patch/external loop recorder/MCOT; 15–25% for a 1–4 week patch/external loop recorder/MCOT; and 30–50% for a ≤36 month ICM [34]. Two randomized controlled trials both demonstrated a higher yield with ICM compared to “conventional diagnostic monitoring.” In the first study, ICM was compared with external loop recorder and tilt and electrophysiology testing, with a diagnosis being achieved in 52% versus 20% (at 12 months), respectively [35]. In the second study, the diagnostic yield of ICM versus conventional diagnostics was 43% versus 6% (at seventeen months), respectively [36]. ICM is particularly useful in detecting bradyarrhythmias, which, with rare exceptions (addressed later), are often not detected with an EP study [35]. Importantly, the use of a prolonged ICM monitoring strategy appears to be a safe approach to the diagnostic assessment of recurrent syncope, in most cases.
4.4. EPS in syncope with structural heart disease
An invasive electrophysiology study (EPS) is rarely indicated in syncope evaluation [37]. The greatest diagnostic yield for EPS occurs in those patients with structural heart disease to assess for ventricular arrhythmias, especially in those with prior MI and LV ejection fraction >35% [37]. Thus, if used in appropriate circumstances, EPS has utility in unmasking those forms of syncope that are at highest SCD risk. Induction of a clinically relevant or hemodynamically significant sustained VT or VF is a class I indication for an ICD [38]. On the other hand, EPS is less useful in the detection of bradyarrhythmias; in certain patients, a His bundle recording can help determine the exact site of a potentially symptomatic AV block. However, long-term ICM is a better choice in this setting, recognizing that some patients may be left at risk of a serious collapse, while awaiting a definitive diagnosis [35].
5. Specific conditions in which syncope has worrisome SCD risk
5.1. Coronary artery disease
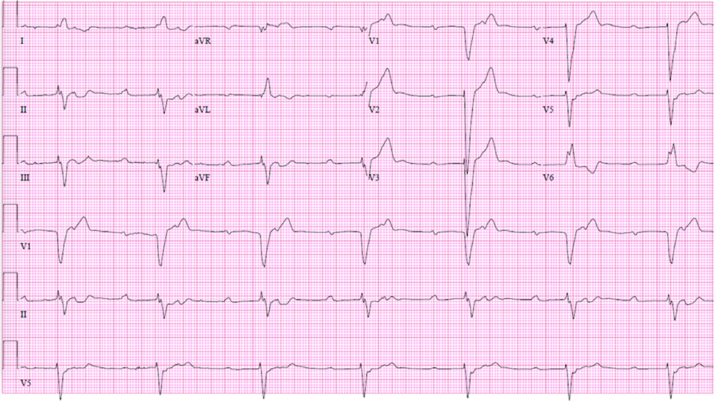
CAD remains the leading cause of SCD worldwide [7], [39]. Syncope is almost never the cause of myocardial infarction (MI), but is not an infrequent consequence of an acute MI event. One mechanism of syncope associated with myocardial ischemia or acute MI, particularly in the case of an inferior MI, includes a reflex faint with both cardioinhibitory and vasodepressor components presumed to be due to the Bezold-Jarisch mechanism. This latter reflex occurs because of the abundance of mechano- and chemo-sensitive receptors in the infero-posterior region of the left ventricle, supplied by the inferior coronary vessels, that activate the afferent neural fibers (generally designated C-fibers) of the vagus nerve, and trigger a predominantly vagal-mediated reflex [40]. However, new high-grade AV block on ECG (Fig. 1), or tachyarrhythmias (particularly paroxysmal monomorphic or non-sustained polymorphic VT) are also potential causes of syncope in the setting of acute ischemia. In the primary percutaneous coronary intervention era, among a registry of >59,000 patients, high-degree AV block occurred in 5.9% of patients with right coronary artery occlusion, and 1.5% of patients with other infarct-related arteries [41].
Fig. 1.
12-lead ECG of a patient with 2:1 AV block. The conducted beats have a long PR interval and a left bundle branch block morphology, indicating severe underlying conduction system disease.
Particularly concerning is syncope occurring in days to weeks after acute MI. In such cases, EPS may be necessary to unmask susceptibility to life-threatening VT [42]. However, it should be noted that EPS with excessively aggressive stimulation protocols may overestimate this risk due to an increased sensitivity for arrhythmia induction during this period. A typical recommended protocol is ventricular extrastimulus testing at 2 basic pacing frequencies between 600 and 400 ms, and no more than 3 premature extrastimuli. The additional use of catecholamine infusion can be considered. EPS may be repeated at the right ventricular outflow tract or left ventricle. Limiting the prematurity of the extrastimuli to a minimum of 180 ms is reasonable if sustained monomorphic VT is considered a positive endpoint, as a very short coupling interval is more likely to induce VF compared to monomorphic VT [43].
In chronic ischemic heart disease, unexplained syncope may be the result of reentrant VT and this circumstance usually merits a myocardial ischemia evaluation with possible revascularization [37]. Nonetheless, because the substrate for arrhythmia may not be sufficiently modified, despite revascularization, these patients should also be considered for an arrhythmia evaluation, including possible EPS. That said, an ICD may be an appropriate choice, despite a negative EPS in these patients (Class IIb)[38]. Alternatively, an ICM may help in better defining the nature of the unexplained syncope [37]. Both the diagnostic yield and safety of ICM monitoring has been demonstrated in those with syncope associated with structural heart disease [44].
