Abstract
Background
Dabigatran is a direct thrombin inhibitor used to decrease the risk of ischemic stroke in patients with non-valvular atrial fibrillation (NVAF). Its prodrug, dabigatran etexilate (DE) is often co-administrated with a proton pump inhibitor (PPI) because of its adverse effects on the gastrointestinal tract. Drug-drug interactions between DE and PPIs in daily clinical practice have not been fully elucidated.
Methods
Changes in blood dabigatran concentration (DC) were investigated using the dilute thrombin time test in a randomized, open-label, two-period crossover study including 34 Japanese patients with NVAF receiving dabigatran therapy with or without PPI.
Results
The average trough DC was significantly higher without PPI than with PPI (83 ± 42.3 vs. 55.5 ± 24.6 ng/mL, respectively; P < 0.001). Similarly, the average peak DC was significantly higher without PPI than with PPI (184.1 ± 107.7 vs. 124 ± 59.2 ng/mL, respectively; P = 0.0029). The average ratio of DC change at the trough and peak levels did not differ significantly among the three PPI types.
Conclusions
PPI administration significantly decreased the trough and peak DCs in patients with NVAF. Therefore, when prescribing PPIs for patients with NVAF in a clinical setting, the possibility that the bioavailability of dabigatran may decrease should be considered.
Keywords: Dabigatran, Proton pump inhibitor, Drug–drug interaction
1. Introduction
Dabigatran is a direct thrombin inhibitor that has been used to decrease the risk of ischemic stroke in patients with non-valvular atrial fibrillation (NVAF) and for direct-current cardioversion and ablation of AF [1], [2], [3]. Since dabigatran is not absorbed orally, its prodrug, dabigatran etexilate (DE), which is rapidly absorbed and converted to dabigatran by esterase-catalyzed hydrolysis, is often used [4]. Similar to other non-vitamin K oral anticoagulants, routine biological monitoring is not usually required in patients receiving dabigatran therapy owing to its predictable pharmacokinetics with limited drug-drug interactions [1]. However, monitoring of the anticoagulant activity might be useful in certain clinical settings [5]. The dilute thrombin time test was reported to show a high degree of linearity at blood dabigatran concentrations (DC) > 50 ng/mL and was useful for quantitation across the entire on-therapy range [5], [6]. The activated partial thromboplastin time (aPTT) is relatively insensitive because the curvilinear response at higher drug levels does not permit accurate quantitation [5]. DE is often co-administered with a proton pump inhibitor (PPI) because of its adverse effects on the gastrointestinal tract [1], [7]. It is known that co-administration of PPI may affect the absorption of DE by raising the gastric pH because an acidic environment is required for the dissolution of DE [8], [9]. However, drug-drug interactions between DE and PPI have not been fully elucidated in daily clinical practice. Therefore, the changes in DC were examined in Japanese patients with NVAF receiving dabigatran therapy with or without PPI, using the dilute thrombin time test.
2. Material and methods
2.1. Subjects
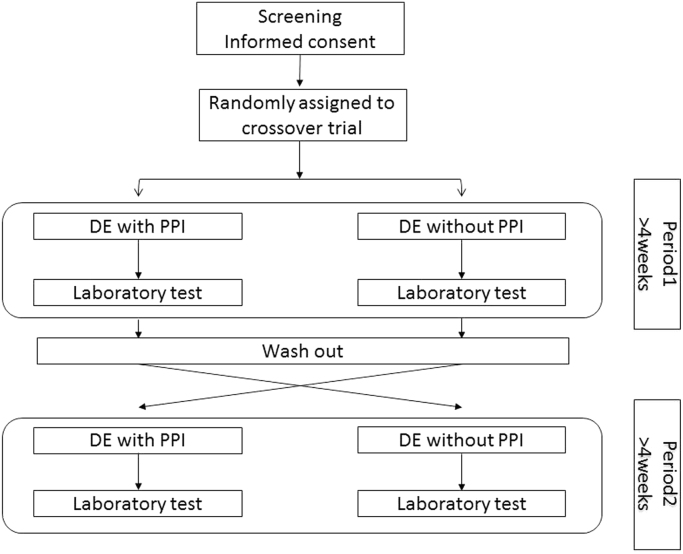
This randomized, open-label, two-period crossover study including Japanese patients with NVAF was conducted at Tosei General Hospital, Aichi, Japan. From May 1, 2015 to July 31, 2015, 37 patients with NVAF were enrolled and assigned randomly to one of two groups, administration of DE with PPI and administration of DE without PPI. Patients with renal insufficiency (creatinine clearance < 30 mL/min) or peptic ulcer were excluded. Each group crossed over, and blood samples at the trough and peak times were obtained during each period more than 4 weeks after the start of treatment, which was preceded by a 2-week wash-out period (Fig. 1). The trough time was defined as the time immediately before the administration of DE, and the peak time was defined as 2 h after the administration of DE [10].
