Abstract
Extracardiac structures can cause distortion of cardiac anatomy particularly in patients presenting with a significantly dilated heart, and/or thoracic deformities. We present the case of a 69-year-old woman with dilated cardiomyopathy who underwent cardiac resynchronization therapy. Preoperative electrocardiography-gated contrast-enhanced computed tomography revealed the inferolateral wall of her significantly dilated and leftward-rotated heart was close to the descending aorta, and the descending aorta compressed the sandwiched inferolateral branch of the coronary vein. Retrograde coronary venography performed at the time of device implantation confirmed focal stenosis of the inferolateral branch of the coronary vein.
Abbreviations: CRT-D, cardiac resynchronization therapy device with defibrillator; CT, computed tomography; LAO, left anterior oblique; NYHA, New York Heart Association; RAO, right anterior oblique
Keywords: Cardiac resynchronization therapy, Computed tomography, Dilated cardiomyopathy
1. Introduction
Extensive research has revealed anatomical variations in the coronary venous system including branching patterns. Preprocedural evaluation of variations in the coronary venous anatomy is important for successful implantation of a cardiac resynchronization therapy device with defibrillator (CRT-D) [1], [2]. However, three-dimensional relationships between the coronary venous anatomy and surrounding extracardiac structures including the descending aorta and the esophagus have rarely been reported.
2. Case report
A 69-year-old woman with dilated cardiomyopathy was referred to our hospital for implantation of a CRT-D. She was classified as presenting with New York Heart Association functional class II heart failure symptoms despite an optimal medication regimen including administration of a beta-blocker and a renin-angiotensin system inhibitor. Her plasma brain natriuretic peptide level was elevated to 309.6 pg/mL. Chest radiography showed cardiomegaly (cardiothoracic ratio of 57%) without congestion or pleural effusion. Electrocardiography showed a normal sinus rhythm with a complete left bundle branch block (QRS duration of 169 ms). Echocardiography revealed a significantly dilated left ventricle (end-diastolic diameter of 66 mm) with reduced left ventricular contraction (ejection fraction of 33%) and apparent dyssynchrony. Based on these findings, we anticipated favorable reverse left ventricular remodeling and clinical outcomes following CRT-D implantation in this patient [3].
We performed electrocardiography-gated contrast-enhanced computed tomography (CT) using a commercially available third-generation dual-source CT scanner (SOMATOM Force, Siemens Healthcare, Forchheim, Germany) for preoperative evaluation of the coronary venous anatomy. Based on this evaluation we could study the anatomical relationships between a significantly dilated and leftward-rotated heart and the surrounding extracardiac structures including the descending aorta (Fig. 1). The inferolateral wall of the left ventricle was compressed and deformed by the adjacent descending aorta (Fig. 1A and B) and the inferolateral branch of the coronary vein sandwiched between the left ventricle and the descending aorta was observed to be compressed by the descending aorta (Fig. 1C). Volume-rendered images showed a three-dimensional orientation of the anatomical relationship between the descending aorta and the coronary veins in combination with the cardiac silhouette (Fig. 2A and B). All image analyses were performed using a commercially available workstation (Ziostation2 version 2.4.2.3, Ziosoft Inc., Tokyo, Japan).
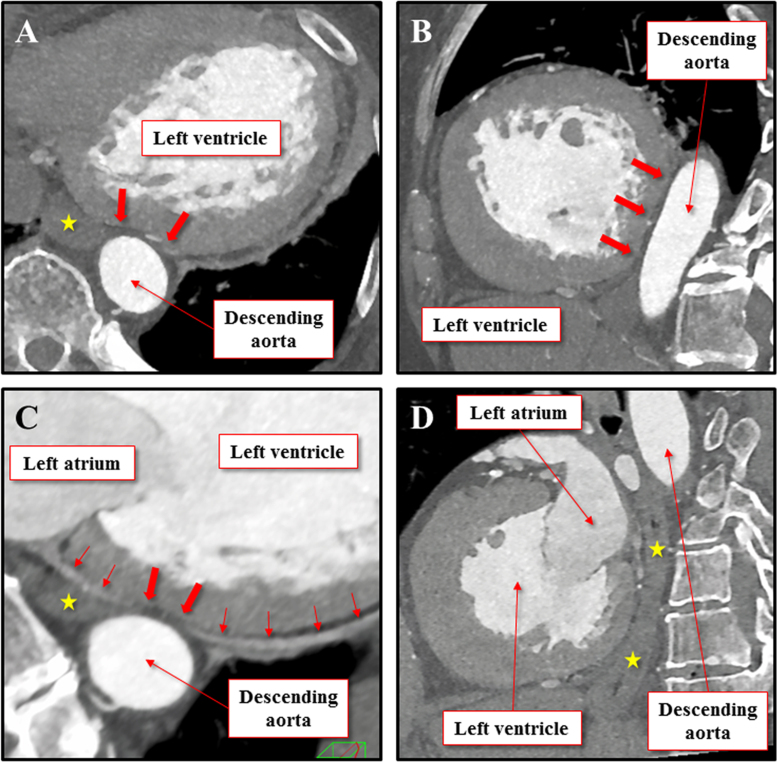
Fig. 1.
