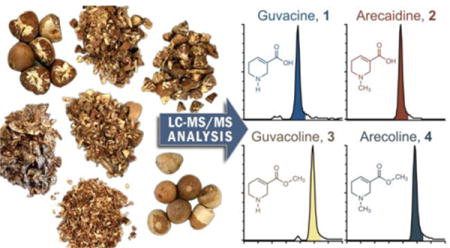
Abstract
Chewing of areca nut in different forms, such as betel quid or commercially-produced pan masala and gutkha, is common practice in the Indian subcontinent and many parts of Asia, and is associated with a variety of negative health outcomes, particularly oral and esophageal cancers. Areca nut-specific alkaloids arecoline, arecaidine, guvacoline, and guvacine have been implicated in both the abuse liability and the carcinogenicity of areca nut. Therefore, variations in the levels of areca alkaloids could potentially contribute to variations in addictive and carcinogenic potential across areca nut-containing products. Here, we have developed an accurate and robust liquid chromatography-tandem mass-spectrometry (LC-MS/MS) method for simultaneous quantitation of all four areca alkaloids and applied this method to the analysis of a range of products obtained from India, China, and USA. The results of the analyses revealed substantial variations in the levels of alkaloids across the tested products, with guvacine being the most abundant (1.39-8.16 mg/g), followed by arecoline (0.64-2.22 mg/g), arecaidine (0.14-1.70 mg/g) and guvacoline (0.17-0.99 mg/g). Substantial differences in the relative contribution of individual alkaloids to the total alkaloid content were also observed among the different products. Our results highlight the need for systematic surveillance of constituent levels in areca nut-containing products and better understanding of the relationship between the chemical profile and the harmful potential of these products.
Keywords: areca nut, arecaidine, arecoline, guvacine, guvacoline, LC-MS/MS
Graphical abstract
INTRODUCTION
Consumption of areca nut, the seed of the Areca catechu tree, is very common in India, Bangladesh, Malaysia, certain parts of China, and some other countries of the Indian subcontinent, Asia and the Pacific region, with over 600 million users globally.1–3 The spectrum of health consequences associated with the use of areca nut-containing products, which includes addiction and a range of toxic and carcinogenic effects, is very similar to the outcomes associated with smokeless tobacco use.1, 2, 4–6 Most notable adverse health effects are pre-cancerous oral lesions (particularly submucuous fibrosis) as well as oral and esophageal cancers.2, 7–10 Based on the evidence from epidemiologic, animal, and mechanistic studies, the International Agency for Research on Cancer classified areca nut as human carcinogen (Group 1).1, 2
Studies aimed at understanding the mechanisms underlying the addictiveness and carcinogenicity of areca nut have identified alkaloids guvacine, 1, arecaidine, 2, guvacoline, 3, and arecoline, 4, as the potential key chemical constituents contributing to these properties (Figure 1).11–14 For instance, arecoline is believed to be the major addictive alkaloid in areca nut and has been shown to modulate a range of cellular enzymes such as matrix metalloproteinases and lysyl oxidase; arecoline is also known to inhibit p53 mRNA expression and DNA repair.1, 2, 15 The contribution of other alkaloids to the addictive potential of areca nut-containing products is not known; however, they have demonstrated bacterial mutagenicity and induced macromolecular changes in mammalian cells.2 In addition, these alkaloids can undergo nitrosation in the oral cavity of users to form areca nut-derived nitrosamines such as N-nitrosoguvacoline, N-nitrosoguvacine, and 3-methylnitrosaminopropionitrile which have the potential to damage DNA.16–18
Figure 1.
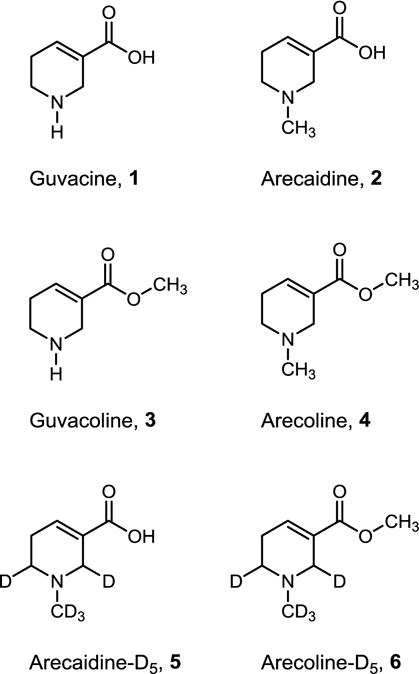
Structures of areca nut alkaloids.
