Abstract
OBJECTIVES
Recognizing the challenges of therapeutic decision-making for heart failure (HF) patients with comorbid conditions, our study objectives were to assess: (1) the clinical effectiveness of beta-blocker therapy in patients with HF and chronic lung disease; and (2) the clinical effectiveness of ACE inhibitors/angiotensin II receptor blockers (ARBs) in patients with HF and chronic kidney disease.
DESIGN
Retrospective cohort study.
SETTING
Large community-based cohorts of HF patients.
PARTICIPANTS
Patients with HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF).
METHODS
We undertook separate new-user cohort studies to assess the effectiveness of: (1) beta-blocker therapy in HF and chronic lung disease; and (2) ACE inhibitors/angiotensin II receptor blockers (ARBs) in HF and chronic kidney disease. For the HF-chronic lung disease dyad group, we included patients who had a chronic lung disease diagnosis (ICD-9 codes 490-496, 518). For the HF-chronic kidney disease dyad group, we included patients who had an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2. The clinical outcomes of interest were death from any cause, hospitalization for HF, and hospitalization from any cause. We fitted pooled logistic marginal structural models (MSM) using inverse probability weighting, stratified by HF type.
RESULTS
Among HFrEF patients with chronic lung disease, beta-blocker therapy was protective for death (RR 0.58, 95% confidence interval (CI) 0.44–0.77) and hospitalization for HF (RR 0.78, 95% CI 0.60–1.00). Among those with HFpEF, no statistically significant associations of beta-blocker therapy use with any of the outcomes were observed. We found ACE inhibitor/ARB use to be protective for all three outcomes of interest for patients with HFrEF including death from any cause, hospitalization for HF, and hospitalization from any cause (RR 0.60, 95% 0.40–0.91; RR 0.43, 95% CI 0.28–0.67; and RR 0.63, 95% CI 0.45–0.89, respectively), as well as for patients with HFpEF (RR 0.52, 95% CI 0.33–0.81; RR 0.35, 95% CI 0.18–0.68; and RR 0.67, 95% CI 0.47–0.95, respectively).
CONCLUSION
Large observational studies may allow for the identification of important subgroups of the HF population that might benefit from existing treatment approaches. Our findings may also better inform the design of more definitive future observational studies and randomized trials.
Keywords: multimorbidity, heart failure, comorbidity, lung disease, kidney disease
INTRODUCTION
The presence of comorbidities in patients with heart failure (HF) can pose diagnostic and treatment challenges substantially increasing the complexity of caring for these patients.1 As practitioners face managing not just a single condition but multiple conditions simultaneously,2 caution is required in applying clinical guidelines to the care of patients with comorbidities, especially older patients, as this could result in undesirable outcomes, including adverse interactions between drugs and diseases.3 Multimorbidity greatly increases the risk of hospitalization and death among HF patients.4,5,6,7 Efforts to reduce these risks are particularly challenging given the limited data available to guide clinical practice.
Chronic lung disease and chronic kidney disease are frequently present in older HF patients,8 and these conditions are among the comorbid conditions that confer the highest excess mortality and morbidity risks.9 Certain HF-related treatments raise special concerns for HF patients with concomitant chronic lung disease or chronic kidney disease. For example, beta-blockers can aggravate bronchospastic symptoms in patients with reactive airway disease, and it has been recommended to use these agents cautiously in such patients.1 Chronic kidney disease frequently complicates HF due to poor renal perfusion, pre-existing intrinsic renal disease, and as a result of the effects of medications used to treat HF. Renal function may worsen with use of ACE inhibitors in patients with HF, although the changes are generally reversible.10 While ACE inhibitors are the first choice for inhibition of the renin-angiotensin system in HF with reduced left ventricular ejection fraction, angiotensin II receptor blockers (ARBs) are considered a reasonable alternative as they have a similar adverse effect profile to ACE inhibitors.1
In the present study, we examined a set of questions relating to the treatment of HF in the setting of two very common comorbidities. Our study objectives were to assess: (1) the clinical effectiveness of beta-blocker therapy in patients with HF and chronic lung disease; and (2) the clinical effectiveness of ACE inhibitors/ARBs in patients with HF and chronic kidney disease. In each case, we sought to separately examine the effects of these therapies in patients with HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF) treated in representative community-based populations.
METHODS
Setting
The source population included members from four participating health plans within the Cardiovascular Research Network (CVRN) from 2005 through 2008.11 Sites included Kaiser Permanente Northern California, Kaiser Permanente Colorado, Kaiser Permanente Northwest, and Fallon Health. Institutional review boards at participating sites approved the study and waiver of consent was obtained due to the nature of the study.