Fig. 1.
Schematic study design. DE, dabigatran etexilate; PPI, proton pump inhibitor.
The PPI selected by the treating physician (lansoprazole [30 mg], rabeprazole [20 mg], or esomeprazole [20 mg]) was used once daily. Creatinine clearance was determined using the Cockcroft-Gault formula [11]. We obtained written, informed consents from all patients. This study was approved by the Ethics Committees of Tosei General Hospital on April 20, 2015 (approval number: 15-03).
2.2. Measurement of DC and aPTT
DC at the trough and peak times was calculated using HemosIL® direct thrombin inhibitor assay (Instrumentation Laboratory, Bedford, MA, USA). This assay is a dilute thrombin time test, which is based on the reaction between dabigatran and exogenous thrombin added to the diluted patient plasma. The associated clotting time was measured using the ACL TOP hemostasis testing system (Instrumentation Laboratory), and then the concentration of dabigatran was estimated from the reference curve of the known plasma standard of dabigatran using HemosIL® dabigatran calibrators (Instrumentation Laboratory). The trough or peak ΔDC ratio was defined as the trough or peak DC in the period without PPI minus the corresponding DC in the period with PPI divided by the trough or peak DC in the period with PPI. Trough and peak aPTTs were measured using HemosIL® APTT-SP (Instrumentation Laboratory).
2.3. Statistical analysis
Categorical variables were presented as numbers and percentages. Continuous variables were expressed as means ± standard deviation. Student's t-test was used to compare parameters between groups. Kruskal-Wallis test was used to compare the trough and peak ΔDC ratios among the three PPI types. All statistical analyses were performed using the Ekuseru-Tokei statistical software program (Ekuseru-Tokei 2010, Social Survey Research Information Co, Tokyo, Japan).
3. Results
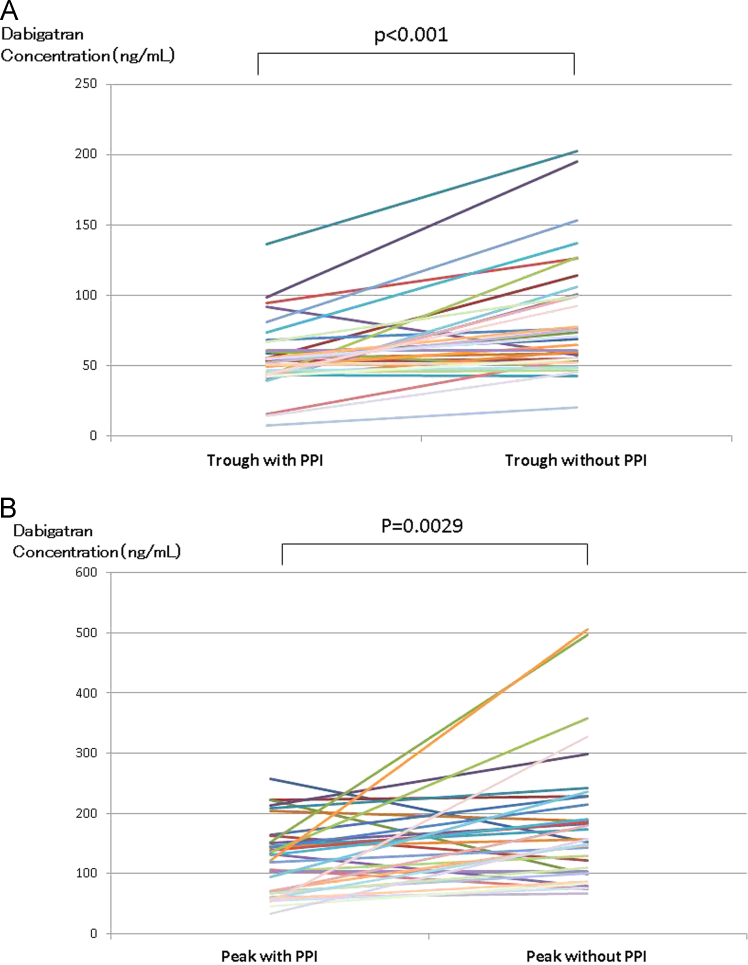
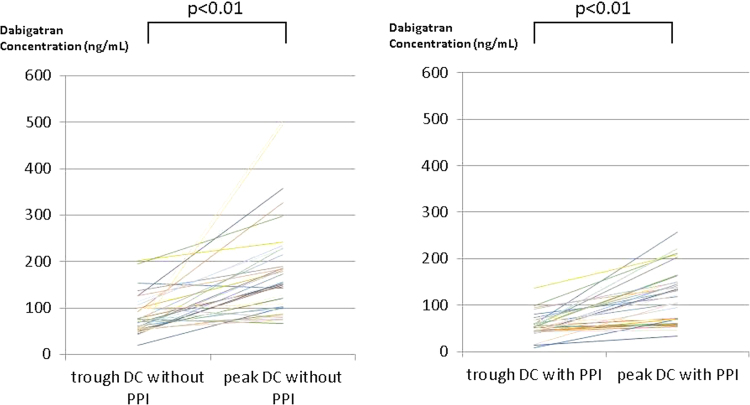
