Abstract
Up to 50% of individuals with major depressive disorder (MDD) do not recover after two antidepressant medication trials and therefore meet criteria for treatment-resistant depression (TRD). Mindfulness based cognitive therapy (MBCT) is one promising treatment, however the extent to which MBCT influences clinical outcomes relative to baseline neural activation remains unknown. The current study investigated baseline differences in amygdala activation between TRD patients and healthy controls (HCs), related amygdala activation to depression symptoms, and examined the impact of MBCT and amygdala activation on longitudinal depression outcomes. At baseline, TRD patients (n = 80) and HCs (n = 37) participated in a functional magnetic resonance imaging task where they identified the emotion of faces (affect labeling), gender of faces (gender labeling), or passively viewed faces (observing). TRD participants then completed 8 weeks of MBCT or a Health Enhancement Program (HEP). Relative to HCs, TRD patients demonstrated less amygdala activation during affect labeling, and marginally less during gender labeling. Blunted amygdala activation in TRD patients during affect labeling was associated with greater depression severity. MBCT was associated with greater depression reductions than HEP directly following treatment, however, at 52 weeks, the treatment effect was not significant and baseline amygdala activation across task conditions predicted depression severity in both groups. TRD patients have blunted amygdala responses during affect labeling that are associated with greater concurrent depression. Furthermore, while MBCT produced greater short term improvements in depression than HEP, overall baseline amygdala reactivity was predictive of long term clinical outcomes in both groups.
Keywords: amygdala, treatment-resistant depression, emotion, mindfulness-based cognitive therapy, affect labeling, faces
Major depressive disorder (MDD) is the most common mental health disorder worldwide (Sartorius, 2001). Antidepressant medication is a standard treatment; yet, treatment failures are frequent—over one third of patients do not fully recover after two or more courses of antidepressants, a condition known as treatment-resistant depression (TRD) (Janicak & Dowd, 2009; Souery, Papakostas, & Trivedi, 2006; Trivedi et al., 2014). Compared to depression that responds to treatment, TRD is associated with greater rates of relapse, prolonged disability, higher medical costs, and lower life quality (Fekadu et al., 2009; Judd et al., 2000; Russell et al., 2004). Consequently, understanding the pathophysiology of TRD is critical for developing effective treatments and reducing its social and personal burden.
MDD has been characterized by enhanced attention to (Gotlib, Krasnoperova, Yue, & Joormann, 2004; Koster, De Raedt, Goeleven, Franck, & Crombez, 2005) and processing of (Leppänen, 2006) emotional information. At the neural level, enhanced processing is often reflected by greater amygdala responses to emotional stimuli (Anand et al., 2005; Sheline et al., 2001), however this is not always the case (Thomas et al., 2001). Individuals with MDD also report difficulty regulating emotions (Beauregard, Paquette, & Levesque, 2006), and demonstrate smaller reductions in amygdala activation than healthy controls (HCs) during emotion regulation (Beauregard et al., 2006; Kanske, Heissler, Schönfelder, & Wessa, 2012). Greater depression severity has been related to increased amygdala activation during passive viewing of emotional stimuli (Lee et al., 2007) and during emotion regulation (Erk et al., 2010). These findings suggest that MDD is characterized by abnormal amygdala activation to affective stimuli that is influenced by depression severity.
Amygdala activation to emotional stimuli can attenuate following the treatment of depression using selective serotonin reuptake inhibitors (SSRIs) (Delaveau et al., 2011; Sheline et al., 2001) or cognitive behavior therapy (CBT) (Fu et al., 2008). Depressed individuals with the greatest amygdala reactivity prior to treatment appear to benefit the most, at least in the short-term. In one study, depressed individuals with the greatest sustained amygdala responses to emotional words demonstrated the greatest depression reductions directly after CBT treatment (Siegle, Carter, & Thase, 2006). Fewer studies have investigated how baseline neural activation impacts long-term effects of antidepressant treatments, however, one study suggested that depressed individuals with the highest amygdala activation during implicit emotional face processing had the lowest depression severity eight months after initial assessment, regardless of treatment received (Canli et al., 2005). These finding suggest that when amygdala hyperreactivity characterizes MDD, SSRIs and CBT treatments can successfully reduce this reactivity and facilitate long-term recovery. However the long-term impact of baseline neural activation on response to treatment and recovery in MDD remain relatively unexplored.
Studies have largely focused on MDD generally; consequently, little is known about amygdala reactivity to affective stimuli or its relation to depressive symptoms specifically in TRD. One recent study reported no baseline differences between TRD patients and HCs in amygdala activation during the processing of sad faces (Murrough et al., 2015). Another study reported that TRD patients did not have differential resting-state amygdala connectivity compared to controls; however, treatment-responsive depressed patients did demonstrated disrupted resting-state amygdala connectivity (Lui et al., 2011). More recently, Jacobs and colleagues reported that individuals with multiple episodes of depression demonstrate differential resting state connectivity between the amygdala and prefrontal regions compared to patients who have only experienced one episode (Jacobs et al., 2016). These limited investigations suggest that TRD may be associated with distinct amygdala reactivity and connectivity; however, additional research is needed. As TRD patients fail to respond to medications that target neural circuitry associated with affective processing, it is critical to establish if TRD patients alone demonstrate the same pattern of hyperactive amygdala response as is seen in MDD patients in general.
