SUMMARY
Background
Tuberculosis (TB) screening in prevention of mother-to-child transmission (PMTCT) programs is important to improve TB detection, prevention and treatment.
Methods
As part of a national PMTCT program evaluation, mother-infant pairs attending 6-week and 9-month immunization visits were enrolled at 141 maternal and child health clinics throughout Kenya. Clinics were selected using population-proportion-to-size sampling with oversampling in a high HIV prevalence region. The World Health Organization (WHO) TB symptom screen was administered to HIV-infected mothers and associations with infant cofactors were determined.
Results
Among 498 HIV-infected mothers, 165 (33%) had a positive TB symptom screen. Positive maternal TB symptom screen was associated with prior TB (p=0.04). Women with a positive TB symptom screen were more likely to have an infant with HIV infection (p=0.02) and non-specific TB symptoms, including cough (p=0.003), fever (p=0.05), and difficulty breathing (p=0.01). TB exposure was reported by 11% of women, and 15% of TB-exposed women received isoniazid preventive therapy.
Conclusions
Postpartum HIV-infected mothers frequently had a positive TB symptom screen. Mothers with a positive TB symptom screen were more likely to have infants with HIV or non-specific TB symptoms. Integration of maternal TB screening and prevention into PMTCT programs may improve maternal and infant outcomes.
Keywords: Tuberculosis screening, PMTCT, HIV
INTRODUCTION
Tuberculosis (TB) is a leading cause of morbidity and mortality among women of childbearing age, and maternal TB during pregnancy and postpartum results in poor maternal and infant outcomes.1–4 The World Health Organization (WHO) recommends routine TB symptom screening (fever, cough, night sweats and weight loss) among HIV-infected individuals because of their increased risk of TB.5 The TB symptom screen is useful to guide clinical evaluation for active TB among those with symptoms and provision of isoniazid preventive therapy (IPT) among patients with a negative symptom screen.5 Integration of TB screening into prevention of mother-to-child-transmission (PMTCT) programs has been proposed as an effective way to improve TB detection and prevention services for both mothers and infants, but has not been widely implemented.6
TB screening and prevention should optimally occur during both antenatal and postnatal services; however, there are no evidence-based models for when and how to integrate maternal TB screening. In research cohorts in high HIV and TB prevalence settings, approximately 20% of HIV-infected pregnant women had a positive TB symptom screen, among whom ~5% had active TB disease.7–9 Fewer studies have assessed postpartum TB screening at child immunization visits, which may be another opportunity for TB detection and prevention, particularly for HIV-exposed infants.10 Within a national survey of mother-infant pairs, we assessed the prevalence and correlates of postpartum TB symptoms and TB exposure among HIV-infected mothers at 6-week and 9-month infant immunization visits, in order to inform future scale-up of TB screening and prevention services within PMTCT programs.
METHODS
Study setting
This study was nested within two surveys of the PMTCT program within the maternal child health (MCH) services in Kenya conducted between June and December 2013. The National PMTCT-MCH Survey used probability proportionate to size sampling to select 120 medium (>500 annual antenatal clinic visits) and large volume (>1,000 annual antenatal clinic visits) maternal-child health (MCH) clinics within seven of the 8 geographic regions of Kenya. All mother-infant pairs attending either 6-week or 9-month infant immunization visits were sampled during a single 5-day period. The PMTCT-Nyanza Survey purposively oversampled HIV-infected women and their infants in 30 large volume MCH clinics in Nyanza province, a region of high HIV prevalence, during a single 10-day period. Overall, 141 unique facilities were visited by a mobile study team for both surveys (Figure 1).
Figure 1.
Maternal and child health clinics surveyed across Kenya. Black circles indicate the National PMTCT-MCH Survey and white circles indicate the PMTCT-Nyanza Survey.
Participants
For this nested analysis, we included data from HIV-infected women and their infants from the National PMTCT-MCH Survey and the PMTCT-Nyanza Survey. Women who were not willing or able to provide informed consent for the study were excluded.