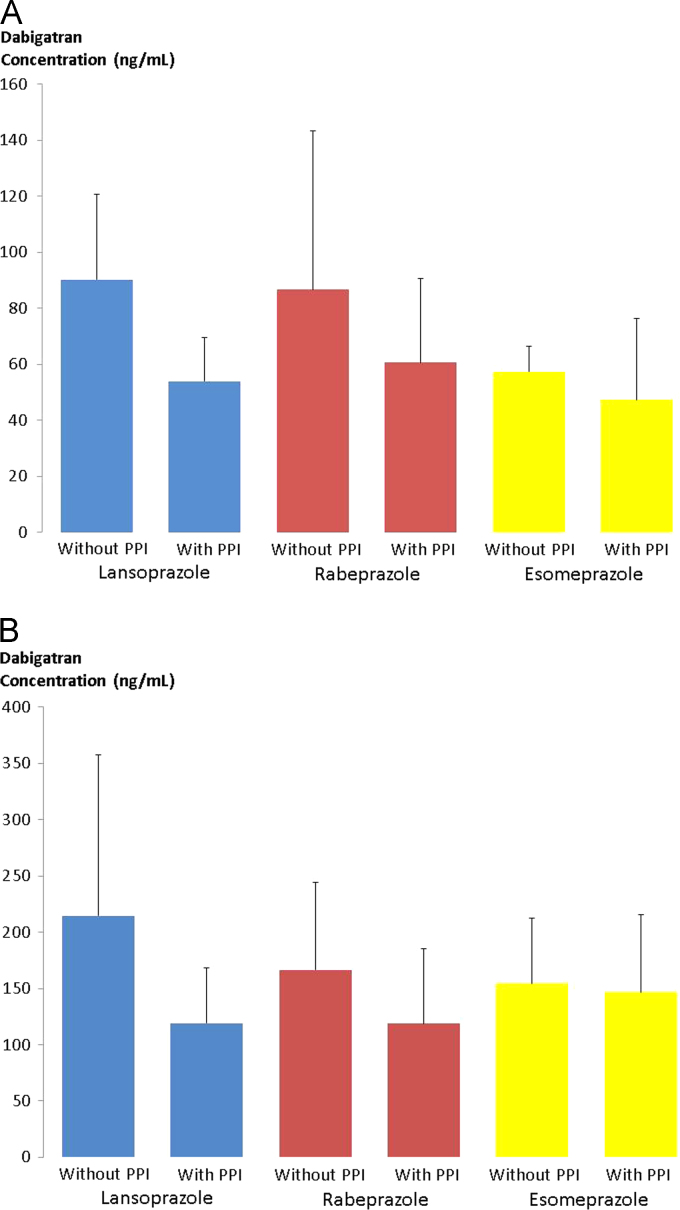
A total of 37 patients with NVAF were enrolled in this study. Two patients dropped out during the first period because of adverse effects. One patient had indigestion and the other had bloody phlegm; both patients were treated with DE + PPI. Furthermore, one patient had acute arterial embolism in the lower limb during the second period of co-administration with PPI. Therefore, results of the remaining 34 patients who completed the entire study were included in the analysis. The characteristics of these 34 patients are presented in Table 1. The average trough DC was significantly higher during the DE without PPI period than during the DE with PPI period (83 ± 42.3 vs. 55.5 ± 24.6 ng/mL, respectively; P < 0.001) (Fig. 2A). Similarly, the average peak DC was significantly higher during the DE without PPI period than during the DE with PPI period (184.1 ± 107.7 vs. 124 ± 59.2 ng/mL, respectively; P = 0.0029) (Fig. 2B). The peak DC was significantly higher than the trough DC during both periods (Fig. 3). The trough and peak DCs with and without co-administration of the three PPI types are presented in Fig. 4A and B, respectively. The average trough and peak ΔDC ratio did not differ significantly among the three PPI types (Table 2). Similar to the results of the dilute thrombin time test, the average trough aPTT was significantly higher during the period without PPI than during the period with PPI (43.7 ± 6.1 vs. 39 ± 4.6 s, respectively; P < 0.001). In addition, the average peak aPTT was significantly higher during the period without PPI than during the period with PPI (56.9 ± 13.5 vs. 48.6 ± 8.8 s, respectively; P = 0.0028). The average control value of aPTT was 30.1 ± 0.5 s.
Table 1.
Patients’ characteristics.
| Patients, n | 34 |
|---|---|
| Male, n (%) | 25 (73.5) |
| Age, years | 71.5 ± 9.7 |
| Body Weight, kg | 63.7 ± 9.2 |
| Serum creatinine, mg/dL | 0.94 ± 0.34 |
