Abstract
A photo-active luminescent rhenium carbonyl complex namely, [Re(CO)3(phen)(pyAl)](CF3SO3) was grafted on a biocompatible carboxymethyl chitosan (CMC) matrix through Schiff base condensation reaction. The light-induced CO delivery from ReCMC has been shown to eradicate human colorectal adenocarcinoma cells (HT-29) very efficiently in a dose-dependent fashion. The onset of CO-induced apoptosis was realized by caspase-3,-7 detection aided by fluorescence confocal microscopy. ReCMC represents a unique example of a biocompatible and biodegradable antineoplastic agent that could find its use in cancer photopharmacology.
The homeodynamic properties of carbon monoxide (CO) in mammalian pathophysiology encouraged the research community to develop molecular CO donors (metal carbonyl complexes and organic CO releasing molecules) that can deliver this noxious yet potentially therapeutic gas to the desired target in a more controlled and sustainable fashion.1–8 Over the past few years our group has been engaged in developing photoactive CO releasing molecules (photoCORMs) using systematic design strategies.9 These molecules release CO only upon illumination of light of varied wavelengths and thereby can deliver CO on demand to the site of interest. CO delivered from such discrete complexes has been shown to eradicate human breast and cervical cancer cells quite effectively in a dose-dependent manner.10,11 However, site-specific delivery of these CO releasing prodrugs remains as a tantalizing challenge. Moreover systemic toxicity that might arise from the photoproduct(s) is another major concern. Previously we have reported construction of mesoporous silica-based CO-donating materials where the metal carbonyl complex is packed within the hexagonal channels of the host material.12 In such composite the photoCORM is locked within the pores through strong electrostatic interactions. Herein we aimed to design a photoCORM-carrying composite material in which the prodrug molecule can be grafted covalently on and within the host matrix. This in turn will ensure better retention of the CO-spent product within the host after CO delivery and thereby will minimize the chances of undesirable systemic exposure. In this work we have chosen carboxymethyl chitosan (CMC) as the host for a luminescent rhenium(I) carbonyl complex namely, [Re(CO)3(phen)(pyAl)](CF3SO3) (1) (phen = 1,10-phenathroline and pyAl = pyridine-4-carboxaldehye) as guest (Scheme 1).
Scheme 1.
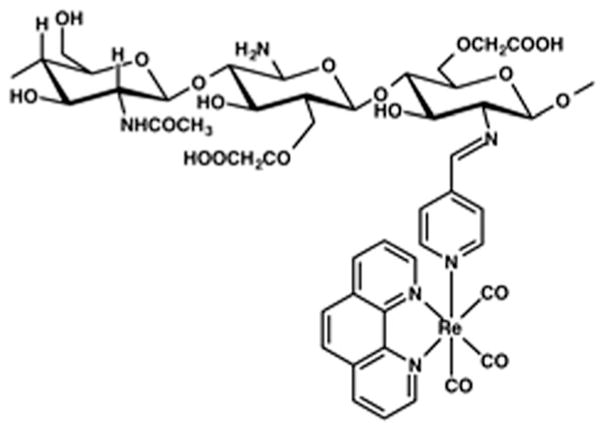
Schematic representation of [Re(CO)3(phen)(pyAl)](CF3SO3) complex covalently attached to CMC frame.