Participants
We identified individuals with diagnosed HF based on either having been hospitalized with a primary discharge diagnosis of HF and/or having ≥3 ambulatory visits coded for HF with at least one visit being with a cardiologist between January 2005 and December 2008, employing International Classification of Diseases, 9th Edition (ICD-9) codes. Previous studies have shown a positive predictive value of >95% for admissions with a primary discharge diagnosis of HF based on these codes when compared against chart review and Framingham clinical criteria.12,13,14
We ascertained information on quantitative and/or qualitative assessments of left ventricular systolic function from the results of echocardiograms, radionuclide scintigraphy, other nuclear imaging modalities, and left ventriculography test results available from site-specific databases complemented by manual chart review. We defined preserved ejection fraction as either a reported left ventricular ejection fraction ≥50% and/or based on a physician’s qualitative assessment of preserved or normal systolic function.15 Reduced ejection fraction was defined either by a reported left ventricular ejection fraction ≤40% and/or based on a physician’s qualitative assessment of moderate, moderate to severe, or severe systolic dysfunction.
We identified two HF subgroups of special interest: (1) patients who had HF and chronic lung disease and (2) patients who had HF and chronic kidney disease.
For the HF-chronic lung disease dyad group, we included patients who had a chronic lung disease diagnosis at or prior to the date relating to the inclusion of the patient in the HF study population (ICD-9 codes 490-496, 518). We excluded patients with history of organ transplant at baseline as well as those who received any beta-blocker therapy within up to 5 years prior to the index date, since we were specifically interested in the clinical effectiveness of beta-blocker therapy in patients with HF and chronic lung disease who were newly initiated on therapy. To ensure an adequate baseline period of time to characterize the patients, we excluded patients with less than 12 months of continuous health plan membership and pharmacy drug benefit before the index date.
For the HF-chronic kidney disease dyad group, we included patients who had an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 according to laboratory results available on or before the date relating to the inclusion of the patient in the HF study population. We excluded patients with a history of organ transplant or dialysis at study entry, defined as up to 5 years before and including the index date. We also excluded patients with estimated glomerular filtration rates (eGFR) ≥60 ml/min/1.73m2 at baseline and during follow-up using the CKD-EPI equation based on outpatient, non-emergency department serum creatinine measures,161 missing eGFR values at baseline, or missing blood hemoglobin measurements at baseline. Similar to the approach described previously for outcomes associated with beta-blockers in the dyad of HF and chronic lung disease, we excluded patients who received any ACE inhibitor or ARB therapy within up to 5 years prior to the index date to focus on the clinical effectiveness of ACE inhibitor/ARB therapy in patients with HF and chronic kidney disease who were newly initiated on therapy. To ensure an adequate baseline period of time to characterize the patients, we excluded patients with less than 12 months of continuous health plan membership and pharmacy drug benefit before the index date.
Study design: exposures, follow-up, and outcomes
For each corresponding dyad, we ascertained use of beta-blocker or ACE inhibitor/ARB therapy from filled outpatient prescriptions from pharmacy database. We employed a “new user” design to eliminate prevalent user bias, by restricting the analysis to persons under observation at the start of treatment.17 We characterized time-varying exposure to therapy using previously validated methods based on estimated days supply information per dispensed prescription and refill patterns found in health plan pharmacy databases.18 Briefly, for any two consecutive prescriptions, we examined the time between the projected end date of the first prescription and the date of the next filled prescription. Since dose adjustment is not uncommon, we allowed a “grace period” of 30 days between prescriptions. If the time between the projected end date of the first prescription and the fill date of the next prescription was ≤30 days, we considered that individual to be continuously receiving the therapy of interest. If the refill interval was >30 days, we considered the individual off therapy starting the day after the projected end date of the first prescription until the date of next filled prescription, if any. If a refill occurred earlier than the predicted end of days’ supply, then any overlap in days’ supply was not added to the end of the next prescription.
Follow-up occurred from the index date through December 31, 2008 for a maximum of 4 years. Median follow-up was 1.6 years (interquartile range [IQR], 0.6–2.8) within the HF-chronic lung disease dyad group, and 1.1 years (IQR, 0.3–2.2) within the HF-chronic kidney disease dyad group. The clinical outcomes of interest were death from any cause, hospitalization for HF, and hospitalization from any cause. Subjects were censored if they disenrolled from the health plan, received an organ transplant, initiated chronic dialysis, or reached the end of study follow-up. Deaths were identified from hospital and billing claims databases, administrative health plan databases, state death certificate registries, and Social Security Administration files as available at each site. These approaches have yielded >97% vital status information in prior studies.12,13
Covariates
We ascertained information on coexisting illnesses based on diagnoses or procedures using relevant ICD-9 codes, laboratory results, or filled outpatient prescriptions from health plan hospitalization discharge, ambulatory visit, laboratory, and pharmacy databases, as well as site-specific cancer registries.19 We defined prevalent HF as having any hospitalization or ambulatory HF diagnosis during a look-back period of up to 5 years prior to the date of the diagnosis that led to inclusion in the study cohort.
We ascertained available ambulatory results for baseline and time-updated systolic and diastolic blood pressure, serum LDL and HDL cholesterol measurements, eGFR, dipstick proteinuria, and blood hemoglobin level. We also collected baseline and time-updated information on receipt of other selected medications including aldosterone antagonists, calcium channel blockers, digoxin, loop diuretics, thiazide diuretics, nitrates, statins, other lipid lowering drugs, anticoagulants, and antiplatelet agents.