Information on the levels of areca alkaloids in specific products is extremely scarce. This is an important gap in knowledge given the high diversity of products that contain areca nut: it can be used directly, fresh or processed (dried or cured), or be part of various complex formulations with other ingredients, including tobacco. For instance in India, areca nut is commonly used as betel quid – a mix that contains areca nut, catechu (an extract from Acacia catechu), slaked lime, and various spices, prepared by vendors or consumers with or without tobacco and wrapped in a fresh betel leaf. Similar ingredients are used in pan masala (without tobacco) and gutkha (with tobacco) which are produced commercially and sold as pre-packaged sachets of dried mixture. On the other hand, in China, commercially produced areca nut is commonly consumed as dried or cured fruit with husk, with or without lime.11 A variety of other forms containing areca nut of varying ripeness and processing (fresh, dried, or cured) are also used worldwide.1, 2 This diversity of areca nut types and formulations can affect the levels of areca alkaloids and the subsequent exposures in users. Understanding the potential ranges of alkaloid variations across products can provide important insights into the potential role of these constituents in the addictive and carcinogenic potential of various formulations.
Our goal was to develop a robust and accurate liquid chromatography-tandem mass-spectrometry (LC-MS/MS) method for simultaneous analysis of arecoline, arecaidine, guvacine, and guvacoline, and to apply the developed method to a range of products in order to provide initial insights into the potential variation of alkaloid levels across different formulations.
MATERIALS AND METHODS
Caution
Areca alkaloids are toxic and potentially carcinogenic and mutagenic, and should be handled with extreme care, using appropriate protective clothing and ventilation at all times.
Chemicals
Arecaidine hydrobromide, arecoline hydrobromide, guvacine hydrochloride, guvacoline hydrobromide, arecaidine-D5 hydrobromide, and arecoline-D5 hydrobromide salts were purchased from Toronto Research Chemicals (North York, Ontario, Canada). All other chemicals and solvents were purchased from either Sigma-Aldrich Chemical Co. (Milwaukee, WI) or Fisher Scientific (Fairlawn, NJ). All aqueous solutions were prepared with water purified on a 0.22 μm Millipore system (Billerica, MA).
Product samples
Products procured for the analysis of areca-specific alkaloids (Figure 2) included various types of areca nut and different manufactured areca nut-containing products purchased from the local markets in India, China and USA. Nine varieties of whole, halved, crushed, chopped, and sliced areca nuts (which are sold in bulk for the use in custom preparations and for traditional offerings) were purchased from the local markets in Mumbai, India. Manufactured pan masala and gutkha were obtained from two locations: Pan Parag, Pan Bahar, Rajnigandha, Rajshree, RMD and Vimal were obtained from Indore, Madhya Pradesh, India and Pan Parag gutkha and Tulsi were purchased in an ethnic shop in Minneapolis, MN. Samples of Chinese commercially produced areca nut products were obtained at three retail shops in one area of Changsha City, Hunan Province, China.
Figure 2.

Bulk areca nut samples from India analyzed in this study (scaled individually to fit the figure).
Moisture content and pH
The measurement of moisture content and pH were performed by following the previously reported method.19 Moisture was determined gravimetrically, via the difference in weight of areca nut sample before and after its drying for 3 h in a heating block set at 99 °C. To measure pH, ~ 200 mg of ground sample was mixed with 5 ml HPLC-grade H2O, extracted at room temperature for 1 h, and centrifuged to pellet areca nut particles. The pH of the aqueous extract was measured with a pH meter.
Sample preparation
Samples were ground into a fine powder using a small coffee grinder (Krups F203), and 400 mg of the powder were extracted with 2 mL of deionized water at room temperature for 1 h with intermittent shaking every 10 min. The areca nut particles were pelleted by centrifugation at 13,000 rpm for 5 min and the supernatant was transferred to a new pre-labeled vial. Samples for LC-MS/MS analysis were prepared by adding 50 ng each of arecaidine-D5 and arecoline-D5 internal standards to the 10 μL of areca nut extract and diluting it to 1000 μL with 1% trifluoroacetic acid (TFA). Eight microliters of the sample was injected into LC-MS/MS for analysis.
Extraction with artificial saliva
To evaluate the potential changes in alkaloid extraction efficiency by salivary components, select product samples were also extracted by using artificial saliva prepared as described by Pappas et al.20 Following the extraction, 10 μL of the salivary extract was mixed with 10 μL of acetonitrile in a 2.0 mL microcentrifuge tube and vortexed vigorously. This was followed by the addition of internal standards (50 ng each), dilution of the mix to 1000 μL with 1% TFA, and centrifugation at 13,000 rpm for 5 min to pellet precipitated proteins. The supernatant was then transferred to LC-MS/MS vials for analysis.