5.2. Idiopathic dilated cardiomyopathy
Syncope is an important risk factor for SCD in advanced heart failure [45], including non-ischemic dilated cardiomyopathy (NIDCM) patients [46]. In such patients, syncope has been shown to have event rates as high as those with sustained arrhythmias [47], [48]. Knight et al. followed 14 consecutive patients with NIDCM, unexplained syncope, and a negative EPS. Half the patients had appropriate ICD shocks for ventricular arrhythmias, compared with 8 of 19 (42%) in a control group with prior cardiac arrest (p=0.1) [47]. Also, Fruhwald et al. followed 23 patients with NIDCM and syncope; 5 of 6 deaths (26% died overall) in the syncope patients were SCDs, whereas only 13 of 41 deaths (20% died overall) in a matched cohort of non-syncope patients were SCDs [46]. For patients with unexplained syncope and NIDCM, it is reasonable to consider ICD therapy according to the most recent guideline recommendations (Class IIa)[38]. Under-recognized causes of NIDCM, such as LMNA-cardiomyopathy, particularly if there are also conduction defects or a family history of NIDCM, must also be considered in those with syncope (Table 4).
Table 4.
Syncope and SCD risk in other cardiac conditions.
| Clinical picture | Refs | |
|---|---|---|
| Infiltrative cardiomyopathies (e.g., Amyloidosis, Sarcoid, Hemochromatosis) | Amyloidosis: | [68], [69] |
| ||
| Early repolarization (ER) | The finding of ER on ECG after syncope is almost always an incidental finding given the high prevalence of ER in the general population (~5 to 13 percent). Thus, the diagnosis is usually made only after an aborted cardiac arrest or VT/VF in a patient who displays ER in the inferior and/or lateral leads on ECG. | [56], [60], [70] |
| ER syndrome is one of the J wave syndromes and has several similarities with Brugada syndrome | ||
| A case control study of idiopathic VF subjects found that VF cases with ER were more likely to have a history of syncope than VF without ER. Also, syncope may be an important predictor in CPVT patients with ER. | ||
| Congenital heart disease | A more aggressive diagnostic approach is recommended when unexplained syncope occurs in congenital heart diseases with “high risk” substrates, including tetralogy of Fallot, TGA after atrial switch surgery, or systemic or single ventricular dysfunction. | [72] |
| Primary pulmonary hypertension | There is no consensus on how to interpret syncope in PPH. | [73], [74] |
| Prevalence is more common in children than adults | ||
| Multiple mechanisms (e.g., atrial arrhythmias, systemic vasodilation, or extreme transient elevations in pulmonary arterial systolic pressure during exertion) | ||
| Laminopathies | LMNA mutation carriers frequently have left ventricular systolic dysfunction and arrhythmias. | [75] |
| In a cohort of 269 Europeans with pathogenic LMNA mutations, unexplained syncope occurred in 11% but was not amongst the independent predictors of malignant VA. | ||
| Short QT Syndrome | This rare channelopathy is associated with both syncope and SCD, but whether syncope confers a greater risk of SCD is unclear. | [55] |
| LV Non-compaction | Unexplained syncope has been reported in 5% of LVNC, but it is unknown if this confers an increased risk of SCD. | [76] |
ARIC, atherosclerosis risk in communities; AV, atrioventricular; CI, confidence interval; ECG, electrocardiogram; ER, early repolarization; HR, hazard ratio; LMNA, Lamin A/C; LV, left ventricular; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; PPH, primary pulmonary hypertension; Refs, references; SCD, sudden cardiac death; TGA, transposition of the great arteries; VA, ventricular arrhythmias; VT, ventricular tachycardia; VF, ventricular fibrillation
5.3. Arrhythmogenic right ventricular cardiomyopathy (ARVC)
Syncope is a frequent issue in ARVC. In one of the largest ARVC series (151 patient), 32% had reported syncope. Unexplained syncope has been associated with an increased arrhythmic risk in some, but not all, studies [49]. For example, in a multicenter study assessing the impact of the ICD for prevention of SCD in 132 patients with ARVC, the rate of appropriate ICD interventions was 15% per year and was similar when patients were stratified by history of cardiac arrest, VT, or unexplained syncope [50]. On the other hand, amongst 84 patients with ARVC who underwent ICD for primary prevention, history of syncope was not predictive of ventricular arrhythmias, despite a baseline prevalence of 27% [51]. Expert consensus guidelines do recommend an ICD for those ARVC patients with unexplained syncope, considering it a major risk factor [49]. Furthermore, ACC/AHA guidelines give a Class IIa recommendation for ICD in those with one or more risk factors, including syncope [52]. Important caveats to ICD placement within the diseased right ventricle of ARVC patients include difficulties with lead implantation and perforation, as well as inadequate pacing, sensing, and defibrillator function (including inappropriate ICD therapies) in the long-term [53].
5.4. Brugada syndrome
Syncope is a relatively common presentation in Brugada syndrome (BrS), an inheritable primary arrhythmia syndrome. Considered one of the J wave syndromes, BrS has several clinical and genetic similarities with early repolarization syndrome (ERS) [54]. The region generally most affected in BrS is the anterior right ventricular outflow tract; in ERS, it is the inferior region of the left ventricle [54]. Guidelines recommend ICD implantation for primary prevention in those with a history of syncope, but recommend against the use of an ICD in patients who are asymptomatic (Class IIa)[38], [55]. In a recent series of 352 consecutive patients with BrS, a prior history of aborted cardiac arrest occurred in 6% and syncope in 35% (28% arrhythmic, 57% non-arrhythmic, and 15% unknown). After 9 years, the probability of aborted cardiac arrest was 53% in those with prior cardiac arrest, 15% with prior suspected arrhythmic syncope, 0% in suspected non-arrhythmic syncope, and 3% in asymptomatic patients (p<0.01) [56]. This speaks to the relatively benign nature of non-arrhythmic syncope in BrS.