| Mean creatinine clearance, mL/min | 65.7 ± 19.8 |
| Dose of dabigatran | |
| 110 mg twice daily, n (%) | 22 (64.7) |
| 150 mg twice daily, n (%) | 12 (35.3) |
| CHADS2 score | 2.6 ± 1.4 |
| Paroxysmal atrial fibrillation, n (%) | 13 (38.2) |
| Type of proton pump inhibitor | |
| Lansoprazole, n (%) | 14 (41.2) |
| Rabeprazole, n (%) | 14 (41.2) |
| Esomeprazole, n (%) | 6 (17.6) |
Fig. 2.
A Plot of the trough dabigatran concentration (DC) during the dabigatran etexilate (DE) with proton pump inhibitor (PPI) period and without PPI period. The average trough DC was significantly higher without PPI than with PPI. B: Plot of the peak dabigatran concentration (DC) during the dabigatran etexilate (DE) with proton pump inhibitor (PPI) period and without PPI period. The average peak DC was significantly higher without PPI than with PPI.
Fig. 3.
Plot of the trough and peak dabigatran concentrations (DCs) during each period, administration of dabigatran etexilate (DE) without PPI or administration of DE with PPI. The peak DC was significantly higher than the trough DC during both periods.
Fig. 4.
A The distribution of trough dabigatran concentration (DC) with and without proton pump inhibitor (PPI) co-administration for the three PPI types. B: The distribution of peak dabigatran concentration (DC) with and without proton pump inhibitor (PPI) co-administration for the three PPI types.
Table 2.
Comparison of the average ratio of dabigatran concentration change at the trough and peak times among the three proton pump inhibitor (PPI) types.
| Type of PPI | Lansoprazole | Rabeprazole | Esomeprazole | P-value |
|---|---|---|---|---|
| Trough ΔDC ratio | 0.75 ± 0.68 | 0.46 ± 0.53 | 0.80 ± 1.25 | 0.55 |
| Peak ΔDC ratio | 0.88 ± 1.03 | 0.72 ± 1.34 | 0.55 ± 1.55 | 0.32 |
Trough ΔDC ratio = The trough dabigatran concentration (DC) in the period without PPI minus the corresponding DC in the period with PPI divided by the trough DC in the period with PPI.