In the current study, we examined amygdala reactivity and its relation to concurrent and longer-term depression severity in TRD patients that were part of a larger randomized controlled trial (RTC) demonstrating that 8 weeks of Mindfulness-Based Cognitive Therapy (MBCT), relative to a structurally equivalent comparator condition, the Health-Enhancement Program (HEP), produced greater improvements in depressive symptoms (Eisendrath et al., 2014; Eisendrath et al., 2016). To probe amygdala reactivity, we used a common emotional face processing task. In healthy participants, labeling the emotion of faces in this task has produced the greatest reduction in amygdala activation relative to observing emotional faces, while labeling gender showed an intermediate effect (Critchley et al., 2000; Lieberman et al., 2007) and this result has been attributed to labeling serving as a form of emotion regulation. The preponderance of MDD studies suggest depressed patients are characterized by exaggerated amygdala activation and difficulties regulating affect (Beauregard et al., 2006; Sheline et al., 2001), and therefore would demonstrate amygdala hyper-reactivity during the observation of emotional faces, as well as a failure to downregulate amygdala activation during gender and affect labeling. However the limited number of studies specifically investigating TRD suggest that amygdala activation in these patients may differ from that of MDD generally. Accordingly, our hypotheses about baseline amygdala activation in TRD are necessarily nondirectional. However, given the previous literature suggesting that heightened amygdala reactivity during the labeling of emotional faces is predictive of treatment response recovery regardless of treatment received (Canli et al., 2005), we predicted that patients with greater amygdala reactivity at baseline would show greater treatment response and better longer-term clinical outcomes.
Methods and Materials
Participants
Eighty-four TRD patients and 37 HCs participated in this study. Participants were recruited from outpatient psychiatry and general medicine clinics at the University of California San Francisco (UCSF), at the outpatient psychiatry clinic at Kaiser Permanente in San Francisco and through clinical referrals, flyers, and advertisements in newspapers and on municipal buses and trains. To qualify, TRD patients needed to meet criteria for unipolar MDD based on the Structured Clinical Interview for DSM-IV Disorders (First, 1995), a Hamilton Rating Scale for Depression (HAM-D17) (Hamilton, 1967) score greater than or equal to 14, and to be taking antidepressant medication with evidence of two or more adequate trials prescribed during the current episode as assessed with the Antidepressant Treatment History Form (ATHF; Sackeim, 2001). Patients were excluded for the following: lifetime history of bipolar disorder, schizophrenia, or any psychotic disorder; substance abuse or dependence within 3 months of study onset; currently suicidal, dangerous to others or self-injurious; psychotherapy that they were unwilling to discontinue during the 8-week treatment portion of the study; or a score of <25 on the Mini Mental Status Exam (Folstein, Folstein, & McHugh, 1975).
The HC group was matched to the TRD group on age, gender, and handedness, and had no history of a major Axis I psychiatric disorder, neurological illness, or current use of psychotropic medication. Participants were required to be at least 18 years of age, fluent in English, no MRI contraindications, and to have normal or corrected-to-normal vision.
Participants were excluded from analysis for mean amygdala activation values greater than 3 times the interquartile range (n = 2), missing behavioral data (n = 1), or task accuracy below 70% for any condition (n = 1). The final sample included 80 TRD and 37 HC participants. Written informed consent approved by the Institutional Review Board at UCSF was obtained for participants. Demographic data are presented in Table 1; information on medication and treatments at each timepoint are presented in Table 2.
Table 1. Demographic data for treatment-resistant depression patients and healthy controls (at baseline).
| Treatment-Resistant Depression Patients (n = 80) | Healthy Controls (n = 37) | |
|---|---|---|
| Demographic Variables | ||
|
| ||
| Age in Years: Mean (Standard Deviation) | 41.81 (9.64) | 42.57 (10.02) |
| Gender: % female | 73% | 73% |
| Handedness: % right | 86% | 89% |
|
| ||
| Baseline Clinical Variables Mean (Standard Deviation) |
||
|
| ||
| Hamilton Rating Scale for Depression: | 17.83 (3.07) | 1.54 (1.40) |
| Age of Depression Onset: | 18.74 (9.57) | -- |
| Duration of Current Episode (months): | 79.34 (96.60) | -- |
| Number of Previous Episodes: | 3.42 (2.31) | -- |
Table 2. Treatment information for treatment-resistant depression patients at baseline, week 8, week 24, week 36 and week 52.
| Baseline n = 80 |
Week 8 n = 65 |
Week 24 n = 62 |
Week 36 n = 61 |
Week 52 n = 47 |
|
|---|---|---|---|---|---|
| % Current Antidepressant | 100 | 98.51 | 98.41 | 98.39 | 95.83 |
| % Not Taking Antidepressant | 0 | 1.49 | 1.59 | 1.61 | 4.17 |
| % One Antidepressant | 37.18 | 32.84 | 33.33 | 33.87 | 33.33 |
| % Two Antidepressants | 47.44 | 47.76 | 46.03 | 45.16 | 39.58 |
| % Three Antidepressants | 15.38 | 17.91 | 19.05 | 19.35 | 22.92 |
| % Current Antianxiety | 29.5 | 24.4 | 23.1 | 21.8 | 17.9 |
| % Current Antipsychotic | 19.2 | 17.9 | 19.2 | 19.2 | 16.7 |
| % Current Mood Stabilizer | 14.1 | 12.8 | 10.3 | 10.3 | 7.7 |
| % Current Therapy | 3.8 | 7.7 | 17.9 | 17.9 | 20.5 |
| Medication Strength Mean (Standard Deviation) | 6.36 (1.93) | 6.23 (2.14) | 6.19 (2.34) | 5.97 (2.31) | 5.72 (2.68) |
| # Medication Changes Mean (Standard Deviation) | .22 (.55) | .61 (1.33) | 1.23 (1.78) | 1.52 (2.04) | 2.28 (2.37) |
Protocol
TRD participants were part of a RTC comparing MBCT to HEP as adjunctive treatments to antidepressant medication. Details regarding treatment programs and the randomization procedure are presented in the published protocol (Eisendrath et al., 2014).