Procedures
After obtaining informed consent, study staff administered a study questionnaire that included questions about sociodemographic information, HIV history (use of combination antiretroviral therapy (cART), CD4 count, use of co-trimoxazole prophylaxis), prior clinical TB history, TB symptoms, TB exposure in the past 12 months, and use of IPT. TB symptom screening included the WHO 4-part symptom screen (fever, cough, weight loss, night sweats)5, as well as other TB symptoms including prolonged cough or fever (>2 weeks), fatigue, anorexia, and hemoptysis. The WHO TB symptom screen was considered positive if a participant reported one or more of the four symptoms. Questions on infant health included sex, birth weight, HIV status, use of antiretroviral prophylaxis, BCG vaccination history, breastfeeding, nonspecific TB symptoms (cough, fever, difficulty breathing, anorexia), and history of hospitalization. Study nurses obtained infant anthropometric measurements (weight and height, and collected a blood sample by heel prick (0.5 ml) for HIV DNA PCR testing. HIV DNA PCR testing was performed at the Centers for Disease Control (CDC)-Kenya Medical Research Institute (KEMRI) HIV research laboratory in Kisumu, Kenya.
National Guidelines
Kenyan National TB guidelines recommend that all HIV-infected patients receive TB symptom screening as part of intensified TB case finding.11 Patients with a positive TB symptom screen are referred for further evaluation, including sputum smear microscopy for acid fast bacilli (AFB), sputum GeneXpert MTB/RIF (where available) and chest X-ray. Our study did not collect data from study participants on results of these evaluations. WHO and Kenyan National TB guidelines recommend provision of IPT for HIV-infected patients >12 months of age with a negative TB symptom screen, HIV-infected children <12 months of age who have had recent contact with active TB disease with no evidence of TB, and for all children <5 years irrespective of HIV status who have had recent close contact with a case of smear positive TB.11, 12 However, these guidelines were not widely implemented during the study period.
Statistical Analysis
Statistical analysis was performed using STATA software, version 13 (StataCorp, College Station, Texas). Maternal and infant characteristics at the 6-week and 9-month study visits were described using proportions or medians. Maternal cofactors of maternal positive WHO TB symptom screen were evaluated using univariate logistic regression, with each cofactor as the predictor variable and maternal positive WHO TB symptom screen as the outcome variable. Infant cofactors of maternal positive WHO TB symptom screen were evaluated using univariate logistic or linear regression as appropriate, with maternal positive WHO TB symptom screen as the predictor variable and each cofactor as the outcome variable. All analyses were adjusted to account for clustering within clinics.
Human subjects approval
This study was approved by ethical review boards at the University of Washington, Kenya Medical Research Institute, and the Centers for Disease Control and Prevention.
RESULTS
Of 2,819 women attending 6-week or 9-month immunization visits, 498 were HIV-infected and included in this analysis; 200 women participated in the National PMTCT-MCH Survey and 298 women participated in the PMTCT-Nyanza Survey. Data were available for 260 HIV-infected mothers at the 6-week immunization visit and 238 mothers from the 9-month immunization visit.
Women were a median age of 28 years (Interquartile Range [IQR] 24–32) and had a median of 8 years education (IQR 7–11). Most women were taking cART (63%) and median CD4 count was 483 cells/mm3 (IQR 346–643 cells/mm3). Eleven percent of women had a history of TB, a median of 4 years (IQR 2–6) prior to enrollment. Eleven percent (52/484) of women reported exposure to a person with active TB in the past year, and 15% (8/52) of TB-exposed women reported taking IPT (Table 1).
Table 1.