Multi-planar reconstruction images focusing on the left ventricular basal inferolateral wall. Horizontal (A) and sagittal (B) sections of multi-planar reconstruction images showing the descending aorta compressing the left ventricular basal inferolateral wall (thick red arrows). (C) Horizontal section of multi-planar reconstruction image showing focal stenosis (thick red arrows) of the inferolateral branch of the coronary vein secondary to extracardiac compression by the descending aorta. Thin red arrows denote the inferolateral branch of the coronary vein. (D) The esophagus (yellow star) is observed adjacent to the inferolateral wall of the left ventricle. Note that the left ventricular inferolateral wall is deformed by the descending aorta but not by the esophagus.
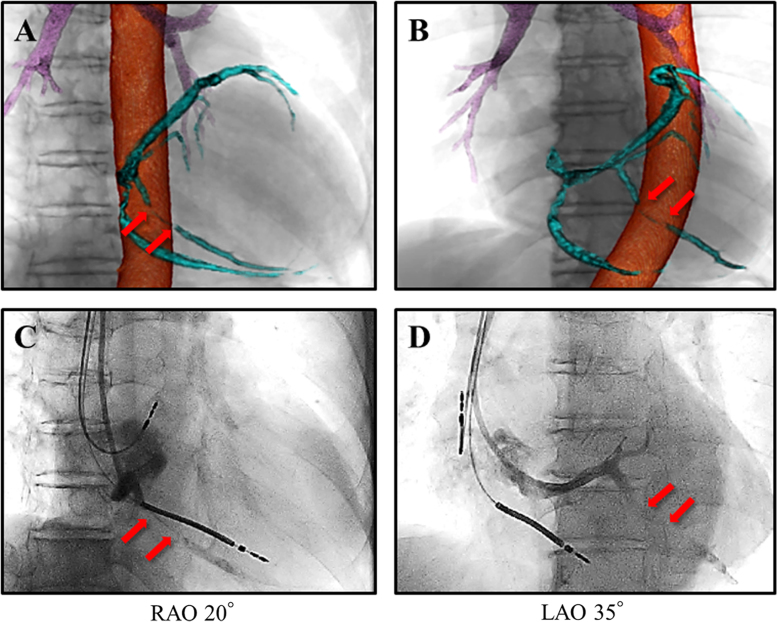
Fig. 2.
Preprocedural volume-rendered computed tomographic images and a retrograde coronary venogram. Volume-rendering reconstruction images of the coronary vein (sky- blue) and the descending aorta (orange) obtained using multidetector-row computed tomography combined with images of the bronchi (purple) and the cardiac silhouette. Focal stenosis of the inferolateral branch (red arrows) of the coronary vein is identified secondary to compression by the descending aorta in the right anterior oblique (RAO) (A) and in the left anterior oblique (LAO) (B) views. Retrograde coronary venogram confirms focal stenosis (red arrows) in the inferolateral branch noted in the RAO (C) and LAO (D) views, corresponding to each upper panel.
Owing to the patient's history of breast cancer, a magnetic resonance imaging-conditional CRT-D system was selected in case she needed to undergo magnetic resonance imaging in future. During the CRT-D implantation, retrograde coronary venography was performed via a 9.0 Fr×50 cm guiding catheter (Selectra Right™, Biotronik Japan Inc., Tokyo, Japan) that was inserted into the coronary sinus. Retrograde coronary venography confirmed focal stenosis of the inferolateral branch (Fig. 2C and D). Thus, we initially tried to insert the left ventricular lead into other branches. However, we found that there was no appropriate branch that could be used except the inferolateral branch. Fortunately, the 4.8 Fr.×80 cm left ventricular lead (Sentus Pro MRI OTW QP L-85™, Biotronik Inc.) could be deployed into the distal inferolateral branch through the stenotic area without complications. The subsequent procedure was completed successfully, and implantation was followed by immediate disappearance of the left ventricular dyssynchrony due to effective biventricular pacing. Her clinical course was uneventful during a 12-month follow-up with significant improvement in symptoms (NYHA Class I), as well as improved laboratory and radiological parameters, primarily, plasma brain natriuretic peptide (20.9 pg/mL), cardiothoracic ratio (54%), QRS duration (148 ms), left ventricular end-diastolic diameter (56 mm), and ejection fraction (45%).