Statistical analysis
Analyses were conducted using SAS, version 9.3 (Cary, N.C.). We compared the baseline characteristics for patients who did or did not initiate the therapies of interest, using ANOVA or the relevant non-parametric test for continuous variables and chi-square tests for categorical variables.
For each dyad and associated therapy of interest, we fitted pooled logistic marginal structural models (MSM)20 stratified by HFrEF or HFpEF at baseline. We first constructed our analytic dataset based on a directed acyclic graph (DAG) of exposure and outcome relationships based on the published literature. Each patient’s follow-up time was then divided into 30-day intervals starting on their index date. Exposures, outcomes, and covariates were then organized into proper time order within and across these time intervals. Next, we calculated inverse probability weights (IPW) to adjust for potential baseline and time-dependent confounders.21 We separately calculated exposure and censoring IPWs, then multiplied them to determine the final summary IPW for each patient. We constructed three models to characterize exposure to the therapy of interest to facilitate generation of weights: probability of exposure at start of follow-up, probability of exposure given previously not exposed in the prior period, and probability of exposure given previously being exposed in the prior period. One censoring model was used to capture censoring due to disenrollment from the health plan or end of administrative study follow-up. After evaluation of the distribution, the final summary IPW was truncated at 2% and 98% for model stability. Our modeling approach was an “as-treated” MSM analysis evaluating the effect of exposure to the therapy of interest on outcomes, with incorporation of IPWs to account for baseline and time-dependent confounders, as well as splines set to 1%, 25%, 50%, 75%, and 99% of overall cohort follow-up time to adjust for the potential effect of time.
RESULTS
Among the 2414 patients with a history of chronic lung disease and no prior beta-blocker use at study entry, there were 986 with HFrEF and 1428 with HFpEF. Details of cohort assembly are provided in Supplementary Figure S1.
Patients with HFrEF and use of beta-blocker therapy were considerably younger than those with no use during follow-up (mean age 69.0 versus 77.7; p<0.001) (Table 1). They were less likely to have previous percutaneous coronary intervention, atrial fibrillation or flutter, ventricular tachycardia or fibrillation, mitral and/or aortic valvular disease, a pacemaker, hospitalized bleeds, dementia, and falls. HFrEF patients with beta-blocker use also had higher eGFR, hemoglobin, and systolic and diastolic blood pressure levels (Supplementary Table S1). In contrast, HFrEF beta-blocker users were less likely to use loop diuretics, nitrates, antiplatelet agents, and/or anticoagulants (Supplementary Table S2). In an MSM analysis among HFrEF patients with chronic lung disease that accounted for potential baseline and time-dependent confounders, beta-blocker therapy was protective for death from any cause (HR 0.58 [95% CI:0.44–0.77]) and hospitalization for HF (HR 0.78 [95% CI:0.60–1.00]), but there was no statistically significant association observed with hospitalization from any cause (Figure 1).
Table 1.
Baseline characteristics of patients with HF and lung disease, stratified by HFrEF or HFpEF status and use of beta-blocker therapy.
| Reduced EF N=986 |
β-blocker N=757 |
No β-blocker N=229 |
p-values | Preserved EF N=1428 |
β-blocker N=705 |
No β-blocker N=723 |
p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr, mean (SD) | 71.0 | 13.4 | 69 | 13.3 | 77.7 | 11.1 | <0.001 | 74.4 | 12.1 | 73.2 | 11.9 | 75.6 | 12.3 | <0.001 |
| Age categories | n | % | n | % | n | % | <0.001 | n | % | n | % | n | % | <0.001 |
| Age <45 | 44 | 4.5 | 41 | 5.4 | 3 | 1.3 | 24 | 1.7 | 13 | 1.8 | 11 | 1.5 | ||