MBCT treatment involved guided meditations and CBT exercises intended to help participants identify cognitive distortions, disengage from rumination, and use non-judgmental present-moment awareness (Segal, Williams, & Teasdale, 2012). HEP treatment involved exercise, functional movement, music therapy, diet education, and guided imagery intended to promote health and improve mood (MacCoon et al., 2012). Both treatments met for 8 weeks in groups of 6-12 once a week for 2h 15m. Participants were assigned 45m of homework 6 days per week. Importantly, HEP treatment was designed to match MBCT treatment on group support, reduction of stigma, improved morale, facilitator attention, treatment duration, and time spent on at-home practice in order to carefully isolate the effects of MBCT.
Participants completed the emotional face processing task at baseline and following treatment; only baseline FMRI data are analyzed in the current report. TRD patients underwent assessments at baseline, and weeks 8, 24, 36, and 52 (for a detailed description of the full battery of measures, see Eisendrath et al., 2014). Changes in depression severity were expressed as the percent change from baseline in HAM-D17 total scores ((Baseline - Post-treatment)/Baseline × 100%). Anxiety was assessed using the self-report State and Trait Anxiety Inventory (Spielberger, Gorush, & Luchene, 1970).
Emotional Face Processing Task
During Shape Matching, participants selected one of two shapes from the bottom of the screen that matched the target shape. During Affect Labeling, participants selected one of two emotional words (e.g. happy, angry) from the bottom of the screen that matched the emotion depicted on the target face. During Gender Labeling, participants selected one of two names (e.g. Sylvia, Allen) from the bottom of the screen that matched the gender of the target face. During Observing, participants passively viewed an emotional face. Stimuli were selected from the NIMSTIM Face Stimulus Set (Tottenham et al., 2009). The faces (half female, half male) depicted negative emotions 80% of the time (fear, anger) and positive emotions 20% of the time (happiness, surprise) to prevent amygdala habituation to negative expressions.
Each block began with a 10s fixation period, followed by 3s of instruction (identify emotion, identify gender, observe, match shape), followed by 10 images presented for 5s each. Four blocks were presented per condition per run. Two runs were presented, each with a run time of 5m 46s.
Neuroimaging Acquisition Methods
Images were acquired on the Siemens 3T TIM TRIO scanner at the UCSF Neuroimaging Center. Acquisition parameters for functional scans were as follows: TR = 2, TE = 30ms, FoV = 220mm; Flip angle = 77°, bandwidth = 2298 Hz/Px; matrix = 64 × 64. Thirty slices (3mm thick, 1mm gap) were acquired in an axial-oblique plane, parallel to the anterior commissure-posterior commissure (AC-PC) line. Acquisition parameters for the high-resolution anatomical scan were as follows: 3D MP-RAGE sequence, scan time: 5m 17s, flip angle =9°, FOV = 220mm, 160 slices per slab, 1.2mm thick, no gap, TR = 2.30s, TE = 2.94ms.
Image Processing
Preprocessing was achieved with Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Image preprocessing entailed motion correction via affine registration; the first image of each run was realigned to the first image of the first run, and then re-alignment proceeded within each run. Images were slice-time corrected to the middle slice. To further denoise the data, we implemented aCompCor (Behzadi, Restom, Liau, & Liu, 2007), a principle component analysis (PCA) based approach to noise reduction of FMRI time-series data. ACompCor derives principal components from the time series of voxels within noise regions of interest (ROIs) defined on eroded white matter and cerebrospinal fluid (CSF) parcels from participants' segmented high-resolution T1-weighted anatomical images coregistered to their functional data. A binary union mask of noise ROIs was generated and co-registered to the mean functional scan. Voxels in the mask that showed even a weak relationship with the task regressors (p < 0.2) were excluded. Time series data for the remaining voxels in the noise ROI mask were then subjected to a PCA, and a number of noise components comprising weighted averages of white matter and CSF voxel time series were identified using a bootstrap procedure.
SPM's canonical hemodynamic response function was convolved with task event vectors to create first-level task regressors representing Affect Labeling, Gender Labeling, Observing, and Shape Matching. Head motion (6 realignment parameters and their derivatives), were included with aCompCor noise regressors in the first level model. Parameters (i.e., beta coefficients) representing the fit of each regressor to a voxel's time series were estimated using the general linear model after applying a high-pass temporal filter (128s cut-off) to remove low-frequency noise. Mean beta images were calculated across runs and contrast images were created by subtracting out the Shape Matching beta image from each condition of interest (Affect Labeling, Gender Labeling, and Observing). The mean functional image from the motion correction pre-processing step was normalized to standard neuroanatomical space (Montreal Neurological Institute's MNI-EPI template; http://www.bic.mni.mcgill.ca), resulting in 3mm3 isotropic voxel dimensions, and the normalization parameters were applied to first-level beta and contrast images, which were then spatially smoothed with a 6mm full-width half-maximum Gaussian kernel.
Data Analysis
Group-based (second-level) random effects analyses were conducted on contrast images to test for statistically significant differences between groups and task conditions. A bilateral amygdala ROI was creating using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) from WFU PickAtlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). Mean amygdala activations were extracted and imported into IBM SPSS Statistics 22 (SPSS Inc., Chicago IL).