Peak ΔDC ratio = The peak dabigatran concentration (DC) in the period without PPI minus the corresponding DC in the period with PPI divided by the peak DC in the period with PPI.
4. Discussion
To the best of our knowledge, this is the first randomized, crossover study on the drug-drug interactions between DE and PPIs in patients with NVAF. In this study, co-administration of PPIs significantly decreased DC at both the trough and peak times.
Results of the RE-LY trial showed that patients cotreated with a PPI exhibited a 12.5% decrease in the area under the plasma concentration-time curve at steady state [8]. Similarly, co-administration of pantoprazole decreased the area under the plasma concentration-time curve and the maximum plasma concentration at steady state by 20–24% in healthy elderly subjects [10]. Although these previous studies depended on the comparison between two groups (with and without PPI administration), their results are consistent with our findings that showed that PPI administration decreased the bioavailability of dabigatran.
Regarding the interactions between DE and PPIs, PPIs are known as moderate inhibitors of the efflux transporter, P-glycoprotein (P-gp) [12], and DE is a substrate of P-gp [8]. Theoretically, PPIs are expected to increase DC, similar to other drugs that strongly inhibit P-gp [8], [13]. However, since PPIs increase the gastric pH, they decrease the dissolution and absorption of DE, which are promoted in the acidic environment [9]. Therefore, PPI administration could decrease DC by affecting its solubility. In healthy subjects, the mean percentage of time maintained with intragastric pH > 4 on day 5 was 61, 76, and 62 for the extensive metabolizer (rabeprazole 20 mg), poor metabolizer (rabeprazole 20 mg), and esomeprazole 20 mg, respectively. For lansoprazole (30 mg), the mean percentage of time maintained with intragastric pH > 4 on day 5 was 48–53 in healthy subjects [14], [15].
PPIs significantly reduced the trough and peak DC in the present study, the latter mechanism seems to control the interaction between DE and PPIs. Contrary to our results, Ollier et al. found no significant interactions between PPIs and DE in a single-center, randomized, open-label study [9]. In this previous study, nine healthy male Caucasian volunteers (median age, 23 years; median weight, 73 kg) were evaluated using a single-dose regimen of DE. The obvious difference in the backgrounds of patients in the present study and those in this previous study, as well as the protocol of repeated-dose DE administration in the present study could explain the different results. Furthermore, in the present study, the DC values were higher with PPI than without PPI in three patients at the trough time and six patients at the peak time. Although the number of patients was too small to clarify their clinical characteristics, the former mechanism might control the interaction between DE and PPIs in a few patients in the present study.
DE contains tartaric acid to increase the extent of absorption, which results in gastrointestinal symptoms (the most frequent adverse effect) [1], [7]. PPIs could be used for prevention of these symptoms. Therefore, when prescribing PPIs with DE in clinical settings, it is necessary to consider the possibility that the bioavailability of dabigatran might decrease.
The clinical outcomes of the interaction of PPIs with DC are not known. In a previous study, co-administration of PPIs resulted in minor to moderate effects, and only minor effects were considered likely in clinical settings [8], [10]. In the present study, the average trough and peak DCs with and without PPI were within the therapeutic range determined in the Japanese subgroup analysis of the RE-LY trial [16]. Thus, the decrease in DC by PPIs might be moderate enough to maintain the efficacy of dabigatran; however, further investigations are needed to confirm the clinical outcomes of the co-administration of PPIs in patients with NVAF receiving dabigatran therapy.
In the present study, the dilute thrombin time was measured to calculate DC because it showed a high degree of linearity at drug levels > 50 ng/mL [5], [6]. This assay is still not available in all clinics; thus, aPTT, the routine and sensitive biomarker of the anticoagulant activity of dabigatran, was also measured [5]. Based on the results of the present study, aPTT could be also useful to evaluate the effect of PPIs on DE to a considerable degree.