5.5. Long QT syndrome
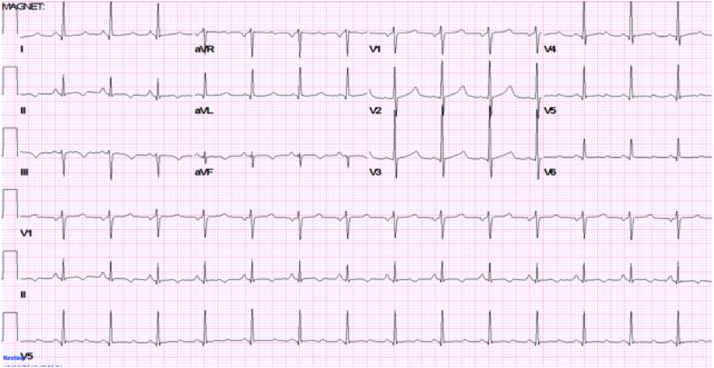
Congenital forms of long QT syndrome (LQTS) are usually inherited in an autosomal dominant manner. LQTS involves a group of channelopathies typically characterized by prolongation of the QT interval on the ECG (Fig. 2). However, ECG manifestations may not always be evident, and there are several different forms of T-wave prolongation depending on the type of LQTS. Even in the presence of syncope, most LQTS patients can be effectively treated with β-blockers, especially those with LQTS type 1 [57]. Less commonly, patients may be considered for stellate ganglion sympathectomy or ICD implantation. The latter is best reserved for those with aborted cardiac arrest and other select situations [58]. For patients with unexplained syncope despite β-blockers, it is reasonable to consider ICD therapy, according to the most recent ACC/AHA guideline recommendations (Class IIa) [38]. In instances of drug-induced LQTS, eliminating the offending agent(s) is the best therapeutic approach. Whether such patients have an underlying susceptibility to LQTS that is unmasked by drug exposure remains controversial, and a reasonable subject for future research.
Fig. 2.
12-lead ECG showing a prolonged QTc in a patient with Long QT type 1 syndrome. The long QT syndromes may be associated with a particular form of polymorphous ventricular tachycardia (i.e., torsade de pointe). Torsades may be non-sustained and, in such cases, could be the cause of syncope. However, torsades may also degenerate into VF and, thereby, be responsible for SCD.
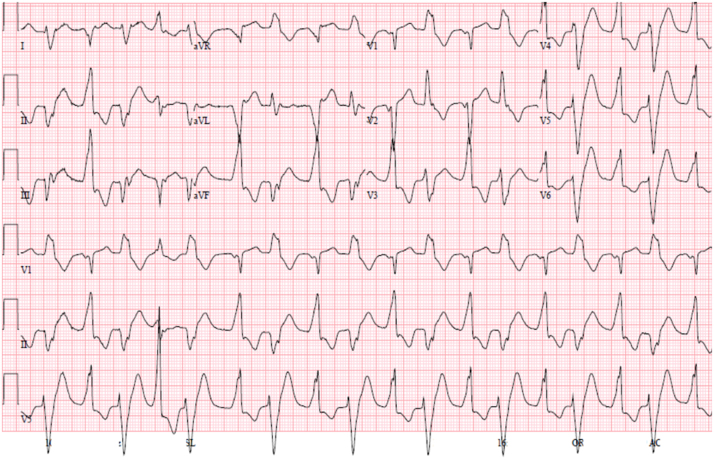
5.6. Catecholaminergic polymorphic ventricular tachycardia (CPVT)
CPVT is a genetic cardiac channelopathy with mutations affecting genes involved in intracellular calcium regulation. In CPVT, syncope and SCD tend to be the result of adrenergically-mediated arrhythmias, induced by emotional stress or exercise (Fig. 3). Recent opinion has migrated towards a relatively conservative therapeutic approach, with avoidance of ICD implantation, if possible.
Fig. 3.
12-lead ECG of a patient with catecholaminergic polymorphic ventricular tachycardia (CPVT). Note that the QRS axis alternates with every other beat, and consequently this arrhythmia is referred to as bidirectional VT. Digitalis toxicity may cause a similar arrhythmia.
Implantable cardioverter-defibrillators (ICDs) may cause significant morbidity in the form of inappropriate shocks and induction of malignant arrhythmias, secondary to both inappropriate and appropriate ICD shocks. Indeed, this has been a particularly important concern in CPVT in which patients have died despite seemingly appropriate ICD therapy. Thus, ICD is recommended as an early treatment strategy, only in those who have had a definite cardiac arrest, recurrent syncope, or polymorphic/bidirectional VT despite seemingly optimal medical management (Class 1) [55]. In CPVT patients with syncope, treatment recommendations start with β-blocker therapy (preferentially nadolol or propranolol) at the highest tolerable dose followed by the addition of flecainide, in the case of recurrent syncope or polymorphic/bidirectional VT while on β-blockers [38], [58], [59]. Lifestyle changes may also be helpful (i.e., diminishing exposure to excessive exertion). In the setting of failed combination drug therapy, one can then consider ICD implantation, although left cardiac sympathetic denervation has emerged as a promising option [55].