Analysis of alkaloids by LC-MS/MS
Alkaloid analysis was performed on a TSQ Vantage mass spectrometer (Thermo Scientific, Waltham, MA) coupled with an Eksigent nanoLC-ultra 2D HPLC (Dublin, CA). The column used for chromatographic separation was a 150 mm × 0.5 mm i.d., 5 μm, Zorbax SB C18 (Agilent, Santa Clara, CA). Water containing 0.1% formic acid (solvent A) and methanol containing 0.1% formic acid (solvent B) were used as mobile phase at a flow rate of 15 μL/min. A linear gradient from 2-95% solvent B was used over 12 min for the alkaloid elution, then the mobile phase was returned to initial condition (2% solvent B) over 1 min and the column was equilibrated at this condition for 7 min before injecting the next sample. The mass spectrometer with electrospray ionization source was set in the positive ion mode with selective reaction monitoring (SRM). The quantitation and confirmation m/z transitions were monitored for the guvacine, guvacoline, arecoline, arecaidine, arecoline-D5 and arecaidine-D5 which are summarized in Table 1 with their respective collision energy. The collision gas (Ar) pressure was set at 1.4 mTorr. Quadrupoles Q1 and Q3 were operated at 0.4 Da mass resolutions. The scan width was set at 0.4 Da with scan time of 0.10 s. The heated capillary temperature was maintained at 350 °C, and the spray voltage was 3500 V.
Table 1.
SRM Transitions for Areca Nut Alkaloids and Internal Standards
| analyte | transition (m/z) |
collision energy (eV) |
internal standard | transition (m/z) |
collision energy (eV) |
|---|---|---|---|---|---|
| guvacine, 1 | 128 → 99a | 15 | arecaidine-D5 | 147 → 48a 147 → 100 |
20 15 |
| 128 → 110 | 12 | ||||
| arecaidine, 2 | 142 → 44a | 20 | |||
| 142 → 99 | 15 | ||||
| guvacoline, 3 | 142 → 113a | 14 | arecoline-D5 | 161 → 48a 161 → 114 |
15 15 |
| 142 → 81 | 20 | ||||
| arecoline, 4 | 156 → 44a | 15 | |||
| 156 → 113 | 15 |
Quantitation transition
Method characterization
Characteristics of the developed method were established using standard solutions of areca nut alkaloids. Arecaidine-D5 and arecoline-D5 were used as internal standards to account for the variability in instrument responses. Due to unavailability of isotope labeled guvacine and guvacoline, arecaidine-D5 was used as an internal standard for guvacine and arecoline-D5 for guvacoline. Accuracy of the method was tested by injecting the different known concentrations of alkaloids in the 0.1 to 500 pg/μL range and back calculating the concentration by using peak area ratios of analyte to internal standard and the external calibration curve. Average percent accuracy was then determined by dividing the calculated and the known concentrations. The precision of the method for each analyte was determined by injecting them at their limit of quantitation (LOQ) concentration in triplicates and determining the coefficient of variation of the three measurements. The LOQ was established as the lowest level of each alkaloid that produced less than 15 percent coefficient of variation whereas the lowest level that produced a distinct signal but greater than 15% CV was set as the limit of detection (LOD).
Statistical analyses
Standard statistics such as means, standard deviations, and paired t-test (for the comparison of alkaloid levels between the salivary and the aqueous extracts) were computed using Sigma Plot v.12.5 (Systat Software).
RESULTS and discussion
Method Development
While a number of methodologies have been used previously for the analysis of areca nut alkaloids, including liquid chromatography or capillary zone electrophoresis with UV detection,14, 21 LC-MS,22 LC-MS/MS,23 and GC-MS.24, 25 Most of these methods offered inadequate analyte selectivity for the analysis of complex extract of areca nut products, while the previously reported LC-MS/MS method has been used to analyze only arecoline and arecaidine, but not other areca alkaloids. The novelty of our developed LC-MS/MS consists of the ability to analyze all four alkaloids in a single run with high selectivity and sensitivity.
A representative LC-MS/MS chromatogram of all four alkaloids and the two internal standards is shown in Figure 3. The presence of highly polar –COOH group in guvacine and arecaidine lead to their early elution at around 3.8 min whereas esterification of –COOH group in guvacoline and arecoline leads to their better retention on the column with elution at around 4.9 min. Due to structural similarity of guvacine with arecaidine and guvacoline with arecoline, which only differ by the methyl group at the pyridine nitrogen, it was not possible to separate their elution times. However, because of different precursor ions and fragmentation patterns there was no interference in their respective analysis. The precursor ion of guvacine, m/z 128 ([M+H]+), produced product ions m/z 110 and m/z 99 due to the loss of H2O and CH3N, respectively, whereas arecaidine with the same retention time had precursor [M+H]+ ion m/z 142 and product ions m/z 99 and m/z 44 due to the loss of C2H5N and C5H6O2, respectively. Similarly, guvacoline with precursor [M+H]+ ion m/z 142 produced product ions m/z 113 and m/z 81 from the loss of CH3N and C2H5O2, respectively, while arecoline with precursor [M+H]+ ion m/z 156 produced product ions m/z 113 and m/z 44 resulting from the loss of C2H5N and C6H8O2, respectively. The internal standard arecaidine-D5 (m/z 147) had the same fragmentation as unlabeled arecaidine, producing product ions m/z 100 (loss of C2D4HN) and m/z 48 (loss of C5DH5O2). Similarly, arecoline-D5 (m/z 161) had the same fragmentation pattern as arecoline, producing product ions m/z 114 (loss of C2D4HN) and m/z 48 (loss of C6DH7O2). For each alkaloid, the precursor-product ion transition that produced higher peak intensity and better signal to noise ratio was used for the quantitation and the second one for the identity confirmation purpose (Table 1).