| Age 45–54 | 91 | 9.2 | 84 | 11.1 | 7 | 3.1 | 76 | 5.3 | 43 | 6.1 | 33 | 4.6 | ||
| Age 55–64 | 171 | 17.3 | 150 | 19.8 | 21 | 9.2 | 214 | 15 | 112 | 15.9 | 102 | 14.1 | ||
| Age 65–74 | 239 | 24.2 | 198 | 26.2 | 41 | 17.9 | 342 | 23.9 | 200 | 28.4 | 142 | 19.6 | ||
| Age 75–84 | 314 | 31.8 | 210 | 27.7 | 104 | 45.4 | 488 | 34.2 | 227 | 32.2 | 261 | 36.1 | ||
| Age ≥ 85 | 127 | 12.9 | 74 | 9.8 | 53 | 23.1 | 284 | 19.9 | 110 | 15.6 | 174 | 24.1 | ||
| Female gender | 406 | 41.2 | 320 | 42.3 | 86 | 37.6 | 0.2 | 861 | 60.3 | 423 | 60 | 438 | 60.6 | 0.82 |
| Race | 0.11 | 0.02 | ||||||||||||
| White | 736 | 74.6 | 559 | 73.8 | 177 | 77.3 | 1112 | 77.9 | 553 | 78.4 | 559 | 77.3 | ||
| Black/African Am. | 87 | 8.8 | 75 | 9.9 | 12 | 5.2 | 104 | 7.3 | 55 | 7.8 | 49 | 6.8 | ||
| Asian | 48 | 4.9 | 39 | 5.2 | 9 | 3.9 | 50 | 3.5 | 29 | 4.1 | 21 | 2.9 | ||
| Native Hawaiian | 5 | 0.5 | 5 | 0.7 | 0 | 0 | 2 | 0.1 | 2 | 0.3 | 0 | 0 | ||
| Other | 2 | 0.2 | 2 | 0.3 | 0 | 0 | 4 | 0.3 | 4 | 0.6 | 0 | 0 | ||
| Missing/Unknown | 108 | 11.0 | 77 | 10.2 | 31 | 13.5 | 156 | 10.9 | 62 | 8.8 | 94 | 13 | ||
| Medical History | ||||||||||||||
| Prevalent HF | 424 | 43.0 | 285 | 37.6 | 139 | 60.7 | <0.001 | 785 | 55 | 345 | 48.9 | 440 | 60.9 | <0.001 |
| AMI | 34 | 3.4 | 22 | 2.9 | 12 | 5.2 | 0.09 | 44 | 3.1 | 14 | 2 | 30 | 4.1 | 0.02 |
| Unstable angina | 8 | 0.8 | 6 | 0.8 | 2 | 0.9 | 0.91 | 31 | 2.2 | 14 | 2 | 17 | 2.4 | 0.64 |
| CABG | 9 | 0.9 | 7 | 0.9 | 2 | 0.9 | 0.94 | 23 | 1.6 | 12 | 1.7 | 11 | 1.5 | 0.79 |
| PCI | 29 | 2.9 | 17 | 2.2 | 12 | 5.2 | 0.02 | 39 | 2.7 | 20 | 2.8 | 19 | 2.6 | 0.81 |
| Stroke/TIA | 41 | 4.2 | 27 | 3.6 | 14 | 6.1 | 0.09 | 88 | 6.2 | 34 | 4.8 | 54 | 7.5 | 0.04 |
| Other thromboembolic event | 5 | 0.5 | 3 | 0.4 | 2 | 0.9 | 0.37 | 13 | 0.9 | 6 | 0.9 | 7 | 1 | 0.82 |
| AF or flutter | 248 | 25.2 | 171 | 22.6 | 77 | 33.6 | 0.001 | 536 | 37.5 | 242 | 34.3 | 294 | 40.7 | 0.01 |
| V tach or V fib | 28 | 2.8 | 17 | 2.2 | 11 | 4.8 | 0.04 | 16 | 1.1 | 4 | 0.6 | 12 | 1.7 | 0.05 |
| Mitral and/or AV disease | 166 | 16.8 | 110 | 14.5 | 56 | 24.5 | <0.001 | 353 | 24.7 | 153 | 21.7 | 200 | 27.7 | 0.009 |
| PAD | 60 | 6.1 | 40 | 5.3 | 20 | 8.7 | 0.06 | 104 | 7.3 | 48 | 6.8 | 56 | 7.7 | 0.5 |
| Rheumatic heart | 16 | 1.6 | 9 | 1.2 | 7 | 3.1 | 0.05 | 63 | 4.4 | 24 | 3.4 | 39 | 5.4 | 0.07 |
| ICD | 33 | 3.3 | 23 | 3 | 10 | 4.4 | 0.3274 | 5 | 0.4 | 3 | 0.4 | 2 | 0.3 | 0.63 |
| Pacemaker | 45 | 4.6 | 27 | 3.6 | 18 | 7.9 | 0.006 | 67 | 4.7 | 31 | 4.4 | 36 | 5 | 0.6 |
| Dyslipidemia | 523 | 53.0 | 402 | 53.1 | 121 | 52.8 | 0.94 | 790 | 55.3 | 412 | 58.4 | 378 | 52.3 | 0.02 |
| Hypertension | 599 | 60.8 | 460 | 60.8 | 139 | 60.7 | 0.99 | 1043 | 73 | 514 | 72.9 | 529 | 73.2 | 0.91 |
| Diabetes mellitus | 236 | 23.9 | 173 | 22.9 | 63 | 27.5 | 0.15 | 340 | 23.8 | 162 | 23 | 178 | 24.6 | 0.47 |
| Hospitalized bleeds | 40 | 4.1 | 22 | 2.9 | 18 | 7.9 | 0.001 | 101 | 7.1 | 31 | 4.4 | 70 | 9.7 | <0.001 |
| Dx dementia | 58 | 5.9 | 33 | 4.4 | 25 | 10.9 | <0.001 | 118 | 8.3 | 54 | 7.7 | 64 | 8.9 | 0.41 |
| Dx depression | 170 | 17.2 | 130 | 17.2 | 40 | 17.5 | 0.92 | 325 | 22.8 | 161 | 22.8 | 164 | 22.7 | 0.94 |
| Liver disease | 43 | 4.4 | 33 | 4.4 | 10 | 4.4 | 0.99 | 77 | 5.4 | 39 | 5.5 | 38 | 5.3 | 0.82 |
| Fall | 18 | 1.8 | 10 | 1.3 | 8 | 3.5 | 0.03 | 65 | 4.6 | 25 | 3.5 | 40 | 5.5 | 0.07 |
| Systemic cancer | 76 | 7.7 | 58 | 7.7 | 18 | 7.9 | 0.92 | 131 | 9.2 | 63 | 8.9 | 68 | 9.4 | 0.76 |
Native Hawaiian includes Other Pacific Islanders
HF = Heart failure; AMI = Acute myocardial infraction; CABG= Coronary artery bypass graft surgery; PCI= Percutaneous coronary intervention; TIA = Transient ischemic attack; AF = Atrial fibrillation; V tach/V fib = Ventricular tachycardia/Ventricular fibrillation; AV = Aortic valve; PAD = Peripheral arterial disease; ICD = Implantable cardioverter defibrillator; Dx = Diagnosed
Figure 1.