Task accuracy and reaction time (RT; median across trials for each condition) data were analyzed using a mixed-design analysis of variance (ANOVA) with Group as the between-subjects and Condition as the within-subjects factor. Amygdala activation data were analyzed using a mixed-design ANOVA to test for Group (TRD, HC), Condition (Affect Labeling, Gender Labeling, Observing), and Hemisphere effects. If there were no significant Hemisphere effects in the overall ANOVA model, bilateral amygdala ROIs were used for subsequent analyses. Significant interaction effects were followed by repeated measures ANOVAs examining Condition effects within each group, and independent t-tests examining Group effects within each condition. Greenhouse-Geisser corrected results are presented if Mauchly's Test of Sphericity indicated a violation. The relationships between amygdala activation across the three conditions (Affect Labeling, Gender Labeling, Observing) were examined using Pearson correlations. In order to assess the relationship amygdala activation across the three task conditions and baseline depression severity without the confounding influence of anxiety, HAM-D17 depression scores were first residualized on anxiety severity scores (STAI-Trait Anxiety). Next, these residualized depression scores were regressed on the three task condition-evoked amygdala activation measures in a multiple regression model in order to determine whether any of them were uniquely associated with depression severity while accounting for the covariation between them. If the three amygdala activation measures were significantly correlated and failed to uniquely account for variance in baseline depression, the relationship with depression was reassessed using the mean amygdala activation across the three conditions. The effect of MBCT versus HEP treatment on percent change in depression severity was assessed using independent t-tests. In order to assess whether baseline amygdala activation differentially predicted treatment response in the MBCT versus HEP groups, HAM-D17 change scores at each follow-up assessment (percent change from baseline) were separately regressed on the three task condition-evoked amygdala activation measures, group, and the three group × amygdala interaction terms in a multiple regression model. The increment in variance accounted for by adding the three interaction terms was tested as a block in order to determine whether the slopes of the depression change-amygdala relationships significantly differed between the groups. If the addition of the interaction terms did not result in a significant improvement in the fit of the model, the interaction terms were omitted and a common slope across the groups was assumed. The unique contributions of each of the three task-condition-evoked amygdala activation were then evaluated in the common slope model, accounting for the covariation between them. If the three amygdala activation measures were significantly correlated and did not uniquely contribute to the prediction of depression change within the treatment groups, the prediction of depression change was re-assessed using the mean amygdala activation across the three conditions. For each separate family of tests, FWER was set to 0.05.
Medication strength was quantified for the TRD patients at each time point according the procedure described in the ATHF (Sackeim, 2001). Briefly, this strength index takes various sources of information into account including drug type, dose, duration of treatment, and compliance. Estimates of medication strength at each time point are presented in Table 2.
Results
Task Accuracy and RT
Task Accuracy
Mean accuracy exceeded 95% for each condition. Accuracy did not differ between HCs and TRD patients (F(1, 115) = 1.13, p = .29, ηp2 = .01) and the Group by Condition interaction was not significant (F(2, 230) = 1.10, p = .33, ηp2 = .01). However, there was a significant main effect for Condition (F(2, 230) = 15.60, p < .001, ηp2 = .12). Follow-up tests revealed that participants were more accurate during Gender Labeling compared to Affect Labeling (t(116) = -5.79, p < .001, d = .69), and Shape Matching (t(116) = 5.75, p < .001, d = .69); there were no differences in accuracy between Affect Labeling and Shape Matching (t(116) = -.75, p < .46, d = .09). Condition means by Group are reported in Table 3.
Table 3. Task accuracy and reaction time means (and standard deviations) for each condition of the emotional face processing task for treatment-resistant depression patients and healthy controls.
| Mean (Standard Deviation) | Treatment-Resistant Depression Patients (n = 80) | Healthy Controls (n = 37) |
|---|---|---|
| Affect Labeling Accuracy | 96% (6.34) | 95% (5.79) |
| Affect Labeling Reaction Time (ms) | 1734.21 (356.23) | 1646.82 (320.69) |
| Gender Labeling Accuracy | 99% (2.97) | 99% (1.73) |
| Gender Labeling Reaction Time (ms) | 1398.57 (243.31) | 1321.78 (244.25) |
| Shape Matching Accuracy | 96% (5.34) | 97% (3.84) |
| Shape Matching Reaction Time (ms) | 1244.60 (198.74) | 1115.14 (185.52) |
Task RT
There was not a significant Group by Condition interaction (F(2, 230) = .66, p = .52, ηp2 = .01). However there was a significant main effect of Condition (F(2, 230) = 226.39, p < .001, ηp2 = .66), with faster RTs during Gender Labeling than Affect Labeling (t(116) = 14.51, p < .001, d = 1.11), and Shape Matching (t(116) = 9.47, p < .001, d = .76), and faster RTs during Shape Matching than Affect Labeling (t(116) = 19.23, p < .001, d = 1.77). There was also a significant main effect of Group (F(1, 115) = 4.64, p = .03, ηp2 = .04); TRD patients (mean RT = 1459.13) were slower across all conditions than HCs (mean RT: 1361.25). Condition means by Group are reported in Table 3.