Figure 3.
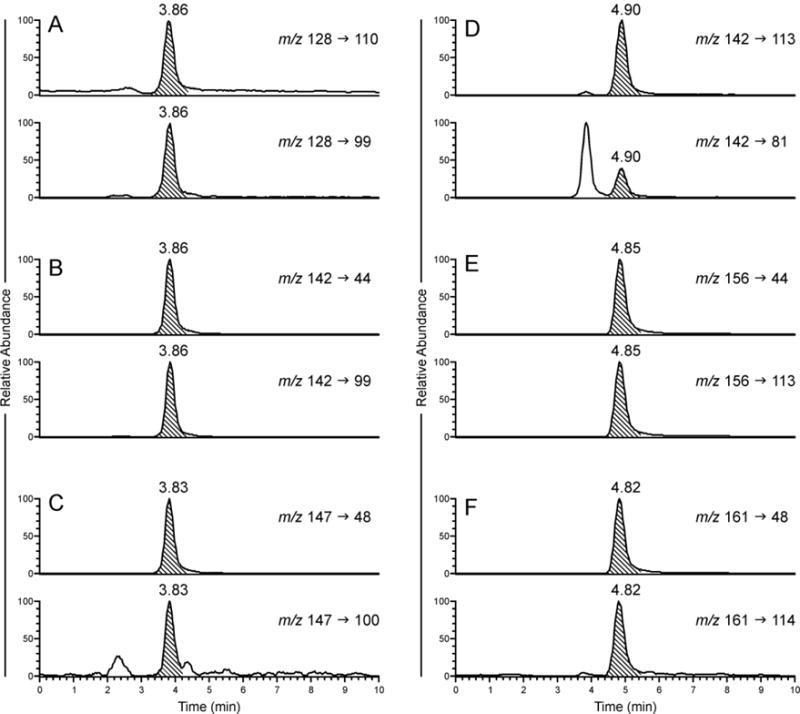
Typical LC-MS/MS chromatogram obtained upon analysis of Pan Parag (pan masala). Two transitions were monitored for each (A) guvacine, 1; (B) arecaidine, 2; (C) arecaidine-D5, 5; (D) guvacoline, 3; (E) arecoline, 4; and (F) arecoline- D5, 6.
Prior to applying the developed method to the analysis of various products, we optimized the extraction procedure to achieve its maximum efficiency. This was necessary primarily due to the variation in size of areca nut pieces in these products, which led to inconsistent extraction efficiency across samples. Therefore, the samples were ground into a fine powder to provide more uniformity in the extraction procedure. The effect of extraction duration and temperature was also investigated by using different product types. The optimal procedure (1 h incubation at room temperature) was selected for the alkaloid extraction as longer incubation and higher temperature did not produce significant change in the alkaloid yields.
Method characteristics
Accuracy, precision, LOQ, and LOD for each alkaloid are summarized in Table 2. All the four alkaloids exhibited good linearity in instrument response (R2 > 0.99) across 10-500 pg/μL concentration range with average accuracy between 98 to 104 percent. The LOQ for arecaidine and arecoline was 0.5 pg (on column) whereas for guvacine and guvacoline it was 250 and 5 pg (on column), respectively. Method precision at the LOQ of each alkaloid was less than 15% CV. The LOD was as low as 0.1 pg (on column) for arecaidine and arecoline, and 2.5 pg (on column) for guvacoline (Table 2). Guvacine showed a much higher LOD (50 pg on column), mainly due to its poor ionization efficiency and higher background noise in the low molecular weight transitions. Despite the higher LOD for guvacine, sensitivity of the method is excellent for all four alkaloids given the high levels at which they are present in areca nut-containing products.
Table 2.