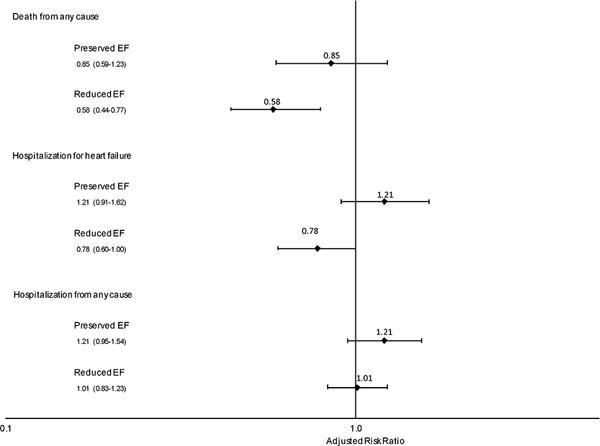
Effect of beta-blockers on risks of death from any cause, hospitalization for heart failure, and hospitalization from any cause among adults with heart failure and chronic lung disease, stratified by left ventricular ejection fraction.
Patients with HFpEF and use of beta-blocker therapy were significantly younger than those with no use (mean age 73.2 versus 75.6; p<0.001) (Table 1). They were less likely to have prior acute myocardial infarction, ischemic stroke or transient ischemic attack, atrial fibrillation or flutter, mitral and/or aortic valvular disease, and hospitalized bleeds. HFpEF patients with beta-blocker use were more likely to have a diagnosis of dyslipidemia, receive statin therapy and have higher systolic and diastolic blood pressure and lower HDL levels (Supplementary Table S1), but were less likely to use aldosterone receptor antagonists, digoxin, loop diuretics, and anticoagulants (Supplementary Table S2). In contrast to results seen in HFrEF patients, among eligible patients with HFpEF and chronic lung disease, there were no statistically significant associations of beta-blocker therapy use with any of the outcomes of interest in MSM analyses (Figure 1).
Among the 1,492 patients with chronic kidney disease and no prior ACE inhibitors/angiotensin II receptor blockers use, 482 had HFrEF and 1,010 patients had HFpEF (Supplementary Figure S2).
Patients with HFrEF and use of ACE inhibitor/ARB therapy were less likely than non-users to have a prior acute myocardial infarction, percutaneous coronary intervention, ischemic stroke or transient ischemic attack, ventricular tachycardia or fibrillation, implantable cardioverter defibrillator, or liver disease (Table 2). Patients with ACE inhibitor/ARB use had higher eGFR levels and were less likely to have dipstick proteinuria (Supplementary Table S3). They were also less likely to use aldosterone receptor antagonists, calcium channel blockers, loop diuretics, and nitrates (Supplementary Table S4). In MSM models among eligible HFrEF patients with concomitant chronic kidney disease that adjusted for baseline and time-dependent confounders, ACE inhibitor/ARB therapy was protective for death from any cause (HR 0.60 [95%CI: 0.40–0.91]), hospitalization for HF (HR 0.43 [95%CI: 0.28–0.67]), and hospitalization from any cause (HR 0.63 [95%CI: 0.45–0.89]) (Figure 2).
Table 2.