Natural polysaccharides have been widely studied as drug delivery agents and in tissue engineering applications for their non-toxic nature, biocompatibility and bio-degradability. Among others, chitosan is one of the most exhaustively used polysaccharides as drug delivery vehicles and in tissue repair and reconstructions.13 However, limited solubility of chitosan in both aqueous and organic media has impeded its exploration in pharmacological settings.14 Among various derivatives, carboxymethyl chitosan (CMC), a water-soluble polysaccharide, has attracted significant attention in several areas such as, diagnostics, theranostics, bioimaging, biosensors and gene therapy.15,16 CMC exhibits superior solubility over a wide range of pH,15,16 and much like chitosan, exhibits enhanced epithelial permeability in intestinal, nasal and buccal tissues by opening up tight cellular junctions and thereby facilitating drug transport.16 The toxicity profile of CMC has been thoroughly evaluated with mouse model experiments. CMC has been shown to be safe in vivo with marginal growth inhibition of sarcoma 180 and improved immunity by elevation of serum IL-2 and TNF-α levels when treated in mice.16 No acute toxicity was detected in the blood circulation of the animals after treatment with CMC and when CMC was absorbed and degraded gradually. A chitosan–doxorubicin composite material has been proposed to enter cells through endocytic mechanisms.17 Composite materials derived from chitosan or chitosamino-oligosaccharide (COS) conjugated with 5-fluorouracil have been shown to exhibit strong growth inhibition effects on Met-A fibrosarcoma and MH-134Y hepatoma.18 None of these composite materials showed any systemic toxicity even at higher concentrations. These reports prompted us to employ CMC as the delivery vehicle for the luminescent CO donor (complex 1). Complex 1 was synthesized in excellent yield through a four-step procedure using [Re(CO)5Cl] as precursor material (see ESI†).
The molecular structure of 1 (Fig. 1) has been determined by X-ray crystallography. The structure reveals the coordination geometry of Re(I) as distorted octahedral with three CO ligands disposed facially.
Fig. 1.
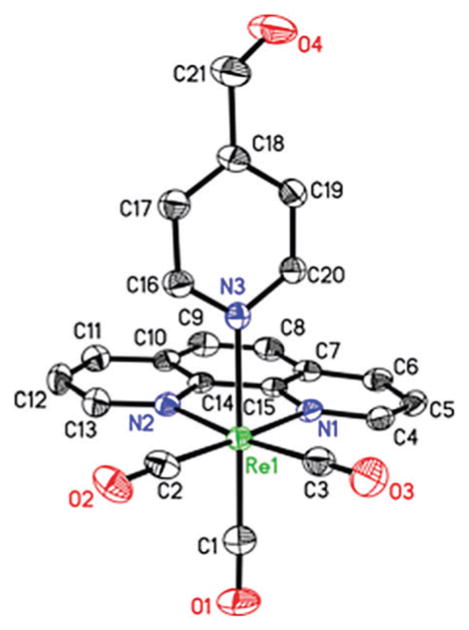
Molecular structure of 1 with atom labeling scheme. The thermal ellipsoids are shown at 50% probability level.
Complex 1 is indefinitely stable in MeCN, CH2Cl2, and CHCl3 solutions under dark conditions. It also exhibits some solubility in aerated aqueous media (deionised water and phosphate buffer saline (PBS)) where it is stable for at least 48 h in absence of light. All solutions of complex 1 are stable under both aerobic and anaerobic conditions. However upon exposure to low power UV-B light (λ < 313 nm, 5 mW cm−2), complex 1 exhibits systematic changes in the electronic absorption spectrum (quantum yield value at 305 nm: 0.027 ± 0.001). These changes can be attributed to the CO photorelease as confirmed by standard Myoglobin (Mb) assay (see ESI†). Ishitani and co-workers clearly delineated the requirement of UV-B radiation to trigger CO release from rhenium carbonyl complexes of type [Re(CO)3(bpy)(X)] (where the X is either σ-donating or moderately π-accepting ancillary ligands).19 This requirement presumably arises from the weaker trans labilizing capacity of chloride and pyridine as coligand. The photo behaviour of 1 is consistent with such observation. In the myoglobin assay experiment first, complex 1 was added to a PBS solution of reduced Mb (by sodium dithionite) under dark condition (pH 7.4). This solution showed no change over time as monitored by spectrophotometry, thus indicating the stability of this photo-CORM in presence of dithionite. However, upon exposure to UV-B light for 30 s resulted in a prominent shift of the Soret band of reduced Mb from 435 to 424 nm clearly suggesting the formation of carboxymyoglobin (COMb). This experiment demonstrated that 1 does not exhibit any CO release in aqueous media under aerobic condition, even in presence of a reductant, and only liberate CO under illumination (Fig. 2).