Method Characteristics
| analyte | average accuracy (%) | precision, % CV (N = 3) |
LOQ pg (on column) | LOD pg (on column) | linearity (R2) |
|---|---|---|---|---|---|
| guvacine, 1 | 97.8 | 5.4 | 250 | 50 | 0.9993 |
| arecaidine, 2 | 103.5 | 14.2 | 0.5 | 0.1 | 0.9999 |
| guvacoline, 3 | 101.4 | 14.4 | 5.0 | 2.5 | 0.9983 |
| arecoline, 4 | 103.3 | 7.2 | 0.5 | 0.1 | 0.9961 |
Analysis of areca nut-containing products
Application of the developed method to the analysis of various areca nut-containing products revealed substantial variation in the levels of individual alkaloids across the products. The results expressed per dry weight product are summarized in Table 3. In all products, levels of guvacine, with the levels ranged from 1.39 to 8.16 mg/g dry weight, accounting for more than 50% of the total alkaloid content in most products. The levels of arecaidine ranged from 0.14 to 1.70 mg/g dry weight. The levels of arecoline and guvacoline ranged from 0.64 to 2.22 mg/g dry weight and 0.17 to 0.99 mg/g dry weight, respectively.
Table 3.
Alkaloid Levels Measured in Areca Nut-Containing Products
| product | pH | % moisture | alkaloids (mg/g product dry weight)
|
||||
|---|---|---|---|---|---|---|---|
| Guvacine 1 |
Arecaidine 2 |
Guvacoline 3 |
Arecoline 4 |
total alkaloids | |||
| dry areca nuts | |||||||
| sample 1 | 4.5 | 12.9 | 1.39 | 0.14 | 0.37 | 0.96 | 2.86 |
| sample 2 | 4.4 | 13.0 | 1.49 | 0.17 | 0.40 | 1.19 | 3.26 |
| sample 3 | 5.3 | 12.0 | 4.06 | 0.33 | 0.43 | 1.60 | 6.41 |
| sample 4 | 5.0 | 11.6 | 2.48 | 0.15 | 0.99 | 2.22 | 5.84 |
| sample 5 | 5.6 | 11.7 | 2.61 | 0.24 | 0.17 | 1.02 | 4.04 |
| sample 6 | 4.4 | 12.1 | 1.79 | 0.18 | 0.32 | 0.91 | 3.19 |
| sample 7 | 5.3 | 9.7 | 2.43 | 0.28 | 0.34 | 1.36 | 4.40 |
| sample 8 | 5.4 | 8.2 | 2.55 | 0.41 | 0.24 | 1.20 | 4.40 |
| sample 9 (roasted) | 5.3 | 8.9 | 8.16 | 0.42 | 0.35 | 0.98 | 9.91 |
|
| |||||||
| pan masala | |||||||
| Pan Parag | 8.5 | 7.9 | 2.60 | 1.04 | 0.19 | 0.64 | 4.48 |
| Pan Bahar | 8.8 | 7.8 | 3.23 | 1.70 | 0.30 | 1.03 | 6.25 |
| Rajnigandha | 9.2 | 7.2 | 2.56 | 1.26 | 0.24 | 0.79 | 4.85 |
| Rajshree | 9.4 | 6.1 | 2.10 | 0.95 | 0.25 | 1.25 | 4.55 |
| RMD | 8.6 | 8.4 | 3.50 | 1.68 | 0.40 | 0.94 | 6.51 |
| Vimal | 8.8 | 6.1 | 3.11 | 1.10 | 0.24 | 0.93 | 5.36 |
|
| |||||||
| gutkha | |||||||
| Tulsi Gutkha | 8.6 | 7.1 | 3.19 | 1.21 | 0.31 | 1.16 | 5.86 |
| Pan Parag Gutkha | 8.5 | 7.6 | 2.48 | 1.10 | 0.23 | 0.74 | 4.55 |
|
| |||||||
| Chinese areca nut | |||||||
| product 1 | 7.9 | 20.2 | 2.34 | 0.64 | 0.37 | 1.44 | 4.79 |
| product 2 | 8.3 | 17.9 | 4.49 | 1.46 | 0.18 | 1.07 | 7.20 |
| product 3 | 7.9 | 19.8 | 2.05 | 0.76 | 0.40 | 1.31 | 4.52 |
Factors such as the maturity of the nut, differences in its processing methods and storage conditions, and potentially the geographical location or other characteristics of the Areca catechu trees from which these particular samples of areca nut originated, could be responsible for the observed variations of alkaloid levels across products. The highest levels of guvacine were found in the roasted areca nut sample from India (sample #9) (Table 3, Figure 4); however, it is not clear whether this is due to the roasting process or other factors. Among other alkaloids, arecaidine levels vary most noticeably, approximately 12-fold, across products. Overall, higher levels of this alkaloid were present in pan masala and gutkha compared to the bulk areca nut samples (Figure 4). The high pH of pan masala and gutkha is most likely the major factor contributing to these differences, as it leads to the hydrolysis of methyl ester in arecoline to form arecaidine.9 However, the sum of arecoline and arecaidine is also higher in pan masala and gutkha than in the bulk areca nut samples (Figure 4), suggesting that other factors, for instance the processing of areca nut during the preparation of manufactured products, may play a role. Similarly, hydrolysis of guvacoline to guvacine is expected to take place in the high-pH manufactured products, which is supported by the generally lower guvacoline levels in pan masala and gutkha than in the bulk areca nut samples (Table 3). However, there are no noticeable differences in guvacine content between these two product types, potentially because of its relatively high levels and wide variability across individual samples. In the areca nut product from China, the effect of pH on alkaloid profile is more pronounced: product 2 had higher pH, higher guvacine and arecaidine, and lower guvacoline and arecoline, than the other two products, in agreement with the ester hydrolysis hypothesis. Overall, the sum of all four alkaloids varied from 2.86 to 9.91 mg/g dry weight, or more than 3.5-fold, in this limited set of products. Further studies are needed to better characterize the levels of these and other chemical constituents in the areca nut-containing product varieties used worldwide.