Characteristics of HIV+ women and their infants
| 6 weeks Postpartum N=260§ |
9 months Postpartum N=238§ |
Overall N=498§ |
||||
|---|---|---|---|---|---|---|
| n or Median | (% or IQR) | n or Median | (% or IQR) | n or Median | (% or IQR) | |
| Mothers | ||||||
| Age (years) | 27 | (24–32) | 28 | (24–33) | 28 | (24–32) |
| Years of education | 8 | (7–11) | 8 | (7–12) | 8 | (7–11) |
| Number of people in home | 4 | (4–5) | 5 | (4–6) | 4 | (4–6) |
| Partner HIV positive | 112/158 | (70.9) | 116/164 | (70.7) | 228/322 | (70.8) |
| CD4 count (cells/mm3) | 430α | (324–576) | 550ε | (368–720) | 483θ | (346–643) |
| Combination ART | 168 | (64.6) | 145 | (60.9) | 313 | (62.9) |
| Cough (past 24 hours) | 47 | (18.1) | 47 | (19.7) | 94 | (18.9) |
| Cough > 2 weeks | 13 | (5.0) | 18 | (7.6) | 31 | (6.2) |
| Fever | 43 | (16.5) | 29 | (12.2) | 72 | (14.5) |
| Fever > 2 weeks | 12 | (4.6) | 11 | (4.6) | 23 | (4.6) |
| Weight loss | 28 | (10.8) | 26 | (10.9) | 54 | (10.8) |
| Night sweats | 29/259 | (11.2) | 16 | (6.7) | 45/497 | (9.1) |
| Fatigue | 70/259 | (27.0) | 53 | (22.3) | 123/497 | (24.7) |
| Anorexia | 51 | (19.6) | 47 | (19.7) | 98 | (19.7) |
| Hemoptysis | 0/256 | (0.0) | 1/236 | (0.4) | 1/492 | (0.2) |
| Positive WHO TB symptom screen* | 82 | (31.5) | 83 | (34.9) | 165 | (33.1) |
| Any TB symptom** | 123 | (47.3) | 109 | (45.8) | 232 | (46.6) |
| History of TB | 24 | (9.2) | 31 | (13.0) | 55 | (11.0) |
| Years since TB | 4.5β | (2.5–6.5) | 3ζ | (2–5) | 4ι | (2–6) |
| TB exposure | 33/256 | (12.9) | 19/228 | (8.3) | 52/484 | (10.7) |
| Isoniazid preventive therapy | 6/33 | (18.2) | 2/19 | (10.5) | 8/52 | (15.4) |
| Infants | ||||||
| Sex (male) | 131 | (50.4) | 113/238 | (47.5) | 244 | (49.0) |
| Birth weight (kg) | 3.1γ | (2.8–3.5) | 3.3η | (2.8–3.5) | 3.2κ | (2.8–3.5) |
| BCG vaccination | 256/259 | (98.8) | 237 | (99.6) | 493/497 | (99.2) |
| BCG scar | 214/259 | (82.6) | 235 | (98.7) | 449/497 | (90.3) |
| Breastfed (primarily) | 245/254 | (96.5) | 38 | (16.2) | 283/489 | (57.9) |
| Weight-for-age Z score | −0.37δ | (−1.1–0.42) | −0.32 | (−1.2–0.64) | −0.32λ | (−1.2–0.51) |
| Received antiretroviral prophylaxis | 251 | (96.5) | 232 | (97.5) | 483 | (97) |
| HIV infected | 12/251 | (4.8) | 10/213 | (4.7) | 22/464 | (4.7) |
| Hospitalization | 8 | (3.1) | 27 | (11.3) | 35 | (7.0) |
| Tuberculosis | 0/8 | (0.0) | 0/27 | (0.0) | 0/35 | (0.0) |
| Cough | 65/259 | (25.0) | 94 | (39.5) | 159/497 | (31.9) |
| Fever | 38/259 | (14.7) | 73 | (30.7) | 111/497 | (22.3) |
| Difficulty breathing | 27 | (10.4) | 22 | (9.2) | 49 | (9.8) |
| Anorexia | 6 | (2.3) | 30 | (12.6) | 36 | (7.2) |
WHO TB symptom screen defined as any cough, fever, weight loss or night sweats reported
Any TB symptom: to include any cough, fever, weight loss, night sweats, fatigue, anorexia, or hemoptysis
Total N, except where otherwise noted;
N=153;
N=24;
N=220;
N=259;
N=167;
N=31;
N=200;
N=320;
N=55;
N=420;
N=497
Of infants, 49% were male and median birth weight was 3.2 kg (IQR 2.8–3.5 kg). Nearly all infants were BCG-vaccinated (99%), and most (90%) had a BCG scar. Five percent of infants were HIV-infected, and 58% of infants were breastfed. Most infants (97%) received antiretroviral prophylaxis. Median weight-for-age Z score was −0.32 (IQR −1.19–0.51). Seven percent of infants had been hospitalized; none were hospitalized for TB. Nonspecific symptoms that could be TB-related were prevalent among infants, including cough (32%), fever (22%), difficulty breathing (10%), and anorexia (7%). Infants at 9 months of age had a higher prevalence of cough (40% vs 25%, p=0.007), fever (31% vs 15%, p<.001), and anorexia (13% vs 2%, p<.001) compared to infants at 6 weeks of age (Table 1).