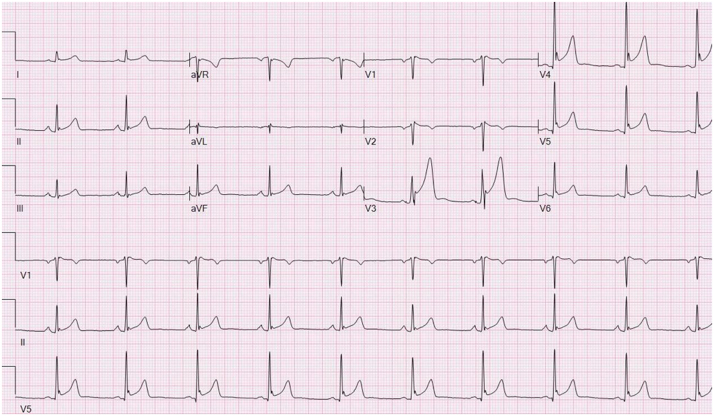
Among 51 patients with CPVT, early repolarization (ER) was present in an unexpected large proportion of 45% (versus the general population of 5 to 13%). A history of syncope was present in 78% of those with ER versus 39% without ER (p=0.005) (Fig. 4) [60].
Fig. 4.
12-lead ECG of a patient with early repolarization of the inferolateral leads. This ECG finding has recently been associated with increased SCD propensity.
5.7. Hypertrophic cardiomyopathy (HCM)
Syncope occurs in 15–25% of those with HCM [61]; it is most common in younger patients with smaller ventricles [62], and is often provoked by exercise (during or after) or by postural change. The principal causes of syncope in HCM are broadly divided into arrhythmias and those that result from primary hemodynamic compromise. Fananapazir et al. performed an EPS in 155 patients with HCM, of which 22, 55, and 37 had prior SCD, syncope, and presyncope, respectively [63]. Remarkably, 81% had abnormalities, including sinus node dysfunction (66%), His-Purkinje disease (30%), inducible atrial re-entrant tachycardia (10%), atrial fibrillation (11%), ventricular arrhythmias (43%), and non-sustained VT (14%). Of note, atrial fibrillation is common and may be responsible for clinical deterioration, including syncope and heart failure due to reduced diastolic filling in an already hypertrophic ventricle and reduced cardiac output [61].
Presyncope or syncope in HCM necessitates urgent workup and treatment as such occurrences indicate a high risk of SCD, particularly when recent and occurring in the young. Spirito et al. found that patients with unexplained syncope within 6 months of diagnosis had a 5-fold increase in risk compared to those without syncope [64]. This was most prominent in the young, where those <18 years old had SCD event rates of 293 versus 55 versus 13 per 1000 person-years for recent (<6 mo), remote, and no syncope, respectively. In those 18–39 years old, event rates remained high at 46 versus 5 per 1000 person-years for recent versus remote syncope, respectively. In those ≥40 years old, event rates were 20 versus 3 per 1000 person-years in those with recent versus remote syncope, respectively.
5.8. Myotonic and muscular dystrophies
The muscular dystrophies are a heterogeneous group of conditions commonly associated with high rates of left ventricular dysfunction and arrhythmias [65]. However, these conditions are often overlooked in the syncope/electrophysiology literature.
Type 1 myotonic dystrophy (DM1) may be the most important of the muscular dystrophies in terms of high rates of arrhythmias and conduction defects, while having a relatively lower incidence of left ventricular dysfunction. In a recent retrospective study of 1388 adults with DM1, sudden cardiac death occurred in 3.6%, accounting for 15.4% of all deaths. Major conduction defects developed in 19.3% and sustained ventricular arrhythmias in 2.3% [66]. Syncope was an independent predictor of major conduction defects and death, but not sudden death or ventricular arrhythmias. Myocardial fibrosis and cardiac-conduction abnormalities are considered to be important mechanisms in the risk of sudden death in DM1, and prophylactic pacing is being used in asymptomatic individuals who have conduction abnormalities on ECG. Abnormal sodium current properties have been implicated in these abnormalities in both human and animal studies, which may, in part, account for the observation that the type 1 Brugada ECG pattern is 50-fold more prevalent in DM1 than the general population. Atrial arrhythmias are also common in DM1, can cause syncope, and are a marker of worse prognosis. In a study of 161 patients with DM1, 27 (17%) had either atrial fibrillation or flutter (AFL), with two presenting with syncope-related AFL with 1:1 AV nodal conduction [67]. These atrial arrhythmias were associated with an increase in mortality (30% died with AF vs 10% without, p<0.01).
5.9. Other causes of increased SCD risk in syncope
A summary of additional cardiac conditions in which syncope may be associated with SCD, but in which the association is less certain or less frequent, is provided in Table 4 [55], [56], [60], [68], [69], [70], [71], [72], [73], [74], [75], [76].
6. Syncope in patients with ICD
6.1. Syncope before ICD implantation
A non-randomized registry from the Antiarrhythmics Versus Implantable Defibrillators (AVID) Study assessed patients with a history of unexplained syncope and structural heart disease who were excluded from the main trial due to the absence of VT/VF [77]. Eighty patients in the registry had a positive EPS. This unexplained syncope with positive EPS registry (of which 84% had an ICD) had a similar survival to ICD-treated VT patients from the main trial, both groups being superior to drug-treated VT patients (p=0.05). Although the two groups appeared similar in terms of survival, subgroup analyses showed that severe left ventricular dysfunction significantly worsened outcomes in the syncope registry patients, but did not impact the outcomes in ICD-treated VT patients from the main trial. This suggests that syncope itself may not have been the major adverse outcome predictor, but rather LV dysfunction.
In the SCD-HeFT randomized controlled clinical trial, 162 (6%) patients had syncope before randomization [14]. Syncope before randomization was not associated with death (HR 0.98, 95%CI 0.73–1.33; p=0.91). However, 38% of patient with syncope before randomization had an appropriate ICD shock compared to 19% of patients without syncope (HR 1.75, 95%CI 1.10 to 2.80, p=0.019), suggesting syncope before ICD implantation is associated with an increase in appropriate ICD therapies.