Figure 4.
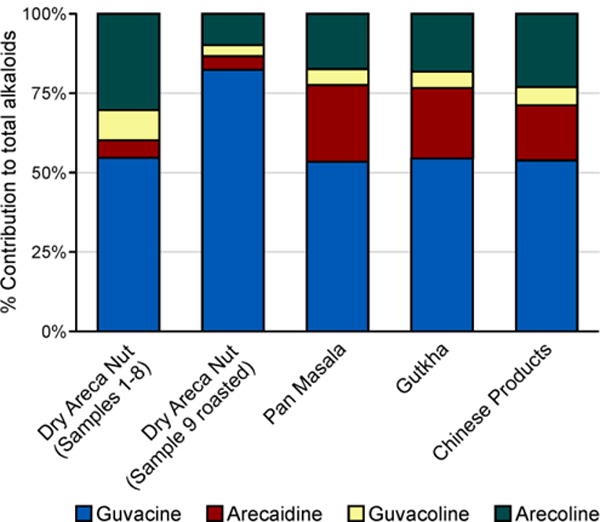
Comparison chart showing the relative contribution of individual alkaloids towards total alkaloid content in different areca nut products which are categorized based on the product type.
Our finding that guvacine is by far the most abundant among the four measured alkaloids, irrespective of the product type, is in contrast with the common assertion in the literature that arecoline is the major alkaloid in areca nut.1, 2, 26–28 This discrepancy can be due to the analyses of raw areca nut in earlier studies and the fact that the total amount and composition of alkaloids varies with the age of areca fruit. For instance, arecoline levels in the nut rise during the maturation period but drop significantly in the matured nut.29 In addition, Franke et al.22 recently analyzed the aqueous extract of young and mature areca nuts and found significant differences in the total alkaloids and relative levels of individual alkaloids between them. Lower level of total alkaloids was observed in the young green nut compared to the mature nut, with arecoline being the major alkaloid. In the mature nut, however, guvacine was the major alkaloid with almost 3-fold higher concentration than arecoline, which is consistent with our data. These observations suggest that the alkaloid profile, i.e. the relative contribution of individual alkaloids to the total alkaloid content, can potentially be used as a tool in product surveillance studies to identify the similarities or differences in characteristics of various areca nut-containing products.
Impact of saliva and tobacco on alkaloid extraction
Given that areca nut-containing products are used orally, and saliva is an enzyme- and electrolyte-rich complex mixture, it is possible that the efficiency of areca alkaloid extraction with saliva may be different from that with water. Our experiment with artificial saliva extraction demonstrated an inconsistent effect on the measured alkaloid levels in various products. Products that share common features (i.e. bulk areca nut pieces, manufactured mixes, and manufactured areca nut from China) exhibited generally similar patterns of the major differences in alkaloid levels between aqueous and salivary extracts, suggesting that product composition plays an important role (Figure 5). Salivary extracts of the bulk areca nut pieces obtained from India contained similar levels of guvacine and arecaidine (p > 0.05 for both alkaloids), but lower levels of guvacoline and arecoline (p = 0.006 and p = 0.007, respectively) than the aqueous extracts of the same products. Salivary extraction of pan masala and gutkha led to higher levels of guvacine as compared to the aqueous extracts of the same products (p < 0.001), while the levels of other alkaloids were not affected. In Chinese manufactured areca nut samples, the decrease in guvacine and guvacoline levels in saliva extracts was not statistically significant, potentially due to the small number of samples, and there was no observable change in the levels of arecaidine or arecoline (p > 0.05 for all comparisons). The observed variations stress the need for biomarker-based studies of areca alkaloid exposures, including salivary measurements, to better understand how the variability of product formulations affects constituent extraction and uptake in users.
Figure 5.