Amygdala Activation at Baseline
Overall Mixed-design ANOVA
None of the main or interaction effects on amygdala activation involving Hemisphere were significant. Accordingly, analyses were conducted using a bilateral amygdala ROI. The Group by Condition interaction was significant after Greenhouse-Geisser correction (F(2, 230) = 6.31, p = .003, ηp2 = .05); TRD patients had less amygdala activation than HCs during Affect Labeling (t(115) = 2.21, p = .03, d = .41). TRD patients also had marginally less amygdala activation during Gender Labeling (t(115) = 1.74, p = .09, d = .29). There were no significant differences between TRD patients and HCs during Observing (t(115) = -.81, p = .42, d = .21). The main effects of Condition (F(2, 230) = 1.75, p = .18, ηp2 = .02) and Group (F(1, 115) = 1.67, p = .20, ηp2 = .01) were not significant. Mean amygdala activation for each Condition is presented in Figure 1A.
Figure 1.
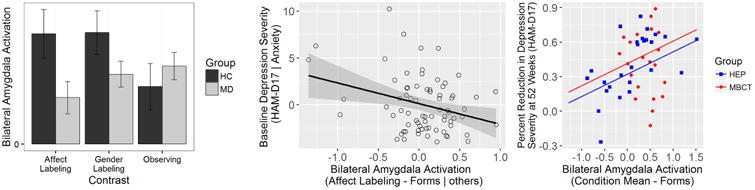
A: A bar graph depicting mean amygdala activation during each condition (Affect Labeling, Gender Labeling, and Observing) for healthy controls (HC) in orange and treatment resistant depression patients (MD) in gray. Error bars: +/- 1 SE. B: A plot depicting the inverse relationship between anxiety-residualized Hamilton Rating Scale for Depression (HAM-D17) and Affect Labeling-evoked amygdala activation (residualized on Gender Labeling- and Observing-evoked amygdala activation) in treatment resistant depression patients at baseline (n = 80). C: A plot depicting the relationship between percent reduction in Hamilton Rating Scale for Depression Scores (HAM-D17) severity and mean amygdala activation averaged across conditions (Affect Labeling, Gender Labeling, and Observing) among treatment resistant depression patients at week 52 (n = 47) following completion of the Mindfulness-Based Cognitive Therapy (MBCT; red dots) or Health-Enhancement Program (HEP; blue squares).
Repeated Measures ANOVA in HCs
The Condition effect was significant (F(2,72) = 3.86, p = .03, ηp2 = .10), with HCs demonstrating greater amygdala activation during both Affect Labeling (t(36) = 2.34, p = .03, d = .37), and Gender Labeling (t(36) = 2.85, p = .007, d = .40) relative to Observing. There were no significant differences between Affect Labeling and Gender Labeling (t(36) = -.05, p = .960, d = .01).
Repeated Measures ANOVA in TRD Patients
For TRD patients, the Condition effect was marginally significant after Greenhouse-Geisser correction (F(2, 158) = 3.06, p = .06, ηp2 = .037) with TRD patients having significantly less activation during Affect Labeling compared to Observing (t(79) = -2.06, p =.04, d = .24), and marginally less activation during Affect Labeling compared to Gender Labeling (t(79) = -1.78, p = .08, d = .18). There were no significant differences between Gender Labeling and Observing (t(79) = -.74, p =.46, d = .07).
Relationships between Amygdala Activation During Each Condition
Despite ANOVA results suggesting that group differences in amygdala activation were relatively specific to Affect Labeling, amygdala activation evoked by the three task conditions were significantly inter-correlated within the TRD patients (Affect Labeling versus Gender Labeling: r = .604, p < .001; Affect Labeling versus Observing: r = .478, p < .001; Gender Labeling versus Observing: r = .662, p < .001). Accordingly, in subsequent analyses examining clinical correlates of amygdala activation, unique contributions of each task condition-evoked amygdala activation were evaluated first taking the covariation among them into account. In the absence of evidence supporting unique contributions, clinical correlation analyses were repeated using the mean amygdala activation across the three conditions.
Relationships between Amygdala Activation and Depression at Baseline
The relationship between baseline depression severity and task-evoked amygdala activation in the TRD patients was assessed after regressing HAM-D17 scores on STAI-Trait Anxiety scores and saving the residuals, thereby deriving depression scores from which shared variation with anxiety had been removed (HAM-D17 versus STAI-Trait Anxiety r = .23, p = .045). Residual HAM-D17 scores were then regressed on amygdala activation measures from the three task conditions in a multiple regression model. While the overall model was significant (multiple R2 = .15, F(4, 69) = 3.06, p = .02), only amygdala activation during Affect Labeling emerged as a significant unique predictor of depression (b = -.39, t(69) = -2.76, p = .007), with Gender Labeling (b = .14, t(69) = .83, p = .41) and Observing (b = .07, t(69) = .47, p = .64) conditions failing to make unique contributions. A plot depicting the inverse relationship between HAM-D17 depression scores (anxiety-residualized) and Affect Labeling-evoked amygdala activation (residualized on Gender Labeling- and Observing-evoked amygdala activation) in TRD patients is presented in Figure 1B.
Across task conditions, blunted amygdala activated was not significantly related to the number of prior depressive episodes or length of illness in the TRD patients (all p's < .05). A table of uncorrected correlations between amygdala activation during each task condition and clinical variables is presented in Table 1 of the Supplemental Materials.
Treatment Response
Following treatment at week 8, independent t-tests indicated greater percent reductions in depression severity in MBCT patients than HEP participants (t(63) = -2.67, p = .01, d = -.67). There were no significant differences in depression reduction between treatment groups at week 24 (t(60) = -1.57, p = .12, d = .41), 36 (t(59) = -0.70, p = .49, d = -0.18), or 52 (t(45) = -1.03, p = .31, d = .31). Percent reduction in depression severity for each group at each timepoint is depicted in Figure 2.