Thirty-three percent of women had a positive WHO TB symptom screen, reporting either current cough, fever, weight loss or night sweats. The most prevalent WHO TB symptom was cough (19%), followed by fever (14%), weight loss (11%), and night sweats (9%). Positive maternal WHO TB symptom screen was associated with household crowding (≥5 vs. ≤4 people in home) [OR=1.14 (1.00–1.29), p=0.05] and history of TB disease [OR=1.66 (1.03–2.68), p=0.04] but not with partner HIV status or reported TB exposure. Women with a positive WHO TB symptom screen had a lower median CD4 count (454 vs 498, p=0.06). Infants born to a mother with a positive WHO TB screen were more likely to have HIV infection (7.6% vs. 2.8%, p=0.02). This association remained significant in multivariate analyses adjusted for maternal cART and infant antiretroviral prophylaxis [OR 3.16 (1.36–7.37), p=0.008]. Mothers with a positive WHO TB screen were more likely to have infants with nonspecific TB symptoms, including cough (44% vs 26%, p=.003), fever (30% vs 19%, p=0.05), difficulty breathing (16% vs 7%, p=0.01) and anorexia (11% vs 5%, p=0.005) (Table 2). Our findings were consistent in a multivariable model adjusting for geographic region and clinic size.
Table 2.
Correlates of maternal WHO TB screen positivity among HIV+ women
| Maternal WHO TB Screen | |||||||
|---|---|---|---|---|---|---|---|
| Negative N=333§ |
Positive N=165§ |
Univariate Analysis | |||||
|
| |||||||
| Correlate | n or Median | (% or IQR) | n or Median | (% or IQR) | Coefficient or ORι | (95% CI) | p^ |
| Mothers | |||||||
| Age (years) | 28 | (24–32) | 28 | (24–33) | 1.01 | (0.97–1.04) | 0.74 |
| Years of education | 8 | (7–12) | 8 | (7–10) | 0.97 | (0.91–1.03) | 0.28 |
| Number of people in home | 4 | (4–6) | 5 | (4–6) | 1.14 | (1.00–1.29) | 0.05 |
| Partner HIV positive | 154/215 | (71.6) | 74/107 | (69.2) | 0.88 | (0.49–1.60) | 0.68 |
| CD4 count (cells/mm3) | 498 α | (348–668) | 454ε | (339–603) | 1.00 | (1.00–1.00) | 0.06 |
| Combination ART | 214 | (64.3) | 99 | (60.0) | 0.83 | (0.56–1.24) | 0.37 |
| History of TB | 31 | (9.3) | 24 | (14.5) | 1.66 | (1.03–2.68) | 0.04 |
| Years since TB | 3β | (2–5) | 4ζ | (3–6) | 1.18 | (0.97–1.43) | 0.09 |
| TB exposure | 32/328 | (9.8) | 20/156 | (12.8) | 1.36 | (0.72–2.56) | 0.34 |
| Isoniazid preventive therapy | 6/32 | (18.8) | 2/20 | (10.0) | 0.48 | (0.04–5.49) | 0.55 |
| Infants | |||||||
| Sex (male) | 160 | (48.0) | 84 | (50.9) | 0.89 | (0.64–1.25) | 0.50 |
| Birth weight (kg) | 3.2γ | (2.8–3.5) | 3.25η | (2.8–3.5) | +0.04 | (−0.08–0.16) | 0.53 |
| BCG vaccination | 329/332 | (99.1) | 164 | (99.4) | 1.50 | (0.15–14.74) | 0.73 |