6.2. Syncope after ICD implantation
ICDs, while effective in reducing SCD, may not prevent syncope. Collapse may occur before the device recognizes and terminates the arrhythmia. In a post-hoc analysis from the SCD-HeFT randomized controlled trial, syncope after ICD implantation occurred in 14% of patients, and was associated with an increase in all-cause mortality, cardiovascular mortality and sudden cardiac death despite randomization to an ICD [14]. However, an important limitation of this study was that the causes of syncope in SCD-HeFT were presumptive. Thus, it is uncertain how many patients had arrhythmic syncope. Interestingly, patients with syncope in the SCD-HeFT were more likely to have appropriate ICD shocks, yet ICDs did not protect these patients from dying. One speculation from this post-hoc analysis is the possibility that both syncope and malignant ventricular arrhythmias are markers for a more advanced cardiovascular disease state, and that a life-threatening event in these cases is less amenable to being successfully reversed.
7. Limitations of our understanding
7.1. Temporal relationship between syncope and death – immediate risk or long-term?
Recent syncope is generally assumed to be more concerning with regard to mortality risk than is remote syncope. In this regard, Sheldon et al. suggest that a long history of fainting is more indicative of reflex faints and, consequently, is of lower concern regarding SCD or mortality [78]. However, although the data are sparse, patients with reflex faints may later develop other causes of syncope as they age, and, thereby, become high-risk individuals. Thus, clinicians should not rely on a ‘long history of fainting’ as being indicative of low future risk.
The strongest evidence for recent syncope denoting higher risk is found in LQTS patients. Data from the LQTS Registry found recent syncope to be the most significant predictor of SCD, even greater than the degree of QT prolongation and genotype [79]. In HCM, recent syncope (<6 mo) is also particularly worrisome, and one could argue that these patients are reasonable candidates for an ICD for primary SCD prevention [64]. On the other hand, recent and remote syncope may not differ in regard to SCD risk in heart failure patients. In a retrospective analysis of 491 consecutive patients with advanced heart failure, no prior cardiac arrest, and a mean left ventricular ejection fraction (LVEF) of 0.20± 0.7, of which 12% had a history of syncope, the risk of SCD did not differ between patients with recent (~6 weeks) or non-recent (>6 weeks) syncope (p=0.68) [45].
The relationship between syncope timing and mortality risk likely varies among disease states and specific syncope etiology. This relationship warrants further study as its answer may directly impact clinical response to a collapse event.
7.2. Does number of episodes of syncope matter in the risk of SCD?
Multiple syncope events over many years suggests a lower mortality/SCD risk based on the evident survivability of the events. In particular, when structural heart disease has been excluded, patients with multiple episodes of syncope are less likely to have been experiencing life-threatening arrhythmias. Krol et al. evaluated 104 patients with unexplained syncope, of which 31 and 73 subjects had a positive or negative EPS, respectively [80]. Rather surprisingly, a negative EPS was associated with significantly more syncopal episodes than a positive study (5.2 versus 2.2, p<0.0001); furthermore, all patients with >6 syncopal episodes had a negative EPS [80]. In another report, ≥4 syncopal events in the preceding year was an independent predictor of psychiatric illness [81] suggesting a greater likelihood of pseudosyncope, and a lesser probability of cardiac syncope.
Regarding these studies, conditions in which recurrent syncope may be ominous may have been under-represented due to limitations in sample size. Furthermore, as patients age the risk of more serious forms of syncope increase, and, therefore, someone who may have initially had a benign form of recurrent syncope could coincidentally develop a more serious, unrelated form of syncope.
8. Future research needs
Syncope remains a vexing challenge, despite the clinical research efforts of the past few decades. While many studies have provided important insights into the evaluation and management of syncope, their limitations are manifest in the inconsistency of clinical decision aids and the inability of consensus guidelines to guide the clinician in, not only establishing the cause of the patient's symptoms, but in also providing an effective treatment strategy and prognosis. The following list offers several topics in which future research may be particularly useful:
-
a)
Syncope in the ED should remain an area of focus given the high cost associated with ED visits, and the impact that ED clinicians have on decisions related to hospital admissions. To better address this problem, Sun et al. have proposed the development of a syncope research agenda that importantly involves multiple specialties and countries [2]. Priorities include 1) appropriately defining syncope, 2) eliminating serious, obvious conditions from future risk prediction tools (e.g., previous studies suggest that a low hemoglobin predicted serious outcomes in syncope, but when patients with obvious bleeding conditions were excluded, hemoglobin level was no longer associated with adverse events), 3) improving diagnostic algorithms for syncope, in part, by adhering to standardized reporting guidelines based on agreed-on data definitions, and 4) determine more specific guidance for the patient's disposition and plan for post-ED care, as most current society guidelines simply recommend admission of “high-risk” patients and discharge of those with “low-risk”.
-
b)
It is not definitive whether syncope, per se, is the cause of increased mortality. The underlying disease process may be the primary driver. As Kapoor et al. reported [82], patients identified as having syncope secondary to cardiac disease had a much higher all-cause mortality and SCD risk than patients with syncope, not felt to be due to a cardiac etiology. However, Kapoor et al. went further. They had shown that in syncope patients those with a cardiac etiology did poorly. They later examined this from the opposite direction: do cardiac patients with syncope do worse than cardiac patients without syncope? They found no significant difference in all-cause mortality between patients with cardiac disease and syncope when compared to patients with similar cardiac disease and no syncope [83]. Similarly, in the EGSYS-2 follow-up study, Ungar et al. found that the likelihood of early mortality after syncope was related to the underlying severity of the cardiac disease and not to syncope, per se, or the etiology of the syncope [84]. Findings such as these highlight the importance of treating the underlying cardiac condition in patients with syncope complicating structural heart disease.