Figure 2.
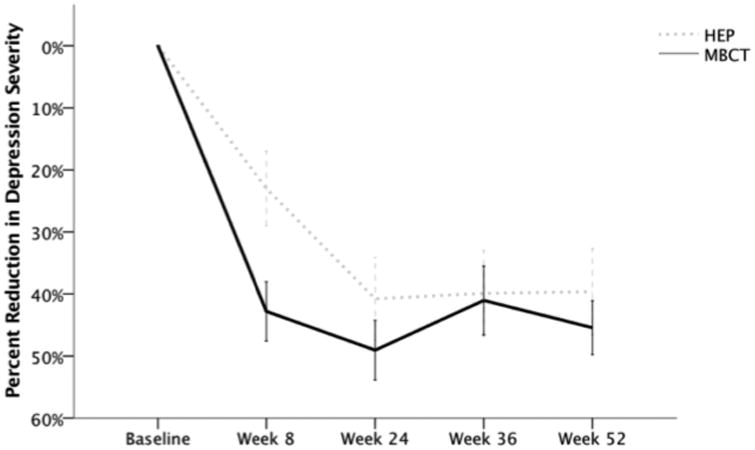
Percent reduction in Hamilton Rating Scale for Depression Scores (HAM-D17) following completion of the Mindfulness-Based Cognitive Therapy (MBCT; solid black line) and Health-Enhancement Program (HEP; dashed grey line) at weeks 8 (MBCT n = 33, HEP n = 32), 24 (MBCT n = 32, HEP n = 30), 36 (MBCT n = 31, HEP n = 30) and 52 (MBCT n = 24, HEP n = 23). Error bars: +/- 1 SE.
Predictors of Treatment Response
For each follow-up assessment, HAM-D17 depression percent change scores were regressed on the three task condition-evoked amygdala activation measures (Affect Labeling, Gender Labeling, and Observing), treatment group (MBCT versus HEP), and their interactions. In no case did the addition of the interaction terms significantly improve the fits of the regression models (all ps > .05), indicating that there were no significant differences in the slopes of the regression lines between the treatment groups. After dropping the interaction terms and assuming common slopes between the groups, tests of the unique contributions of each of the task condition-evoked amygdala activation models failed to show any significant unique contributions to the prediction of depression change at any follow-up assessment (all ps >.05). Accordingly, in order to examine whether amygdala activation more generally predicted treatment within the treatment groups, regression models were repeated using mean amygdala activation across the three task conditions. Again, significant group × amygdala activation interactions were not found for any follow-up assessment (all ps > .05), supporting the evaluation of common slopes across the groups. The only significant common slope relationship between mean amygdala activation and HAM-D17 percent change scores was observed at the 52 week follow-up assessment (b = .19, t(44) = 2.57, p = .0136). As shown in Figure 1C, the greater the mean amygdala activation at baseline, the greater the improvement in depression severity at the 52 week follow-up.
Medication Effects
Estimates of medication strength did not change significantly over the assessment time points in the TRD patients (p = .27). In addition, for all affective face conditions, amygdala activation in the TRD patients was unrelated to variation in medication strength at any time point (all p's < .05).
Discussion
The present study investigated amygdala reactivity during emotional face processing and its relation to concurrent and long-term depression severity in a sample of TRD patients who received MBCT or HEP treatment. Compared to HCs, TRD patients had blunted amygdala activation during affect labeling that was related to greater depression severity. Immediately following treatment, MBCT participants showed greater reductions in depression severity than HEP, but not at any future timepoints. While amygdala activation during affect labeling, relative to gender labeling and passive observing of emotional faces, was not uniquely predictive of treatment outcomes, greater mean amygdala activation across task conditions was predictive of improvement in depression severity across treatment groups at 52 weeks. However, this predictive relationship was not evident at earlier follow-up assessments (8, 24 or 36 weeks). These results suggest that treatment modality differentially influenced short term changes in depression severity, but that baseline amygdala reactivity during affect labeling was predictive of concurrent depressive symptom severity while amygdala reactivity to emotional faces more generally, irrespective of the specific task instructions, was predictive of long-term clinical outcomes.
Compared to HCs, TRD patients exhibited blunted amygdala activation during affect labeling that was associated with greater concurrent depression severity. These findings contrast with literature on MDD that suggested that depressed individuals demonstrate enhanced amygdala activation to emotional stimuli, even in largely medicated samples (Anand et al., 2005; Fournier et al., 2013; Palmer, Crewther, & Carey, 2015; Sheline et al., 2001), that increases with greater depression severity (Lee et al., 2007). However, there have also been reports of normal amygdala activation in MDD patients (Van Den Bulk et al., 2014), as well as blunted amygdala reactivity in depressed adults (Drevets, 2001) depressed children (Thomas et al., 2001), and children with severe mood dysregulation (Brotman et al., 2010). Heterogeneity in emotional reactivity in MDD has been attributed to moderating factors including subtypes of depression, number of previous episodes, and concurrent diagnoses that are not consistently reported (Bylsma, Morris, & Rottenberg, 2008). Very limited neuroimaging studies have investigated TRD specifically; those that have reported no differences in amygdala activation during the processing of sad faces (Murroughs 2015) and no differences in amygdala resting state connectivity (Lui et al., 2011). The current study adds to these limited reports by also demonstrating no differences in amygdala activation during passive viewing of emotional faces between HC and TRD patients, but blunted amygdala activation during affect labeling. Together, these conflicting findings suggest that, although the average pattern of activation in MDD may be one of amygdala hyperactivation to emotional stimuli, there is clinically relevant variation in amygdala reactivity. In particular, some forms of depression, including TRD, may be characterized by blunted amygdala activation during explicit emotional processing that worsens with greater depression severity.