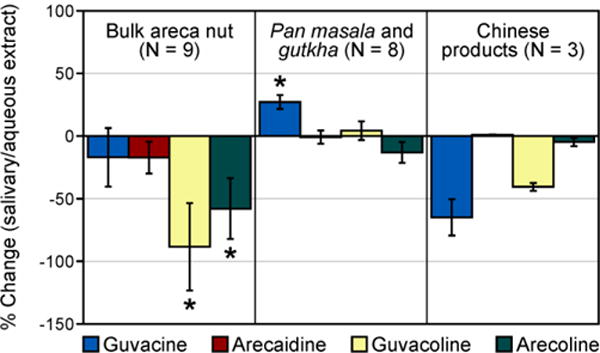
Changes in individual alkaloid levels extracted with artificial saliva expressed as % of the levels measured in aqueous extracts of the same products. Error bars represent standard deviations of the percent change among different products of the same type.
*Statistically significant changes (p < 0.05)
Another potential consumer-driven factor that may affect exposures to areca nut constituents is the concomitant use of areca nut-containing products with other products, such as tobacco. For instance, while pan masala is prepared without the addition of tobacco, tobacco sachets of the same brand are often being manufactured and sold to be mixed with pan masala before use by consumers.30 We analyzed three samples of pan masala with available tobacco sachets of the same brand: Rajshree, RMD, and Vimal. When expressed per gram of pan masala, the levels of alkaloids extracted with or without the addition of tobacco were similar. This lack of tobacco effect on the extracted levels of areca alkaloids is consistent with the very similar levels of all four alkaloids between pan masala Pan Parag and gutkha of the same brand (Table 3). However, it should be noted that tobacco itself is a rich source of carcinogens, including the tobacco-specific potent oral and esophageal carcinogen N′-nitrosonornicotine.31 Therefore, addition of tobacco to areca nut-containing formulations increases total carcinogenic exposures in users.
Summary
A large variety of products containing different types of areca nut and other ingredients are being sold and consumed by hundreds of millions of users worldwide. Areca alkaloids guvacine, arecaidine, guvacoline, and arecaline are implicated in the addictiveness of such products, as well as in the adverse health outcomes associated with their use.2, 3 In this study, we have developed a robust and accurate method for the analysis of these important constituents and for the first time characterized their levels in a variety of product types and formulations obtained from India, China, and USA. Despite the limited number of samples analyzed here, this study represents the first important step towards characterizing the chemical diversity and the associated addictive and carcinogenic potency of various areca nut-containing products. Given the detrimental health outcomes associated with areca nut use and the high number of users worldwide, there is an urgent need to expand such studies. Future work should also incorporate biomarker-based measures of areca nut constituent exposure and effect.
Supplementary Material
Acknowledgments
We thank Xun Ming and Peter Villalta for their help with the mass spectrometry analysis, and Bob Carlson for editorial assistance.
Funding Sources
This study was supported by the contract HHSN261201200392P and grant CA180880 from the National Cancer Institute, grant K23DE023572 from the National Institute of Dental and Craniofacial Research, and by startup funds to IS from the Masonic Cancer Center. LC-MS/MS was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, supported in part by grant CA-77598 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge via the Internet at http://pubs.acs.org. Figure S1a and S1b show LC-MS/MS chromatogram of standard mix of guvacine with guvacoline and arecaidine with arecoline, respectively. Figure S2 shows the linearity of the LC-MS/MS method. Table S1 contains the data on alkaloid levels in salivary extracts and Table S2 the data on the levels of alkaloids extracted with or without the addition of tobacco.
Final version of this manuscript is available online at https://www.ncbi.nlm.nih.gov/pubmed/28190359
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 37. IARC; Lyon, FR: 1985. Tobacco Habits Other than Smoking: Betel-Quid and Areca-Nut Chewing; and Some Related Nitrosamines; pp. 37–202. [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Personal Habits and Indoor Combustions. 100E. IARC; Lyon, FR: 2012. Betel Quid and Areca Nut; pp. 333–372. [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 4.Winstock A. Areca nut-abuse liability, dependence and public health. Addict Biol. 2002;7:133–138. doi: 10.1080/13556210120091509. [DOI] [PubMed] [Google Scholar]
- 5.Garg A, Chaturvedi P, Gupta PC. A review of the systemic adverse effects of areca nut or betel nut. Indian J Med Paediatr Oncol. 2014;35:3–9. doi: 10.4103/0971-5851.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu NS. Effects of betel chewing on the central and autonomic nervous systems. J Biomed Sci. 2001;8:229–236. doi: 10.1007/BF02256596. [DOI] [PubMed] [Google Scholar]