| BCG scar | 305/332 | (91.9) | 144 | (87.3) | 0.61 | (0.31–1.19) | 0.15 |
| HIV infected | 9/319 | (2.8) | 12/158 | (7.6) | 2.83 | (1.16–6.90) | 0.02 |
| Breastfed (primarily) | 189/327 | (57.8) | 94/162 | (58.0) | 1.00 | (0.70–1.47) | 0.96 |
| Weight-for-age Z score | −0.27δ | (−1.12–0.51) | −0.53θ | (−1.22–0.46) | −0.09 | (−0.35–0.17) | 0.50 |
| Hospitalization | 18 | (5.4) | 17 | (10.3) | 2.01 | (0.84–4.80) | 0.12 |
| Tuberculosis | 0/18 | (0.0) | 0/17 | (0.0) | Not run | ||
| Cough | 86/332 | (25.9) | 73 | (44.2) | 2.27 | (1.34–3.84) | 0.003 |
| Fever | 62/332 | (18.7) | 49 | (29.7) | 1.84 | (0.99–3.40) | 0.05 |
| Difficulty breathing | 23/332 | (6.9) | 26 | (15.8) | 2.51 | (1.23–5.11) | 0.01 |
| Anorexia | 17/332 | (5.1) | 19 | (11.5) | 2.41 | (1.30–4.46) | 0.005 |
Total N, except where otherwise noted;
N=216;
N=31;
N=279;
N=332;
N=104;
N=24;
N=141;
N=165;
OR, Odds ratio;
p value by logistic or linear regression as appropriate.
DISCUSSION
In this national survey of 141 MCH clinics, we found that a third (33%) of postpartum HIV-infected women had a positive WHO TB symptom screen at infant immunization visits. Eleven percent of HIV-infected women reported known TB exposure, of whom few received IPT.
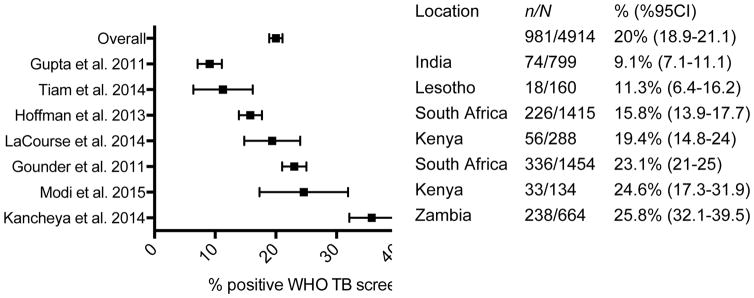
The prevalence of WHO TB screen positivity among postpartum HIV-infected women in this study (33%) was higher than most prior studies in pregnant HIV-infected women (~20%) (Figure 2).7–9, 13–16 TB symptoms may be less frequent in pregnancy than postpartum due to immunologic and physiologic changes.17, 18 Zenner et al. observed a higher incidence of TB disease in the first 6 months postpartum compared to pregnancy and hypothesized that this may reflect subclinical TB disease during pregnancy that becomes clinically-apparent in the postpartum period.19 Other studies have noted that non-specific or absent symptoms in pregnancy can lead to delays in TB diagnosis.20, 21 TB symptom screening during the postpartum period may improve diagnostic yield to identify TB disease compared to screening during pregnancy, which has only modest sensitivity between 28 and 50%.7–9, 13 Variable performance of the WHO TB symptom screen has been observed, with decreased sensitivity in the context of cART and early TB disease.7, 22, 23 To our knowledge, performance of the WHO TB symptom screen during the postpartum period has not been previously evaluated. Our data suggest that postpartum TB screening may be useful to consider. Further studies to compare performance of TB screening during pregnancy and postpartum and to estimate incremental benefit and cost-effectiveness of postpartum TB screening will be helpful to determine its role in PMTCT programs.