Consistent with results from the RCT investigating the effects of MBCT versus HEP on depression outcomes (Eisendrath et al., 2016), MBCT participants in the current study demonstrated greater percent reductions in depression severity compared to HEP participants at week 8 (45% versus 28%). Treatment differences were not observed in longer-term clinical outcomes, although both groups demonstrated long-term sustained reductions in depression severity. By week 52, greater baseline amygdala activation during affect labeling significantly predicted the degree of improvement in depression severity across both treatment groups. Together, these findings suggest that the differential effect of treatment may be relatively transient, and that baseline amygdala reactivity during emotional face processing, irrespective of task instructions, may better predict long-term depression outcomes.
Our findings are consistent with reports suggesting that depressed individuals who demonstrate blunted amygdala reactivity to emotional stimuli prior to treatment have worse long-term clinical outcomes (Phillips et al., 2015). In depressed patients, enhanced amygdale activation during emotional face processing predicted greater depression improvement nearly a year later, independent of treatment received (Canli et al., 2005). Further, exaggerated amygdala response to emotional words predicted greater depression improvement following CBT (Siegle et al., 2006). SSRIs and CBT are thought to target common neural mechanisms, including amygdala activation, and to influence emotional processing (DeRubeis, Siegle, & Hollon, 2008; Sheline et al., 2001). If these treatments function by regulating exaggerated amygdala response to emotional stimuli (DeRubeis et al., 2008), then they may not be particularly effective for TRD patients who demonstrated blunted responses to emotional stimuli.
In healthy individuals, affect labeling has been associated with reduced amygdala activation compared to observing or gender labeling, and has been considered a form of emotion regulation (Lieberman et al., 2007). In contrast, HCs in our study were characterized by greater amygdala activation during affect and gender labeling compared to observing. Decreased amygdala activation during affect compared to gender labeling has not consistently been reported in healthy individuals (Gee et al., 2015; Gorno-Tempini et al., 2001; Lange et al., 2003), and some studies have reported enhanced amygdala activation during affect labeling compared to implicit processing (Gur et al., 2002; Habel et al., 2007). These discrepant findings suggest that while affect labeling may down-regulate amygdala activity in some cases, affect labeling may increase amygdala activation. Indeed, a meta-analysis of fMRI face processing studies concluded that explicit, relative to implicit, processing of emotional faces, was associated with greater amygdala activation (Fusar-Poli et al., 2009). In our sample, enhanced amygdala activation during affect and gender labeling represented the normal response to the task, while blunted amygdala activation not only characterized TRD patients, it was systematically predictive of more severe depression severity.
One limitation of this study is that positive and negative faces were intermixed in a block design, limiting our ability to isolate amygdala effects to either positive or negative expressions. This design was chosen to optimize power, mirror previous studies using this paradigm, and to prevent amygdala habituation to the repeated presentation of negative expressions; however, it also limits our ability to isolate the amygdala effects to either positive or negative facial expressions. Rather than being valence specific, these results reflect emotional processing in general. However, the amygdala is responsive to both pleasant and unpleasant facial expressions, and the bilateral amygdala is responsive to directing attention to the emotional features of the faces (explicit face processing), despite the valence of the emotional expression (Costafreda, Brammer, David, & Fu, 2008; Fusar-Poli et al., 2009). Further studies may wish to independently examine positive and negative faces in order to clarify if blunted amygdala activation in TRD is specific to negatively valenced faces, which comprised the majority of our trials. A second limitation is the lack of a treatment-responsive group of depressed patients, preventing us from directly testing if these findings are unique to TRD patients or if they are characteristic of MDD patients in general. In addition, while these findings suggest that TRD is associated with blunted amygdala activation during affect labeling, in the absence of longitudinal data we are unable to determine if abnormal amygdala activation predates TRD, or if it occurs as a function of illness course, or variables that are secondary to illness, including prolonged exposure to antidepressant medication. In particular, we cannot rule out the possibility that the antidepressant medications taken by all of the TRD patients at the time of the baseline fMRI assessment contributed to dampened emotional responding and blunted amygdala activation. Finally, while both HEP and MBCT groups demonstrated sustained improvements in depression throughout the follow-up period, it is possible that a beneficial effect of MBCT over HEP beyond 8-weeks might emerge if MBCT treatment had been continued for a longer period or if the implemented treatment had been augmented with booster sessions over the subsequent year.
In summary, TRD appears to be characterized by blunted amygdala activation during explicit emotional face processing that worsens with greater depression severity. MBCT is associated with greater reductions in depression severity than HEP directly after treatment; however, after 52 weeks, treatment no longer differentially impacts depression severity, and greater pre-treatment amygdala activation in response to emotional faces, irrespective of specific task conditions, predicts depression improvement in both treatment groups.
TRD is characterized by a lack of response to SSRI medications, which are thought to target abnormal neural circuitry related to affective processing, including hyperactive amygdala activation (DeRubeis et al., 2008). The current study provides evidence that TRD patients do not demonstrate the same affective processing abnormalities as MDD patients in general, which may provide some mechanistic explanation for why medications that target this circuitry are not effective at improving symptoms in TRD patients. As TRD patients represent a major social economic and burden of depression as a whole, understanding specific neurobiological features of this group and their relation to clinical outcomes will add necessary nuance to depression models and may ultimately result in better diagnosis and treatment of TRD.