-
c)
As noted above, based on SCD-Heft data, syncope in patients already having an ICD is associated with a worse prognosis despite the presence of the device. Given the increasing prevalence of patients with ICDs, this may be an area where future research could have an important impact. The causes of syncope in this group, if defined and better understood, could lead to more effective treatment strategies. For example, if further studies with better monitoring reveal the cause of syncope to be slow ventricular arrhythmias below the device detection zone, or hemodynamically significant arrhythmias causing collapse prior to the onset of device therapy, such scenarios could potentially be amenable to different treatment algorithms with the ICD.
9. Conclusions
Syncope is a common medical problem with many potential causes. The highest mortality and SCD risk occur when syncope is associated with underlying cardiac disease (including channelopathies), and, in particular, when the underlying cause of syncope is determined to be of a cardiac etiology. In this regard, although syncope may be the first manifestation of an underlying cardiac disease, it may also present later as a heralding sign for SCD. It remains far from clear in many scenarios whether syncope is, itself, the driver of increased mortality in patients with cardiac diseases. Current understanding indicates that reduction of mortality in these patients requires aggressive management of the underlying cardiac condition, in addition to whatever specific therapy may be indicated for prevention of future syncope events. A further understanding of the relation between syncope and SCD risk in various heart diseases may help to identify those individuals who will benefit most from diagnostic procedures and therapeutic interventions.
Conflict of interest
Dr. Benditt is a consultant to Medtronic Inc., and Zoll Corp.. He is supported in part by a grant from the Dr. Earl E Bakken Family in support of Heart-Brain research.
References
- 1.Kapoor W.N. Current evaluation and management of syncope. Circulation. 2002;106:1606–1609. doi: 10.1161/01.cir.0000031168.96232.ba. [DOI] [PubMed] [Google Scholar]
- 2.Sun B.C., Costantino G., Barbic F. Priorities for emergency department syncope research. Ann Emerg Med. 2014;64 doi: 10.1016/j.annemergmed.2014.04.014. 649–55.e2. [DOI] [PubMed] [Google Scholar]
- 3.Myerburg R., Castellanos A. Cardiac arrest and sudden cardiac death. In: Bonow R.O., Mann D.L., Zipes D.P., Libby P., editors. Braunwald's heart disease- a textbook of cardiovascular medicine. 10th ed. Elsevier Saunders; Philadelphia, PA: 2015. [Google Scholar]
- 4.Zipes D.P., Wellens H.J.J. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 5.Bergum D., Skjeflo G.W., Nordseth T. ECG patterns in early pulseless electrical activity-Associations with aetiology and survival of in-hospital cardiac arrest. Resuscitation. 2016;104:34–39. doi: 10.1016/j.resuscitation.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Kürkciyan I., Meron G., Sterz F. Pulmonary embolism as cause of cardiac arrest. Arch Intern Med. 2000;160:1529. doi: 10.1001/archinte.160.10.1529. [DOI] [PubMed] [Google Scholar]
- 7.Myerburg R.J., Halperin H., Egan D.A. Pulseless electric activity: definition, causes, mechanisms, management, and research priorities for the next decade: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2013;128:2532–2541. doi: 10.1161/CIRCULATIONAHA.113.004490. [DOI] [PubMed] [Google Scholar]
- 8.Reddy K.S., Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 9.Liberthson R.R. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor N. W. Diagnostic evaluation of syncope. Am J Med. 1991;90:91–106. doi: 10.1016/0002-9343(91)90511-u. [DOI] [PubMed] [Google Scholar]
- 11.Soteriades E.S., Evans J.C., Larson M.G. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 12.Greve Y., Geier F., Popp S. The prevalence and prognostic significance of near syncope and syncope: a prospective study of 395 cases in an emergency department (the SPEED study) Dtsch Arztebl Int. 2014;111:197–204. doi: 10.3238/arztebl.2014.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colivicchi F., Ammirati F., Santini M. Epidemiology and prognostic implications of syncope in young competing athletes. Eur Heart J. 2004;25:1749–1753. doi: 10.1016/j.ehj.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Olshansky B., Poole J.E., Johnson G. Syncope predicts the outcome of cardiomyopathy patients. J Am Coll Cardiol. 2008;51:1277–1282. doi: 10.1016/j.jacc.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 15.Glinge C., Jabbari R., Risgaard B. Symptoms before sudden arrhythmic death syndrome: a nationwide study among the young in Denmark. J Cardiovasc Electrophysiol. 2015;26:761–767. doi: 10.1111/jce.12674. [DOI] [PubMed] [Google Scholar]
- 16.Alboni P., Brignole M., Menozzi C. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37:1921–1928. doi: 10.1016/s0735-1097(01)01241-4. [DOI] [PubMed] [Google Scholar]
- 17.Rogers G., O’Flynn N. NICE guideline: transient loss of consciousness (blackouts) in adults and young people. Br J Gen Pract. 2011;61:40–42. doi: 10.3399/bjgp11X548965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Rosso A., Ungar A., Maggi R. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1620–1626. doi: 10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 19.Sheldon R., Rose S., Connolly S. Diagnostic criteria for vasovagal syncope based on a quantitative history. Eur Heart J. 2006:27. doi: 10.1093/eurheartj/ehi584. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon R., Rose S., Ritchie D. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol. 2002;40:142–148. doi: 10.1016/s0735-1097(02)01940-x. [DOI] [PubMed] [Google Scholar]