The present study had several limitations. This study was performed at a single institution for a short observation period and included a limited number of subjects. Since the results of the present study were analyzed at low statistical power owing to the small number of subjects, further studies including a greater number of patients are required to confirm the present results. The clinical outcomes were not compared between the two periods because there were too few events to analyze. One patient had bloody phlegm during the period 1 when he took DE co-administration with a PPI, and another had acute arterial embolism in the lower limb during the period 2 when he took DE co-administration with a PPI. Although dabigatran has a stable pharmacokinetic profile, intra-patient variability in the peak and trough DCs can occur. In a previous study, the intra-patient geometric coefficients of variation in the trough and peak DC over 6 months were 32–40% [10]. Thus, repeated measurements of DC during both periods may be needed to confirm the present results. The peak time was defined uniformly as 2 h after DE administration; however, the actual peak concentration may differ among individual patients. In the present study, the peak DC was slightly lower than the trough DC in one patient during the period of co-administration of PPI and in three patients during the period without PPI. In these patients, it is possible that the increase in DC 2 h after DE administration was not strong enough.
5. Conclusions
In conclusion, PPI co-administration was found to significantly decrease the trough and peak DC measured using the plasma-diluted thrombin time in patients with NVAF in a randomized, crossover study. When prescribing PPIs to patients with NVAF in clinical settings, the possibility that the bioavailability of dabigatran may decrease should be considered.
Conflict of interest
H.O. and M.A. have received lecture fees from Bristol-Myers Squibb, Pfizer, Bayer Yakuhin Ltd, Boehringer Ingelheim, and Daiichi Sankyo.
References
- 1.Connolly S.J., Ezekowitz M.D., Yusuf S. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Yadlapati A., Groh C., Passman R. Safety of short-term use of dabigatran or rivaroxaban for direct-current cardioversion in patients with atrial fibrillation and atrial flutter. Am J Cardiol. 2014;113:1362–1363. doi: 10.1016/j.amjcard.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Sardar S., Nairooz R., Chatterjee S. Meta-analysis of risk of stroke or transient ischemic attack with dabigatran for atrial fibrillation ablation. Am J Cardiol. 2014;113:1173–1177. doi: 10.1016/j.amjcard.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Blech S., Ebner T., Ludwig-Schwellinger E. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- 5.Cuker A., Deborah M., Siegal D.M. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128–1139. doi: 10.1016/j.jacc.2014.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okubo K., Kuwahara T., Takagi K. Relation between dabigatran concentration, as assessed using the direct thrombin inhibitor assay, and activated clotting time/activated partial thromboplastin time in patients with atrial fibrillation. Am J Cardiol. 2015;115:1696–1699. doi: 10.1016/j.amjcard.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Bytzer P., Connolly S.J., Yang S. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol. 2013;11:246–252. doi: 10.1016/j.cgh.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Liesenfeld K.H., Lehr T., Dansirikul C. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9:2168–2175. doi: 10.1111/j.1538-7836.2011.04498.x. [DOI] [PubMed] [Google Scholar]
- 9.Ollier E., Hodin S., Basset T. In vitro and in vivo evaluation of drug-drug interaction between dabigatran and proton pump inhibitors. Fundam Clin Pharmacol. 2015;29:604–614. doi: 10.1111/fcp.12154. [DOI] [PubMed] [Google Scholar]
- 10.Stangier J., Staehle H., Rathgen K. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Pauli-Magnus C., Rekersbrink S., Klotz U. Interaction of omeprazole, lansoprazole with P-glycoprotein. Naunyn Schmiedeberg's Arch Pharmacol. 2001;364:551–557. doi: 10.1007/s00210-001-0489-7. [DOI] [PubMed] [Google Scholar]
- 13.Hartter S., Sennewald R., Nehmiz G. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner P., Jr, Katz P.O., Chen Y. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–2620. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 15.Pisegna J.R., Sostek M.B., Monyak J.T. Intravenous esomeprazole 40 mg vs. intravenous lansoprazole 30 mg for controlling intragastric acidity in healthy adults. Aliment Pharmacol Ther. 2008;27:483–490. doi: 10.1111/j.1365-2036.2007.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori M., Connolly S.J., Ezekowitz M.D. Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation—sub-analysis in Japanese population in RE-LY trial. Circ J. 2011;75:800–805. doi: 10.1253/circj.cj-11-0191. [DOI] [PubMed] [Google Scholar]