Fig. 2.
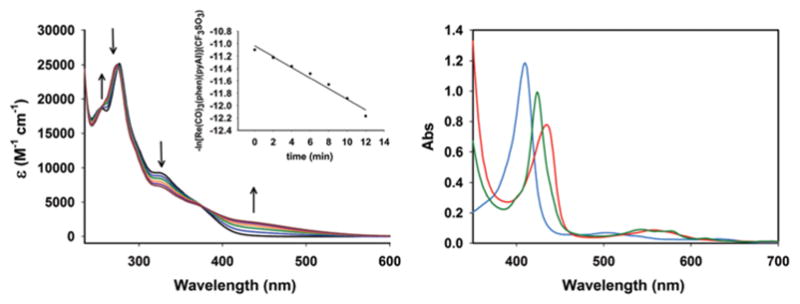
Changes in electronic spectrum of 1 (conc., 7.36 × 10−5 M) in MeCN solution upon exposure to low power UV-B light (λmax = 305 nm, 5 mW cm−2, 2 min intervals) at 298 K (left panel) and UV/Vis traces from the Mb assay for 1 in PBS solution at 298 K (right panel): cyan trace, oxidized Mb; red trace: reduced Mb and green trace: COMb.
The apparent rate of CO photorelease (kCO) from complex 1 has been determined by recording the electronic absorption spectra and monitoring the changes of the spectral traces upon exposure to light at 2 min intervals. The kCO value calculated from ln(C) vs. time (t) plot was found to be 0.09 ± 0.02 min−1. Both PBS and MeCN solutions of 1 display strong emission band (centered at 550 nm) at ambient temperature upon excitation at 350 nm. Such luminescence property of complex 1 provides a convenient means to track this prodrug molecule within cellular targets.
The reactive primary amine group of CMC was utilized to covalently graft 1 on the host matrix. Schiff base condensation reaction between the dangling aldehyde group of the 4-pyridine carboxaldehye moiety of 1 with such NH2 group was achieved by the reaction of 1 with CMC in dry MeOH under reflux condition (details ESI†). The resulting ReCMC composite was thoroughly washed with dry MeCN to ensure the removal of any unreacted 1 that might remain on the surface of the host matrix. FT-IR spectra of CMC before and after the crosslinking clearly displayed the successful conjugation of complex 1 with the polymeric host (Fig. 3). EDX elemental mapping exhibited uniform distribution of the elements within the ReCMC framework (Fig. 4). The percentage of Re was 12% in the composite as determined by sodium peroxide fusion method with aid of inductively-coupled plasma atomic emission spectroscopy (ICP-AES). The solution behaviour of 1 is retained in ReCMC. For example, ReCMC releases CO under low power UV-illumination and displays strong luminescence (λem = 550 nm) in PBS solution under aerobic conditions much like 1. The latter property of ReCMC was utilized to track this composite material within the cellular targets. In PBS solution the ReCMC composite is stable for at least 24 h as studied by spectrophotometry.
Fig. 3.
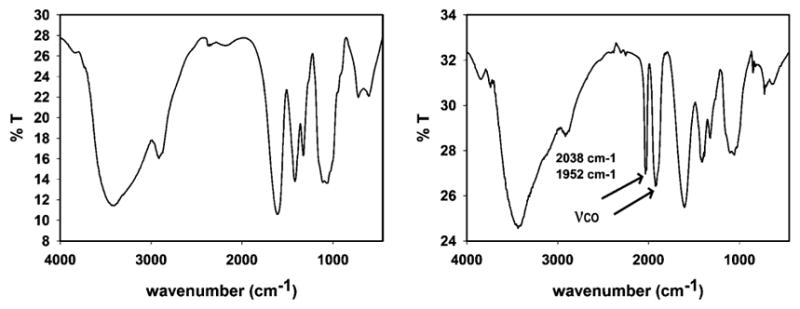
FT-IR spectra of CMCS (left panel) and ReCMCS (right panel) in KBr disk.