3. Discussion
We report a patient with dilated cardiomyopathy who developed focal stenosis in the inferolateral branch of the coronary vein secondary to extracardiac compression between the descending aorta and the enlarged left ventricular wall. Compression of the inferolateral branch of the coronary vein was initially detected when a preprocedural electrocardiography-gated contrast-enhanced CT was performed and was subsequently confirmed using intraprocedural retrograde coronary venography. CT images were reconstructed in mid-diastole, which suggested that the descending aorta could deform the left ventricular basal inferolateral wall because the left ventricular end-diastolic pressure would have been significantly lower than the diastolic blood pressure. Although the procedure was completed successfully and the patient's subsequent clinical course has been favorable, it is important to carefully monitor the patient for the development of long-term consequences, if any, due to the lead being sandwiched between the left ventricle and the descending aorta.
Although lead stability was expected to improve between the enlarged left ventricular mass and the descending aorta, initially, we suspected delivery failure and damage to the left ventricular lead. We suspected that the continued focused force created by the sandwiched lead could cause tissue injury in the surrounding structures in the long term. The compression force ought to be reduced in the chronic phase, owing to negative remodeling of the left ventricle by an effective biventricular pacing. In preparation for possible delivery failure, selecting a left ventricular lead with a smaller diameter should have been considered in this patient. However, there was no other magnetic resonance imaging-conditional system available in Japan at the time of implantation.
Anatomy of the coronary circulation is affected by the rotation and dilation of the whole heart within the thorax [4]. Furthermore, as demonstrated in our patient, cardiac anatomy can be distorted by extracardiac structures, particularly in patients presenting with a markedly dilated heart, or thoracic deformities [5]. CT can precisely evaluate the coronary venous anatomy [1], [2] in addition to the cardiac contour and anatomy of the surrounding structures. A CT obtained prior to invasive cardiac procedures should thoroughly evaluate cardiac anatomy in relation to surrounding structures.
4. Conclusion
Electrophysiologists should consider the role of extracardiac structures in patients presenting with focal stenosis within the coronary venous system when performing procedures around the epicardium.
Conflict of interest disclosures
We declare that Dr. K. Fukuzawa, Dr. K. Kiuchi, and Dr. K. Hirata have received research funding from Medtronic Japan Co. Ltd (Tokyo, Japan) and St. Jude Medical Japan Co. Ltd (Tokyo, Japan).
Acknowledgments
All authors wish to thank the following radiological technologists for their assistance in image acquisition and reconstruction: Erina Suehiro, Wakiko Tani, Toshinori Sekitani, Kiyosumi Kagawa, and Noriyuki Negi.
References
- 1.Malagò R., Pezzato A., Barbiani C. Noninvasive cardiac vein mapping: role of multislice CT coronary angiography. Eur J Radiol. 2012;81:3262–3269. doi: 10.1016/j.ejrad.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Da Costa A., Gate-Martinet A., Rouffiange P. Anatomical factors involved in difficult cardiac resynchronization therapy procedure: a non-invasive study using dual-source 64-multi-slice computed tomography. Europace. 2012;14:833–840. doi: 10.1093/europace/eur350. [DOI] [PubMed] [Google Scholar]
- 3.Yokoshiki H., Mitsuyama H., Watanabe M. Cardiac resynchronization therapy in ischemic and non-ischemic cardiomyopathy. J Arrhythm. 2017 doi: 10.1016/j.joa.2017.03.002. [Article in Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichibori H., Mori S., Takaya T. Slit-like deformation of the coronary sinus orifice due to compression of the inferior pyramidal space by the severely dilated left ventricle. Pacing Clin Electrophysiol. 2016;39:1026–1029. doi: 10.1111/pace.12881. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K., Takaya T., Mori S. Compression of the right ventricular outflow tract due to straight back syndrome clarified by low-dose dual-source computed tomography. Intern Med. 2016;55:3279–3283. doi: 10.2169/internalmedicine.55.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]