Figure 2.
Proportion of HIV-infected women with positive WHO TB symptom screen during pregnancy (%, 95% CI) in published studies to date.
We found that positive maternal WHO TB screen was associated with history of prior TB, household crowing, and immunosuppression, which are known risk factors for active TB.24–27 We additionally observed an association between a positive maternal WHO TB screen and infant HIV infection. Maternal TB disease has previously been shown to be an independent risk factor for infant HIV infection, which may be mediated by immune activation and increased HIV replication.28 A positive maternal WHO TB screen in our cohort may be a proxy for maternal TB disease or be associated with other mother-to-child HIV transmission risk factors that we were not able to adjust for, such as maternal viral load. However, the association between maternal TB symptoms and infant HIV infection remained significant in multivariable analyses adjusted for use of maternal cART and infant antiretroviral prophylaxis. Maternal TB symptoms were also associated with infant nonspecific TB symptoms, including cough, difficulty breathing, fever, and anorexia, which may represent some cases of undiagnosed infant TB. Older infants were more symptomatic at 9 months compared to infants screened at the 6-week immunization visit, which may represent a missed opportunity for infant TB prevention through early maternal TB diagnosis and IPT administration to TB-exposed infants. Infant TB disease is often not recognized clinically and has high fatality, though IPT is effective to prevent TB in young children with a known TB contact.29,30 Our study suggests that integration of maternal TB symptom screening into MCH immunization programs could prompt infant TB and HIV screening and prevention.
Few women received IPT in this study, which is consistent with limited implementation of IPT observed in high TB burden countries.31 IPT is an effective intervention to prevent TB disease; in a modeling study, treatment of latent infection had higher impact on decreasing global TB incidence compared to improved vaccinations or diagnostics.32 Lack of IPT implementation is due to many factors, including clinician decision-making, poor dissemination of local guidelines and erratic drug supply chain.33 Improved uptake of IPT is a key priority identified in Kenya’s National Tuberculosis, Leprosy, and Lung Health strategic plan.34 Clinical providers may be hesitant to use IPT among pregnant and postpartum women given lack of adequate safety data and prior reports of maternal deaths due to hepatotoxicity in pregnancy and postpartum.35 An ongoing clinical trial (IMPAACT 1078) that assesses safety and toxicity of IPT during pregnancy and postpartum, will better inform future use of IPT in this population.36
Our study had several strengths and limitations. In the context of a national survey, our study presented data for a large number of postpartum HIV-infected women in Kenya. Our data were not nationally representative since we included the PMTCT-Nyanza Survey to increase our sample size of HIV-infected women. Clinical and microbiologic TB diagnostic data were not available for mothers or infants; therefore we could not assess performance of the WHO TB symptom screen for TB diagnosis in the postpartum period or assess if infants with nonspecific TB symptoms had TB disease. Our data on IPT use was limited to women with known TB exposure, which may have underestimated overall IPT use.
In conclusion, we found that postpartum HIV-infected mothers in Kenya frequently reported TB exposure and positive TB symptom screen, but few received IPT. Maternal positive TB symptom screen was associated with infant HIV and nonspecific infant TB symptoms. Integration of maternal TB screening and prevention into PMTCT programs may improve maternal and infant outcomes.
Acknowledgments
We thank participants of the study and clinical and laboratory staff at CDC-KEMRI. We appreciate critical review of the manuscript by Dr. David Horne.