Fig. 4.
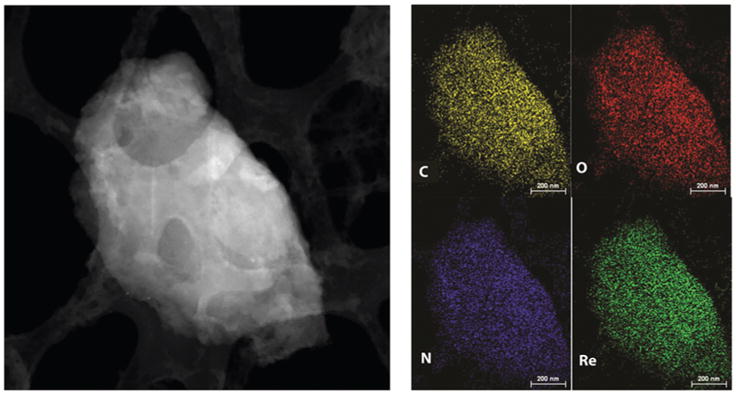
STEM image and SEM-EDX elemental maps of ReCMC material.
In order to evaluate cellular uptake properties of the ReCMC composite, MDA-MB-231 (human breast cancer) and HT-29 (human colorectal cancer) cells were treated with sonicated ReCMC for 1 h in DMEM and McCoy’s 5A media (both devoid of fetal bovine serum and phenol red) respectively. After 1 h of incubation the media were aspirated, and the cells were washed three times with PBS very carefully. The images of the cells were then captured with the aid of a fluorescence confocal microscope. Trypan blue was added prior to imaging to quench any extracellular luminescence. The images as shown in Fig. 5 clearly suggest rapid internalization of ReCMC by these two different malignant cells.
Fig. 5.
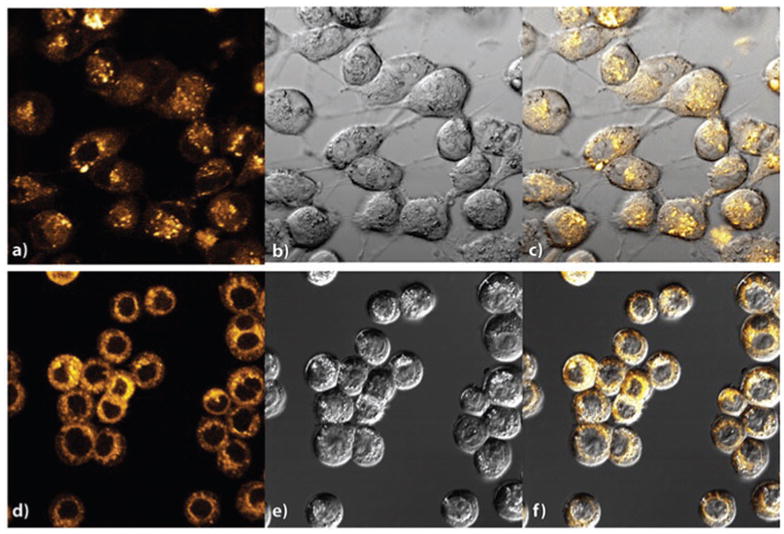
Confocal images of MDA-MB-231 cells showing the internalization of ReCMC composite (a) fluorescence image, (b) bright-field image and (c) overlaid DIC image, and HT-29 cells (d) fluorescence image, (e) bright-field image and (f) overlaid DIC image after incubation with ReCMC for 1 h (λex = 405 nm and λem = 550 nm).