- 21.Del Rosso A., Alboni P., Brignole M. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005;96:1431–1435. doi: 10.1016/j.amjcard.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 22.O’Dwyer C., Bennett K., Langan Y. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011:13. doi: 10.1093/europace/eur069. [DOI] [PubMed] [Google Scholar]
- 23.Colivicchi F., Ammirati F., Melina D. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J. 2003;24:811–819. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 24.Quinn J.V., Stiell I.G., McDermott D.A. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43:224–232. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 25.Grossman S.A., Fischer C., Lipsitz L.A. Predicting adverse outcomes in syncope. J Emerg Med. 2007;33:233–239. doi: 10.1016/j.jemermed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costantino G., Perego F., Dipaola F. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008;51:276–283. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 27.Sun B.C., Derose S.F., Liang L.-J. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54:769–778-5. doi: 10.1016/j.annemergmed.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed M.J., Newby D.E., Coull A.J. The ROSE (Risk Stratification of Syncope in the Emergency Department) study. J Am Coll Cardiol. 2010;55:713–721. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 29.Thiruganasambandamoorthy V., Kwong K., Wells G.A. Development of the Canadian syncope risk score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289–E298. doi: 10.1503/cmaj.151469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes D.G., Kus T., Sant’anna R.T. Simple risk stratification score for prognosis of syncope. J Interv Card Electrophysiol. 2016;47:153–161. doi: 10.1007/s10840-016-0165-y. [DOI] [PubMed] [Google Scholar]
- 31.Quinn J., McDermott D., Stiell I. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med. 2006;47:448–454. doi: 10.1016/j.annemergmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Shen W.-K., Sheldon R.S., Benditt D.G. ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Hear Rhythm. 2017:2017. doi: 10.1016/j.hrthm.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Costantino G., Casazza G., Reed M. Syncope risk stratification tools vs clinical judgment: an individual patient data meta-analysis. Am J Med. 2014;127:1126.e13–1126.e25. doi: 10.1016/j.amjmed.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg J.S., Varma N., Cygankiewicz I. ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Hear Rhythm. 2017;2017(14):e55–e96. doi: 10.1016/j.hrthm.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Krahn A.D., Klein G.J., Yee R. Randomized assessment of syncope trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46–51. doi: 10.1161/01.cir.104.1.46. [DOI] [PubMed] [Google Scholar]
- 36.Farwell D.J., Freemantle N., Sulke A.N. Use of implantable loop recorders in the diagnosis and management of syncope. Eur Heart J. 2004;25:1257–1263. doi: 10.1016/j.ehj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Moya A., Sutton R., Ammirati F. Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Circulation. 2008;117:2820–2840. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization . World Health Organization; 2015. Global atlas on cardiovascular disease prevention and control [Internet]http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ [cited 2017 month day] n.d. [Google Scholar]
- 40.Wei J.Y., Markis J.E., Malagold M. Cardiovascular reflexes stimulated by reperfusion of ischemic myocardium in acute myocardial infarction. Circulation. 1983;67:796–801. doi: 10.1161/01.cir.67.4.796. [DOI] [PubMed] [Google Scholar]
- 41.Singh S.M., FitzGerald G., Yan A.T. High-grade atrioventricular block in acute coronary syndromes: insights from the global registry of acute coronary events. Eur Heart J. 2015;36:976–983. doi: 10.1093/eurheartj/ehu357. [DOI] [PubMed] [Google Scholar]
- 42.Olshansky B., Sullivan R.M. Sudden death risk in syncope: the role of the implantable cardioverter defibrillator. Prog Cardiovasc Dis. 2013;55:443–453. doi: 10.1016/j.pcad.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Priori S.G., Blomström-Lundqvist C., Mazzanti A. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;2015(36):2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 44.Solano A., Menozzi C., Maggi R. Incidence, diagnostic yield and safety of the implantable loop-recorder to detect the mechanism of syncope in patients with and without structural heart disease. Eur Heart J. 2004;25:1116–1119. doi: 10.1016/j.ehj.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Middlekauff H.R., Stevenson W.G., Stevenson L.W. Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope. J Am Coll Cardiol. 1993;21:110–116. doi: 10.1016/0735-1097(93)90724-f. [DOI] [PubMed] [Google Scholar]
- 46.Fruhwald FM, Eber B, Schumacher M, et al. Syncope in dilated cardiomyopathy is a predictor of sudden cardiac death. Cardiology n.d.;87. p. 177–80. [DOI] [PubMed]
- 47.Knight B.P., Goyal R., Pelosi F. Outcome of patients with nonischemic dilated cardiomyopathy and unexplained syncope treated with an implantable defibrillator. J Am Coll Cardiol. 1999;33:1964–1970. doi: 10.1016/s0735-1097(99)00148-5. [DOI] [PubMed] [Google Scholar]
- 48.Phang R.S., Kang D., Tighiouart H. High risk of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy presenting with syncope. Am J Cardiol. 2006;97:416–420. doi: 10.1016/j.amjcard.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 49.Corrado D., Wichter T., Link M.S. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrado D., Leoni L., Link M.S. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 51.Bhonsale A., James C.A., Tichnell C. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–1496. doi: 10.1016/j.jacc.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 52.Tracy C.M., Epstein A.E., Darbar D. ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2012;2013(61):e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Mugnai G., Tomei R., Dugo C. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular cardiomyopathy: the course of electronic parameters, clinical features, and complications during long-term follow-up. J Interv Card Electrophysiol. 2014;41:23–29. doi: 10.1007/s10840-014-9920-0. [DOI] [PubMed] [Google Scholar]