- 7.Thomas S, Kearsley J. Betel quid and oral cancer: a review. Eur J Cancer B Oral Oncol. 1993;29B:251–255. doi: 10.1016/0964-1955(93)90044-f. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SJ, Bain CJ, Battistutta D, Ness AR, Paissat D, Maclennan R. Betel quid not containing tobacco and oral cancer: a report on a case-control study in Papua New Guinea and a meta-analysis of current evidence. Int J Cancer. 2007;120:1318–1323. doi: 10.1002/ijc.22304. [DOI] [PubMed] [Google Scholar]
- 9.Sharan RN, Mehrotra R, Choudhury Y, Asotra K. Association of betel nut with carcinogenesis: revisit with a clinical perspective. PLoS One. 2012;7:e42759. doi: 10.1371/journal.pone.0042759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One. 2014;9:e113385. doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 85. IARC; Lyon, FR: 2004. Betel-quid and Areca-nut Chewing and Some Areca-nut-derived N-Nitrosamines; pp. 41–300. [PMC free article] [PubMed] [Google Scholar]
- 12.Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 13.Dave BJ, Trivedi AH, Adhvaryu SG. In vitro genotoxic effects of areca nut extract and arecoline. J Cancer Res Clin Oncol. 1992;118:283–288. doi: 10.1007/BF01208617. [DOI] [PubMed] [Google Scholar]
- 14.Huang JL, McLeish MJ. High-performance liquid chromatographic determination of the alkaloids in betel nut. J Chromatogr A. 1989;475:447–450. [Google Scholar]
- 15.Tsai CH, Chou MY, Chang YC. The up-regulation of cyclooxygenase-2 expression in human buccal mucosal fibroblasts by arecoline: a possible role in the pathogenesis of oral submucous fibrosis. J Oral Pathol Med. 2003;32:146–153. doi: 10.1034/j.1600-0714.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 16.Sundqvist K, Liu Y, Nair J, Bartsch H, Arvidson K, Grafstrom RC. Cytotoxic and genotoxic effects of areca nut-related compounds in cultured human buccal epithelial cells. Cancer Res. 1989;49:5294–5298. [PubMed] [Google Scholar]
- 17.Nair J, Ohshima H, Friesen M, Croisy A, Bhide SV, Bartsch H. Tobacco-specific and betel nut-specific N-nitroso compounds: occurrence in saliva and urine of betel quid chewers and formation in vitro by nitrosation of betel quid. Carcinogenesis. 1985;6:295–303. doi: 10.1093/carcin/6.2.295. [DOI] [PubMed] [Google Scholar]
- 18.Prokopczyk B, Rivenson A, Bertinato P, Brunnemann KD, Hoffmann D. 3-(Methylnitrosamino)propionitrile: occurrence in saliva of betel quid chewers, carcinogenicity, and DNA methylation in F344 rats. Cancer Res. 1987;47:467–471. [PubMed] [Google Scholar]
- 19.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tobacco Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 21.Lord GA, Lim CK, Warnakulasuriya S, Peters TJ. Chemical and analytical aspects of areca nut. Addict Biol. 2002;7:99–102. doi: 10.1080/13556210120091455. [DOI] [PubMed] [Google Scholar]
- 22.Franke AA, Mendez AJ, Lai JF, Arat-Cabading C, Li X, Custer LJ. Composition of betel specific chemicals in saliva during betel chewing for the identification of biomarkers. Food Chem Toxicol. 2015;80:241–246. doi: 10.1016/j.fct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu CW, Chang YZ, Wang HW, Chao MR. High-throughput simultaneous analysis of five urinary metabolites of areca nut and tobacco alkaloids by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Cancer Epidemiol Biomarkers Prev. 2010;19:2570–2581. doi: 10.1158/1055-9965.EPI-10-0483. [DOI] [PubMed] [Google Scholar]
- 24.Self R, Jones R, Holdworth D. Gas chromatography/mass spectrometry analysis of alkaloids in betel nut (Areca catechu) Eur J Mass Spectrom. 1999;5:213. [Google Scholar]
- 25.Hocart CH, Frankhauser B. Betel nut residues in archaeological samples of human teeth from the Mariana Islands. Experientia. 1996;52:281–285. [Google Scholar]
- 26.Chang BE, Liao MH, Kuo MY, Chen CH. Developmental toxicity of arecoline, the major alkaloid in betel nuts, in zebrafish embryos. Birth Defects Res A Clin Mol Teratol. 2004;70:28–36. doi: 10.1002/bdra.10136. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YS, Lee KW, Huang JL, Liu YS, Juo SH, Kuo WR, Chang JG, Lin CS, Jong YJ. Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology. 2008;249:230–237. doi: 10.1016/j.tox.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Shih YT, Chen PS, Wu CH, Tseng YT, Wu YC, Lo YC. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic Biol Med. 2010;49:1471–1479. doi: 10.1016/j.freeradbiomed.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Wang C-K, Lee W-H, Peng C-H. Contents of phenolics and alkaloids in areca catechu linn. during maturation. J Agric Food Chem. 1997;45:1185–1188. [Google Scholar]
- 30.Nair S, Schensul JJ, Bilgi S, Kadam V, D’Mello S, Donta B. Local responses to the Maharashtra gutka and pan masala ban: a report from Mumbai. Indian J Cancer. 2012;49:443–447. doi: 10.4103/0019-509X.107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