Baseline characteristics of patients with HF and CKD stratified by HFrEF or HFpEF status and use of ACE inhibitors/Angiotensin II receptor blockers (ACEi/ARB)
| Reduced EF N=482 |
ACEi/ARB N=282 |
No ACEi/ARB N=200 |
p-values | Preserved EF N=1010 |
ACEi/ARB N=362 |
No ACEi/ARB N=648 |
p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr, mean (SD) | 81.8 | 9.5 | 81.2 | 8.9 | 82.7 | 10.1 | 0.08 | 83.0 | 8.7 | 82.3 | 8.8 | 83.3 | 8.7 | 0.09 |
| Age categories | n | % | n | % | n | % | 0.02 | n | % | n | % | n | % | 0.17 |
| Age <45 | 1 | 0.2 | 0 | 0.0 | 1 | 0.5 | 2 | 0.2 | 0 | 0.0 | 2 | 0.3 | ||
| Age 45–54 | 7 | 1.5 | 4 | 1.4 | 3 | 1.5 | 12 | 1.2 | 7 | 1.9 | 5 | 0.8 | ||
| Age 55–64 | 20 | 4.1 | 11 | 3.9 | 9 | 4.5 | 26 | 2.6 | 8 | 2.2 | 18 | 2.8 | ||
| Age 65–74 | 68 | 14.1 | 44 | 15.6 | 24 | 12.0 | 104 | 10.3 | 42 | 11.6 | 62 | 9.6 | ||
| Age 75–84 | 194 | 40.2 | 128 | 45.4 | 66 | 33.0 | 439 | 43.5 | 166 | 45.9 | 273 | 42.1 | ||
| Age ≥ 85 | 192 | 39.8 | 95 | 33.7 | 97 | 48.5 | 427 | 42.3 | 139 | 38.4 | 288 | 44.4 | ||
| Female gender | 156 | 32.4 | 93 | 33.0 | 63 | 31.5 | 0.73 | 564 | 55.8 | 183 | 50.6 | 381 | 58.8 | 0.01 |
| Race | 0.38 | 0.27 | ||||||||||||
| White | 406 | 84.2 | 242 | 85.8 | 164 | 82.0 | 857 | 84.9 | 312 | 86.2 | 545 | 84.1 | ||
| Black/African Am. | 15 | 3.1 | 10 | 3.5 | 5 | 2.5 | 33 | 3.3 | 9 | 2.5 | 24 | 3.7 | ||
| Asian | 27 | 5.6 | 12 | 4.3 | 15 | 7.5 | 34 | 3.4 | 14 | 3.9 | 20 | 3.1 | ||
| Native Hawaiian | 1 | 0.2 | 1 | 0.4 | 0 | 0.0 | 7 | 0.7 | 4 | 1.1 | 3 | 0.5 | ||
| Other | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 0.3 | 2 | 0.6 | 1 | 0.2 | ||
| Missing/Unknown | 33 | 6.8 | 17 | 6.0 | 16 | 8.0 | 76 | 7.5 | 21 | 5.8 | 55 | 8.5 | ||
| Medical History | ||||||||||||||
| Prevalent HF | 201 | 41.7 | 85 | 30.1 | 116 | 58.0 | <0.001 | 505 | 50.0 | 137 | 37.8 | 368 | 56.8 | <0.001 |
| AMI | 49 | 10.2 | 21 | 7.4 | 28 | 14.0 | 0.02 | 69 | 6.8 | 19 | 5.2 | 50 | 7.7 | 0.14 |
| Unstable angina | 12 | 2.5 | 7 | 2.5 | 5 | 2.5 | 0.99 | 37 | 3.7 | 13 | 3.6 | 24 | 3.7 | 0.93 |
| CABG | 13 | 2.7 | 6 | 2.1 | 7 | 3.5 | 0.36 | 29 | 2.9 | 14 | 3.9 | 15 | 2.3 | 0.16 |
| PCI | 33 | 6.8 | 12 | 4.3 | 21 | 10.5 | 0.008 | 45 | 4.5 | 11 | 3.0 | 34 | 5.2 | 0.1 |
| Stroke/TIA | 33 | 6.8 | 13 | 4.6 | 20 | 10.0 | 0.02 | 92 | 9.1 | 32 | 8.8 | 60 | 9.3 | 0.82 |
| Other thromboembolic | 1 | 0.2 | 0 | 0.0 | 1 | 0.5 | 0.23 | 9 | 0.9 | 3 | 0.8 | 6 | 0.9 | 0.87 |
| AF or flutter | 169 | 35.1 | 94 | 33.3 | 75 | 37.5 | 0.34 | 477 | 47.2 | 150 | 41.4 | 327 | 50.5 | 0.006 |
| V tach or V fib | 13 | 2.7 | 4 | 1.4 | 9 | 4.5 | 0.04 | 13 | 1.3 | 1 | 0.3 | 12 | 1.9 | 0.03 |
| Mitral and/or AV disease | 74 | 15.4 | 39 | 13.8 | 35 | 17.5 | 0.27 | 247 | 24.5 | 84 | 23.2 | 163 | 25.2 | 0.49 |
| PAD | 40 | 8.3 | 20 | 7.1 | 20 | 10.0 | 0.25 | 99 | 9.8 | 28 | 7.7 | 71 | 11.0 | 0.1 |
| Rheumatic heart | 22 | 4.6 | 11 | 3.9 | 11 | 5.5 | 0.41 | 35 | 3.5 | 13 | 3.6 | 22 | 3.4 | 0.87 |
| ICD | 13 | 2.7 | 4 | 1.4 | 9 | 4.5 | 0.04 | 4 | 0.4 | 0 | 0.0 | 4 | 0.6 | 0.13 |
| Pacemaker | 43 | 8.9 | 22 | 7.8 | 21 | 10.5 | 0.31 | 65 | 6.4 | 21 | 5.8 | 44 | 6.8 | 0.54 |
| Dyslipidemia | 251 | 52.1 | 142 | 50.4 | 109 | 54.5 | 0.37 | 536 | 53.1 | 195 | 53.9 | 341 | 52.6 | 0.7 |