The only other biocompatible discrete rhenium carbonyl-based photoCORM has been reported by Ford and co-workers where the pro-drug can be tracked within prostatic cancer cells.20 Colon-specific drug delivery systems have been gaining considerable research interest since in most cases the chemotherapeutic agents are unable to survive the aggressive conditions within the upper gastrointestinal tract (GI-tract). CMC-based delivery systems have been shown to protect the guest therapeutics from such hostile conditions.21 In addition, mucoadhesive properties of the CMC host ensures longer residence time at the target site and thereby maintaining an sustainable drug release profile22 (CO release from ReCMC in the present case). The excellent mucoadhesive and permeation enhancing properties of CMC are also expected to increase preferential accumulation of the ReCMC composite at the colonic target. Upon CO delivery the photoproduct is expected to be retained within the CMC matrix and later be biodegraded by the colonic bacterial flora.23 In a relatively recent study, a chitosan-derived composite material containing 5-fluorouracil and curcumin has shown significant anticancer effect in HT-29 cells and also led to improved plasma concentration of the drug in vivo in Swiss Albino mouse model.24 These results confirm greater bioavailability of the chemotherapeutics upon conjugation to chitosan. Another motivation to choose HT-29 cells for screening with the ReCMC prodrug arises from the convenient means available for illumination of the colonic sites to initiate CO release from this material. The relatively recent Endoscopic Fiber Optic Shape Tracker (EFOST) technology has emerged as a minimally invasive means for application toward endovascular space.25 In fact the newly developed fiber-optic illumination sources are able to deliver sufficient light through very thin optical fiber to activate CO release from photoactive drugs like ReCMC following their accumulation at the colonic target. Keeping this in mind, we have assessed the viability of HT-29 cells in vitro upon photo-initiated CO delivery from ReCMC via MTT assay.
Results of the cell viability assay clearly indicate that CO delivery from 150 μg mL−1 and 300 μg mL−1 of ReCMC composite for 20 min was enough to eradicate about 60 and 50% of the HT-29 cells respectively (Fig. 6, grey bars). Furthermore, UV light and CMC host itself (light or dark conditions) show no cytotoxi-city under same experimental settings. The ReCMC material under dark condition was only minimally detrimental toward these malignant cells. These observations confirm the efficacy of the ReCMC material toward CO-induced eradication of human colon cancer cells. Cell viability studies with complex 1 clearly showed no apparent cytotoxicity towards HT-29 cells without illumination. However, a dose dependent eradication of the cells was noted upon CO delivery under illumination (see ESI†). In a previous account Schatzschneider and co-workers have shown CO-induced eradication of HT-29 cells utilizing a Mn-carbonyl based photoCORM.26 We have been curious to find out whether or not in the present case the cell deaths occur through apoptosis (programmed cell death).27 It is now established that two apoptotic initiators namely, caspase-8 and caspase-9 activate the executioner caspases-3, -6 and -7, which subsequently interact with key proteins and gradually promote DNA fragmentation, membrane blebbing, and controlled disassembly of various cellular components. In the present work we followed the onset of apoptosis (caspases-3, -7 activation) in the HT-29 cells upon CO delivery with the aid of confocal microscopy (Fig. 7). In the caspase-3/7 detection assay, the cells were plated in similar way as for cellular internalization experiments (see ESI†). Following incubation of the HT-29 cells with 300 μg mL−1 of ReCMC for 1 h, the cells were exposed to 2, 10 and 30 min of low power (5 mW cm−2) UV light. After such exposure, the cells were further incubated for 1 h at 37 °C upon addition of 5 μM of Caspase 3/7 detection substrate (green) and cell images were recorded individually under same experimental settings. The results, shown in Fig. 7, clearly indicated activation of caspase-3 and caspase-7 within the cancer cells upon exposure to CO.
Fig. 6.