Funding
This study was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through U.S. Centers for Disease Control and Prevention, Division of Global HIV/AIDS [5U2GPS002047-09]. This work was also supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development at the National Institutes of Health [K24 HD054314-06 to GJS, K12 HD000850 to LMC, T32 AI07140 to SML, T32 AI007140 to CM, T32 CA080416 to KR]. LC was a Fellow of the Pediatric Scientist Development Program (PSDP) supported by an American Pediatric Society and American Academy of Pediatrics grants.
Footnotes
Conflicts of Interest:
The authors have no other funding or conflicts of interest to disclose.
CDC Disclaimer
The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention/Government of Kenya.
Author Contributions:
LC and GJ-S designed the study. AL, CM, BS, AK, LN, JK implemented the study. KR performed data analysis. LC drafted the manuscript. AL, CM, JP, BO, BS, AK, LN, JK, SL and GJ-S revised the manuscript.
References
- 1.Khan M, Pillay T, Moodley JM, Connolly CA. Maternal mortality associated with tuberculosis-HIV-1 co-infection in Durban, South Africa. AIDS. 2001;15:1857–1863. doi: 10.1097/00002030-200109280-00016. [DOI] [PubMed] [Google Scholar]
- 2.Pillay T, Khan M, Moodley J, et al. The increasing burden of tuberculosis in pregnant women, newborns and infants under 6 months of age in Durban, KwaZulu-Natal. S Afr Med J. 2001;91:983–987. [PubMed] [Google Scholar]
- 3.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 4.Jana N, Vasishta K, Saha SC, Ghosh K. Obstetrical outcomes among women with extrapulmonary tuberculosis. N Engl J Med. 1999;341:645–649. doi: 10.1056/NEJM199908263410903. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for intensified tuberculosis case finding and isoniazid preventive therapy for people living with HIV in resource contrained settings. 2011. [Google Scholar]
- 6.Kali PB, Gray GE, Violari A, Chaisson RE, McIntyre JA, Martinson NA. Combining PMTCT with active case finding for tuberculosis. J Acquir Immune Defic Syndr. 2006;42:379–381. doi: 10.1097/01.qai.0000218434.20404.9c. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann CJ, Variava E, Rakgokong M, et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One. 2013;8:e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi S, Cavanaugh S, Shiraishi RW, et al. Symptom-based Screening for Tuberculosis among Pregnant Women Living with HIV in Kenya. Proceedings of Conference Symptom-based Screening for Tuberculosis among Pregnant Women Living with HIV in Kenya; 2014. [Google Scholar]
- 9.LaCourse S, Cranmer LM, Matemo D, Kinuthia J, John-Stewart G, Horne DJ. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr. 2015;71:219–227. doi: 10.1097/QAI.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndwiga C, Birungi H, Undie CC, Weyenga H, Sitienei J. Feasibility and effect of integrating tuberculosis screening and detection in postnatal care services: an operations research study. BMC Health Serv Res. 2013;13:99. doi: 10.1186/1472-6963-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenya Ministry of Health Guidelines for Management of Tuberculosis and Leprosy in Kenya. Division of Leprosy Tuberculosis and Lung Disease; 2013. [Google Scholar]
- 12.Kenya Ministry of Health Guidelines for antiretroviral therapy in Kenya. Ministry of Medical Services, Republic of Kenya; 2011. [Google Scholar]
- 13.Kancheya N, Luhanga D, Harris JB, et al. Integrating active tuberculosis case finding in antenatal services in Zambia. Int J Tuberc Lung Dis. 2014;18:1466–1472. doi: 10.5588/ijtld.14.0920. [DOI] [PubMed] [Google Scholar]