| Hypertension | 303 | 62.9 | 167 | 59.2 | 136 | 68.0 | 0.05 | 800 | 79.2 | 284 | 78.5 | 516 | 79.6 | 0.66 |
| Diabetes mellitus | 85 | 17.6 | 43 | 15.2 | 42 | 21.0 | 0.1 | 186 | 18.4 | 62 | 17.1 | 124 | 19.1 | 0.43 |
| Hospitalized bleeds | 26 | 5.4 | 11 | 3.9 | 15 | 7.5 | 0.08 | 85 | 8.4 | 20 | 5.5 | 65 | 10.0 | 0.01 |
| Dx dementia | 49 | 10.2 | 25 | 8.9 | 24 | 12.0 | 0.26 | 130 | 12.9 | 37 | 10.2 | 93 | 14.4 | 0.06 |
| Dx depression | 58 | 12.0 | 35 | 12.4 | 23 | 11.5 | 0.76 | 170 | 16.8 | 68 | 18.8 | 102 | 15.7 | 0.22 |
| Lung disease | 154 | 32.0 | 81 | 28.7 | 73 | 36.5 | 0.07 | 438 | 43.4 | 144 | 39.8 | 294 | 45.4 | 0.09 |
| Liver disease | 18 | 3.7 | 6 | 2.1 | 12 | 6.0 | 0.03 | 31 | 3.1 | 9 | 2.5 | 22 | 3.4 | 0.42 |
| Fall | 18 | 3.7 | 10 | 3.5 | 8 | 4.0 | 0.8 | 53 | 5.2 | 15 | 4.1 | 38 | 5.9 | 0.24 |
| Systemic cancer | 42 | 8.7 | 23 | 8.2 | 19 | 9.5 | 0.61 | 92 | 9.1 | 23 | 6.4 | 69 | 10.6 | 0.02 |
Native Hawaiian includes Other Pacific Islanders
HF = Heart failure; AMI = Acute myocardial infraction; CABG= Coronary artery bypass graft surgery; PCI= Percutaneous coronary intervention; TIA = Transient ischemic attack; AF = Atrial fibrillation; V tach/V fib = Ventricular tachycardia/Ventricular fibrillation; AV = Aortic valve; PAD = Peripheral arterial disease; ICD = Implantable cardioverter defibrillator; Dx = Diagnosed
Figure 2.
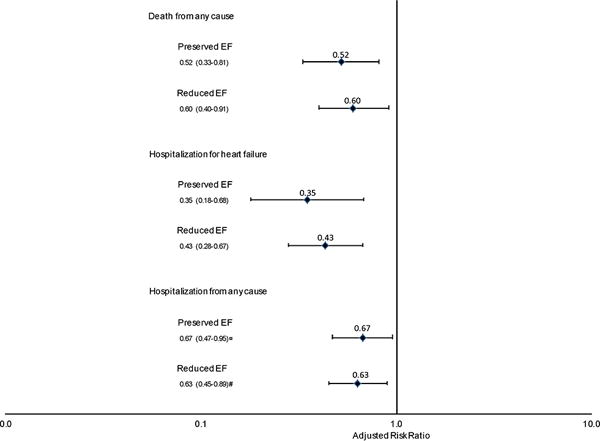
Effect of ACE inhibitor/angiotensin II receptor blocker therapy on risks of death from any cause, hospitalization for heart failure, and hospitalization from any cause among adults with heart failure and chronic kidney disease, stratified by left ventricular ejection fraction.
Patients with HFpEF and use of ACE inhibitor/ARB therapy were less likely to be female and to have a history of atrial fibrillation or flutter, ventricular tachycardia or fibrillation, hospitalized bleeds, and cancer than non-users (Table 2). Patients with ACE inhibitor/ARB use had higher eGFR and diastolic blood pressure, lower LDL cholesterol levels, and were less likely to have dipstick proteinuria (Supplementary Table S3). They were also less likely to use calcium channel blockers, and nitrates, and more likely to use thiazide diuretic therapy (Supplementary Table S4). Similar to results seen in HFrEF patients, MSM model results revealed a favorable impact of ACE inhibitor/ACE therapy on all three outcomes among eligible patients with HFpEF and chronic kidney disease (RR 0.52 [95% CI 0.33–0.81]; RR 0.35 [95% CI 0.18–0.68]; and RR 0.67 [95% CI 0.47–0.95], respectively) (Figure 2).
DISCUSSION
Among community-based patients with HFrEF and chronic lung disease, we found that beta-blocker therapy was associated with a reduced risk for death from any cause and hospitalization, but there were no statistically significant associations of beta-blocker therapy use with any of the outcomes of interest in those with HFpEF and chronic lung disease. Among patients with HFpEF or HFrEF complicated by chronic kidney disease, ACE inhibitor/ARB use was independently associated with reduced risks for death from any cause, hospitalization for HF, and hospitalization for any cause.