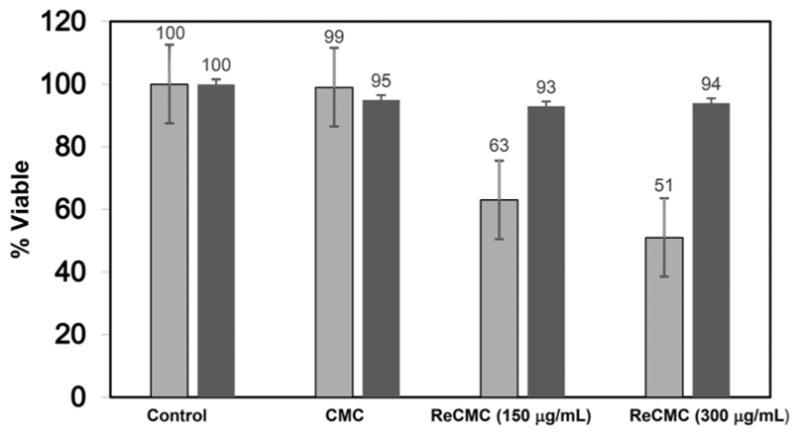
Results of the MTT assay with HT-29 cells (grey bars represent cell viability upon exposure to light, while the black bars represent cell viability under dark conditions).
Fig. 7.
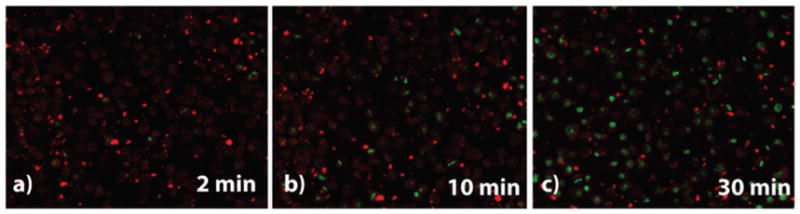
Results of detection of caspase activation within HT-29 cells with light exposure of (a) 2 min, (b) 10 min, and (c) 30 min.
Finally to confirm that CO in similar doses does not induce apoptosis in normal cells, we examined the viability of human embryonic kidney cells (HEK-293) upon exposure to CO released from ReCMC under illumination. Results of the MTT assay (see ESI†) clearly demonstrated marginal (less than 5%) reduction of cell viability under the same experimental conditions as with the HT-29 cells.
In summary, a CMC-based photoCORM with a photoactive Re(I) carbonyl complex namely ReCMC has been synthesized and the composite material has been characterized by several spectroscopic and electron microscopic techniques. This highly biocompatible and luminescent composite material is rapidly internalized by different cancer cells (as evidenced by live-cell confocal microscopy) and promotes CO-induced apoptotic eradication of human colorectal adenocarcinoma cells (HT-29) quite efficiently. The excellent mucoadhesive and permeation enhancing properties of ReCMC could be used to achieve preferential accumulation of ReCMC material within colonic tumors and their CO-induced eradication through application of light with the aid of EFOST technology (as used in endoscopic/colonoscopic procedures). Taken together, ReCMC is a promising biocompatible and biodegradable CO-releasing micropolymer that could find its application as alternative antineoplastic material in the near future.
Supplementary Material
Acknowledgments
Financial support from NSF (Grant DMR-1409335) is gratefully acknowledged. We thank Dr Jhoti Somanah of University of Technology Mauritius for helpful technical suggestion.