Supplementary Material
Supplemental Table 1. A table of uncorrected correlations between mean amygdala activation during each condition (Affect Labeling, Gender Labeling, and Observing) and clinical variables (age of depression onset, number of previous depressive episodes, and current depressive episode length) at baseline.
Acknowledgments
This work was supported by National Institutes of Health/National Center for Complimentary & Alternative Medicine Grant R01 AT004572-02S1 awarded to Stuart J. Eisendrath and Daniel H. Mathalon and from the National Institutes of Health/National Institute of Mental Health Grant T32 MH089920 awarded to Judith M Ford.
Footnotes
Daniel Mathalon is a consultant for Boehringer Ingelheim. All other authors report no biomedical financial interests or potential conflicts of interest.
Trial Title: Practicing Alternative Techniques to Heal From Depression: The PATH-D Study
Trial Number: NCT01021254
URL: https://clinicaltrials.gov/ct2/show/NCT00871299?term=NCT01021254&rank=1
References
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17(8):843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Pine DS. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16(12):1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. Journal of Affective Disorders. 2011;130(1):66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Eisendrath SJ, Gillung EP, Delucchi KL, Chartier M, Mathalon DH, Sullivan JC, et al. Feldman MD. Mindfulness-based cognitive therapy (MBCT) versus the health-enhancement program (HEP) for adults with treatment-resistant depression: a randomized control trial study protocol. BMC complementary and alternative medicine. 2014;14(1):95. doi: 10.1186/1472-6882-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendrath SJ, Gillung EP, Delucchi KL, Segal ZV, Nelson JC, McInnes LA, et al. Feldman MD. A Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy for Treatment-Resistant Depression. Psychotherapy and Psychosomatics. 2016 doi: 10.1159/000442260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience. 2010;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. Journal of Affective Disorders. 2009;116(1):4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- First MB. Structured Clinical Interview for the DSM (SCID) The Encyclopedia of Clinical Psychology 1995 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fournier JC, Keener MT, Mullin BC, Hafeman DM, LaBarbara EJ, Stiffler RS, et al. Phillips ML. Heterogeneity of amygdala response in major depressive disorder: the impact of lifetime subthreshold mania. Psychological Medicine. 2013;43(02):293–302. doi: 10.1017/S0033291712000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Steiner H. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Politi P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gee DG, McEwen SC, Forsyth JK, Haut KM, Bearden CE, Addington J, et al. Cornblatt BA. Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Human Brain Mapping. 2015 doi: 10.1002/hbm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Nichelli P. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14(2):465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):127. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16(3):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Habel U, Windischberger C, Derntl B, Robinson S, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45(10):2369–2377. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British journal of social and clinical psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. Langenecker SA. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. 2016;46(5):1055–1067. doi: 10.1017/s0033291715002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicak PG, Dowd SM. Treatment-Resistant Depression: An Update on Diagnosis and Management. Psychopharm Review. 2009;44(6):41–48. [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, et al. Keller MB. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? American Journal of Psychiatry. 2000;157(9):1501–1504. doi: 10.1176/appi.ajp.157.9.1501. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage. 2012;61(3):686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion. 2005;5(4):446. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SCR, et al. Phillips ML. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53(3):226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Lee BT, Cho SW, Khang HS, Lee BC, Choi IG, Lyoo IK, Ham BJ. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(7):1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current Opinion In Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, et al. Gong Q. Resting-state functional connectivity in treatment-resistant depression. American Journal of Psychiatry. 2011;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, et al. Davidson RJ. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behaviour Research And Therapy. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Murrough J, Collins K, Fields J, DeWilde K, Phillips M, Mathew S, et al. Iosifescu D. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Translational psychiatry. 2015;5(2):e509. doi: 10.1038/tp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SM, Crewther SG, Carey LM. A meta-analysis of changes in brain activity in clinical depression. Frontiers in Human Neuroscience. 2015;8:1045. doi: 10.3389/fnhum.2014.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JRC, Deckersbach T, Trivedi MH. Identifying Predictors, Moderators, and Mediators of Antidepressant Response in Major Depressive Disorder: Neuroimaging Approaches. The American Journal of Psychiatry. 2015;172(2):124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, et al. Rush AJ. The cost consequences of treatment-resistant depression. The Journal of clinical psychiatry. 2004;65(3):341–347. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry. 2001 [PubMed] [Google Scholar]
- Sartorius N. The economic and social burden of depression. Journal of Clinical Psychiatry. 2001 [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression. Guilford Press; 2012. [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle G, Carter C, Thase M. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. Journal of Clinical Psychiatry. 2006;67:16. [PubMed] [Google Scholar]
- Spielberger C, Gorush R, Luchene R. State-Trait Anxiety Inventory. Palo Alto Consulting Psychologists Press; 1970. [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Casey B. Amygdala response to fearful faces in anxious and depressed children. Archives Of General Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. McGrath PJ. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Den Bulk BG, Meens PH, van Lang ND, de Voogd L, Van Der Wee NJ, Rombouts SA, et al. Vermeiren RR. Amygdala activation during emotional face processing in adolescents with affective disorders: the role of underlying depression and anxiety symptoms. Frontiers In Human Neuroscience. 2014;8:393. doi: 10.3389/fnhum.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. A table of uncorrected correlations between mean amygdala activation during each condition (Affect Labeling, Gender Labeling, and Observing) and clinical variables (age of depression onset, number of previous depressive episodes, and current depressive episode length) at baseline.