- 54.Antzelevitch C., Yan G.-X., Ackerman M.J. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. J Arrhythmia. 2016;32:315–339. doi: 10.1016/j.joa.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Hear Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Olde Nordkamp L.R.A., Vink A.S., Wilde A.A.M. Syncope in Brugada syndrome: prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm. 2015;12:367–375. doi: 10.1016/j.hrthm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz P.J., Spazzolini C., Priori S.G. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 58.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. J Arrhythm. 2014;30:1–28. [n.d.] [Google Scholar]
- 59.Watanabe H., Chopra N., Laver D. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tülümen E., Schulze-Bahr E., Zumhagen S. Early repolarization pattern: a marker of increased risk in patients with catecholaminergic polymorphic ventricular tachycardia. Europace. 2016;18:1587–1592. doi: 10.1093/europace/euv357. [DOI] [PubMed] [Google Scholar]
- 61.Williams L., Frenneaux M. Syncope in hypertrophic cardiomyopathy: mechanisms and consequences for treatment. Europace. 2007;9:817–822. doi: 10.1093/europace/eum093. [DOI] [PubMed] [Google Scholar]
- 62.Nienaber C.A., Hiller S., Spielmann R.P. Syncope in hypertrophic cardiomyopathy: multivariate analysis of prognostic determinants. J Am Coll Cardiol. 1990;15:948–955. doi: 10.1016/0735-1097(90)90222-b. [DOI] [PubMed] [Google Scholar]
- 63.Fananapazir L., Tracy C.M., Leon M.B. Electrophysiologic abnormalities in patients with hypertrophic cardiomyopathy: a consecutive analysis in 155 patients. Circulation. 1989;80:1259–1268. doi: 10.1161/01.cir.80.5.1259. [DOI] [PubMed] [Google Scholar]
- 64.Spirito P., Autore C., Rapezzi C. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 65.Freyermuth F., Rau F., Kokunai Y. Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy. Nat Commun. 2016;7:11067. doi: 10.1038/ncomms11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wahbi K., Babuty D., Probst V. Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur Heart J. 2017;38:751–758. doi: 10.1093/eurheartj/ehw569. [DOI] [PubMed] [Google Scholar]
- 67.Brembilla-Perrot B., Schwartz J., Huttin O. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in myotonic dystrophy. Pacing Clin Electrophysiol. 2014;37:329–335. doi: 10.1111/pace.12260. [DOI] [PubMed] [Google Scholar]
- 68.Dubrey S., Cha K., Anderson J. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 69.Bejar D., Colombo P.C., Latif F. Infiltrative cardiomyopathies. Clin Med Insights Cardiol. 2015;9:29–38. doi: 10.4137/CMC.S19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haïssaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 71.Sacher F., Arsac F., Wilton S.B. Syncope in Brugada syndrome patients: prevalence, characteristics, and outcome. Hear Rhythm. 2012;9:1272–1279. doi: 10.1016/j.hrthm.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Khairy P. Ventricular arrhythmias and sudden cardiac death in adults with congenital heart disease. Heart. 2016;102:1703–1709. doi: 10.1136/heartjnl-2015-309069. [DOI] [PubMed] [Google Scholar]
- 73.McGoon M.D., Kane G.C. Pulmonary hypertension: diagnosis and management. Mayo Clin Proc. 2009;84:191–207. doi: 10.4065/84.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikhail G.W., Gibbs J.S., Yacoub M.H. Pulmonary and systemic arterial pressure changes during syncope in primary pulmonary hypertension. Circulation. 2001;104:1326–1327. doi: 10.1161/hc3601.095274. [DOI] [PubMed] [Google Scholar]
- 75.van Rijsingen I.A.W., Arbustini E., Elliott P.M. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers. J Am Coll Cardiol. 2012;59:493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 76.Brescia S.T., Rossano J.W., Pignatelli R. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127:2202–2208. doi: 10.1161/CIRCULATIONAHA.113.002511. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg J.S., Beckman K., Greene H.L. Follow-up of patients with unexplained syncope and inducible ventricular tachyarrhythmias: analysis of the AVID registry and an AVID substudy. Antiarrhythmics versus implantable defibrillators. J Cardiovasc Electrophysiol. 2001;12:996–1001. doi: 10.1046/j.1540-8167.2001.00996.x. [DOI] [PubMed] [Google Scholar]
- 78.Sheldon R. Syncope diagnostic scores. Prog Cardiovasc Dis. 2013;55:390–395. doi: 10.1016/j.pcad.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Hobbs J.B., Peterson D.R., Moss A.J. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 80.Krol R.B., Morady F., Flaker G.C. Electrophysiologic testing in patients with unexplained syncope: clinical and noninvasive predictors of outcome. J Am Coll Cardiol. 1987;10:358–363. doi: 10.1016/s0735-1097(87)80019-0. [DOI] [PubMed] [Google Scholar]
- 81.Kapoor W.N., Fortunato M., Hanusa B.H. Psychiatric illnesses in patients with syncope. Am J Med. 1995;99:505–512. doi: 10.1016/s0002-9343(99)80227-7. [DOI] [PubMed] [Google Scholar]
- 82.Kapoor W.N., Karpf M., Wieand S. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309:197–204. doi: 10.1056/NEJM198307283090401. [DOI] [PubMed] [Google Scholar]
- 83.Kapoor W.N., Hanusa B.H. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med. 1996;100:646–655. doi: 10.1016/s0002-9343(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 84.Ungar A., Del Rosso A., Giada F. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J. 2010;31:2021–2026. doi: 10.1093/eurheartj/ehq017. [DOI] [PubMed] [Google Scholar]