Footnotes
Electronic supplementary information (ESI) available: X-ray crystallographic files in CIF format. Experimental details, crystallographic data and refinement parameters details of biological experiments, additional figures. CCDC 1524183. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc02842c
Notes and references
- 1.Motterlini R, Otterbien LE. Nat Rev Drug Discovery. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Gallego S, Bernardes GJL. Angew Chem, Int Ed. 2014;53:9712–9721. doi: 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- 3.Romao CC, Blatter WA, Seixas JD, Bernardes GJ. Chem Soc Rev. 2012;41:3571–3583. doi: 10.1039/c2cs15317c. [DOI] [PubMed] [Google Scholar]
- 4.Schatzschneider U. Br J Pharmacol. 2015;172:1638–1650. doi: 10.1111/bph.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schatzschneider U. Inorg Chim Acta. 2011;374:19–23. [Google Scholar]
- 6.Heinemann SH, Hoshi T, Westerhausen M, Schiller A. Chem Commun. 2014;50:3644–3660. doi: 10.1039/c3cc49196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crespy D, Landfester K, Schubert US, Schiller A. Chem Commun. 2010;46:6651–6662. doi: 10.1039/c0cc01887b. [DOI] [PubMed] [Google Scholar]
- 8.Rimmer RD, pierri AE, Ford PC. Coord Chem Rev. 2012;256:1509–1519. [Google Scholar]
- 9.Chakraborty I, Carrington SJ, Mascharak PK. Acc Chem Res. 2014;47:2603–2611. doi: 10.1021/ar500172f. [DOI] [PubMed] [Google Scholar]
- 10.Carrington SJ, Chakraborty I, Mascharak PK. Chem Commun. 2013;49:11254–11256. doi: 10.1039/c3cc46558f. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty I, Carrington SJ, Roseman G, Mascharak PK. Inorg Chem. 2017;56:1534–1545. doi: 10.1021/acs.inorgchem.6b02623. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty I, Carrington SJ, Hauser J, Oliver SRJ, Mascharak PK. Chem Mater. 2015;27:8387–8397. [Google Scholar]
- 13.Bernkop-Schnurch A, Dunnhaupt S. Eur J Pharm Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Casettari L, Vllasaliu D, Castagnino E, Stolnik S, Howdle S, Illum L. Prog Polym Sci. 2012;37:659–685. [Google Scholar]
- 15.Mourya VK, Inamdar NN, Tiwari A. Adv Mater Lett. 2010;1:11–33. [Google Scholar]
- 16.Upadhyaya L, Singh J, Agarwal V, Tiwari RP. J Controlled Release. 2014;186:54–87. doi: 10.1016/j.jconrel.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Kean T, Thanou M. Adv Drug Delivery Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Dodane V, Vilivalam VD. Pharm Sci Technol Today. 1998;1:246–253. [Google Scholar]
- 19.Sato S, Matubara Y, Koike K, Falkenstrom M, Katayama T, Ishibashi Y, Miyasaka H, Taniguchi S, Chosrowjan H, Mataga N, Fukazawa N, Koshihara S, Onda K, Ishitani O. Chem – Eur J. 2012;18:15722–15734. doi: 10.1002/chem.201202734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierri AE, Pallaoro A, Wu G, Ford PC. J Am Chem Soc. 2012;134:18197–18200. doi: 10.1021/ja3084434. [DOI] [PubMed] [Google Scholar]
- 21.Hejari R, Amiji M. J Controlled Release. 2003;89:151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 22.Berscht PC, Nies B, Liebendorfer A, Kreuter J. Biomaterials. 1994;15:593–600. doi: 10.1016/0142-9612(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 23.Pantaleone D, Yalpani M, Scollar M. Advances in chitin and chitosan. Princeton; New York: 1991. [Google Scholar]
- 24.Anitha A, Sreeranganathan M, Chennazhi KP, Lakhsmanan VK, Jayakumar R. Eur J Pharm Biopharm. 2014;88:238–251. doi: 10.1016/j.ejpb.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Cao CGL, Wong PY, Lilge L, Gavalis RM, Xing H, Zamarripa N. Proc SPIE. 2008:6935. doi: 10.1117/12.776276. [DOI] [Google Scholar]
- 26.Niesel J, Pinto A, N’Dongo HWP, Merz K, Ott I, Gust R, Schatzschneider U. Chem Commun. 2008:1798–1800. doi: 10.1039/b719075a. [DOI] [PubMed] [Google Scholar]
- 27.Elmore S. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