- 14.Gounder CR, Wada NI, Kensler C, et al. Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr. 2011;57:e77–84. doi: 10.1097/QAI.0b013e31821ac9c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Chandrasekhar A, Gupte N, et al. Symptom screening among HIV-infected pregnant women is acceptable and has high negative predictive value for active tuberculosis. Clin Infect Dis. 2011;53:1015–1018. doi: 10.1093/cid/cir605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiam A, Machekano R, Gounder CR, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr. 2014;67:e5–e11. doi: 10.1097/QAI.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 17.Hamadeh MA, Glassroth J. Tuberculosis and pregnancy. Chest. 1992;101:1114–1120. doi: 10.1378/chest.101.4.1114. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 19.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185:779–784. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- 20.Kothari A, Mahadevan N, Girling J. Tuberculosis and pregnancy--Results of a study in a high prevalence area in London. Eur J Obstet Gynecol Reprod Biol. 2006;126:48–55. doi: 10.1016/j.ejogrb.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Llewelyn M, Cropley I, Wilkinson RJ, Davidson RN. Tuberculosis diagnosed during pregnancy: a prospective study from London. Thorax. 2000;55:129–132. doi: 10.1136/thorax.55.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangaka MX, Wilkinson RJ, Glynn JR, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012;55:1698–1706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28:1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahey T, Mackenzie T, Arbeit RD, et al. Recurrent tuberculosis risk among HIV-infected adults in Tanzania with prior active tuberculosis. Clin Infect Dis. 2013;56:151–158. doi: 10.1093/cid/cis798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amoakwa K, Martinson NA, Moulton LH, Barnes GL, Msandiwa R, Chaisson RE. Risk factors for developing active tuberculosis after the treatment of latent tuberculosis in adults infected with human immunodeficiency virus. Open Forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark M, Riben P, Nowgesic E. The association of housing density, isolation and tuberculosis in Canadian First Nations communities. Int J Epidemiol. 2002;31:940–945. doi: 10.1093/ije/31.5.940. [DOI] [PubMed] [Google Scholar]
- 27.Zenteno-Cuevas R, Montes-Villasenor E, Morales-Romero J, Coronel-Martin del Campo G, Cuevas B. Co-infection and risk factors of tuberculosis in a Mexican HIV+ population. Rev Soc Bras Med Trop. 2011;44:282–285. doi: 10.1590/s0037-86822011005000034. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203:358–363. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu KH. Isoniazid in the prevention and treatment of tuberculosis. A 20-year study of the effectiveness in children. JAMA. 1974;229:528–533. [PubMed] [Google Scholar]
- 30.Cranmer LM, Kanyugo M, Jonnalagadda SR, et al. High prevalence of tuberculosis infection in HIV-1 exposed Kenyan infants. Pediatr Infect Dis J. 2014;33:401–406. doi: 10.1097/INF.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Granich R, Date A, et al. Review of policy and status of implementation of collaborative HIV-TB activities in 23 high-burden countries. Int J Tuberc Lung Dis. 2014;18:1149–1158. doi: 10.5588/ijtld.13.0889. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Raddad LJ, Sabatelli L, Achterberg JT, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Date AA, Bitoria M, Granich R, Banda M, Fox MY, Gilks C. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–259. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenya Ministry of Health National Strategic Plan for Tuberculosis, Leprosy and Lung Health, 2015–2018. Division of Leprosy Tuberculosis and Lung Disease; 2014. [Google Scholar]
- 35.Franks AL, Binkin NJ, Snider DE, Jr, Rokaw WM, Becker S. Isoniazid hepatitis among pregnant and postpartum Hispanic patients. Public Health Rep. 1989;104:151–155. [PMC free article] [PubMed] [Google Scholar]
- 36.International Maternal Pediatric Adolescent AIDS Clinical Trials Network P1078: A Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Safety (Hepatotoxicity) of Immediate (Antepartum-Initiated) vs. [Accessed January 12, 2017];Deferred (Postpartum-Initiated) Isoniazid Preventive Therapy Among HIV-Infected Women in High TB Incidence Settings. See http://impaactnetwork.org/studies/P1078.asp for further details.