The findings of two recent meta-analyses are intriguing in relation to our results. Bavishi and colleagues undertook a meta-analysis to evaluate the efficacy of beta-blockers on mortality and morbidity in patients with HFpEF.22 Observational studies included in this meta-analysis suggested a favorable association between receipt of beta-blockers and all-cause mortality, but not for HF-related hospitalization. No subgroup analyses were performed relevant to patients with specific comorbidities and potential time-dependent confounding was not addressed. A recent meta-analysis of randomized controlled trials assessing the efficacy of inhibition of the renin-angiotensin aldosterone system (RAAS) in HF suggested that the relative beneficial effects decreased with increasing mean left ventricular ejection fraction for outcomes including mortality and HF hospitalization.23 However, although no benefit of RAAS inhibition on all-cause mortality was observed above a mean ejection fraction of 50%, a significant benefit on HF-related hospitalization was observed (RR 0.88 [95% CI:0.80 to 0.97]). Again, no subgroup analyses were performed relevant to patients with specific comorbidities.
Our study has several strengths and limitations. Insured populations receiving care in the health care delivery systems relevant to our study may not be fully representative of all HF populations. This limitation is counterbalanced by the breadth of geographic and demographic diversity, as well at the community-based nature of health care delivery, suggesting that findings of our investigations are likely to be highly generalizable to patients with HF in “real-world” practice settings in relation to the treatments they receive and the effectiveness of these treatments. In addition, individual diagnoses of HF were not adjudicated through medical record abstraction and expert review. However, prior studies in our population have shown a high positive predictive value for the approach we used to identify patients with HF.12,13 We also used both quantitative and qualitative assessments of left ventricular systolic function to characterize patients as having HFrEF versus HFpEF. While chronic kidney disease diagnoses were based on the estimated glomerular filtration rate, diagnoses of chronic lung disease were based on diagnosis codes and were not validated.
Small sample sizes in subgroups of interest adversely impacted the precision of our estimates of effect. We also did not study HF patients with an EF of 41–49 percent, who were relatively few in number. These patients, who fall into a “borderline”, “intermediate”, or “mid-range” group, may comprise a separate group with regard to characteristics, pathophysiology, and treatment.24
Our new-user study design addressed the problem of prevalent user bias that plagues many observational studies of treatment effectiveness in the “real world,” when information about baseline medication use is lacking.17 However, an inherent limitation of observational studies is the potential for residual confounding and selection bias. Of particular concern is “healthy user bias,” which we attempted to mitigate through our analytic approach. However, residual confounding may still exist, because users of beta-blockers or ACE inhibitor or ARB therapy had a lower comorbidity burden than non-users. Strategies to address healthy user bias include having an active control,25 which was not possible in these studies. We combined this design approach with marginal structural modeling and inverse probability weighting to address potential time-dependent confounding, overcoming the limitations of several commonly used methods such as propensity score matching and Cox regression.26
Trials of treatments for HF have excluded the types of patients most commonly presenting with this condition, particularly older adults and those with high burdens of comorbidity,27,28,29 posing an ongoing challenge for clinical decision-making in these growing numbers of high risk HF patients.3,5,8,30 In addition, the current non-evidence-based approach to the care of patients with HFpEF remains extremely frustrating.31 The imperative to address this situation has never been greater, as the majority of older patients who present with a new diagnosis of HF have HFpEF.32 With the aging of the US population, it is likely that the numbers of patients affected by this form of HF will increase dramatically over the coming decades, with many if not most, burdened by additional chronic conditions. The findings of our efforts suggest that large observational studies may allow for the identification of important subgroups of the HF population that might benefit from existing treatment approaches and inform the design of more definitive future randomized controlled trials.
Supplementary Material
Supplementary Figure S1. Assembly of cohorts with dyads of heart failure and chronic lung disease.
Supplementary Figure S2. Assembly of cohorts with dyads of heart failure and chronic kidney disease.
Supplementary Table S1. Additional baseline characteristics of patients with HF and lung disease, stratified by HFrEF or HFpEF status and use of beta-blocker therapy.
Supplementary Table S2. Baseline medication use characteristics of patients with HF and lung disease, stratified by HFrEF or HFpEF status and use of beta-blocker therapy.
Supplementary Table S3. Additional baseline characteristics of patients with HF and CKD stratified by HFrEF or HFpEF status and use of ACE inhibitors/ Angiotensin II receptor blockers (ACEi/ARB).
Supplementary Table S4. Baseline medication use characteristics of patients with HF and CKD stratified by HFrEF or HFpEF status and use of ACE inhibitors/Angiotensin II receptor blockers (ACEi/ARB).
IMPACT STATEMENT.
1. We certify that this work is novel or confirmatory of recent novel clinical research. 2. The potential impact of this research on clinical care or health policy includes the following: informing the design of more definitive future observational studies and randomized trials relevant to the treatment of heart failure patients with multiple chronic conditions.
Acknowledgments
Dr. Allen reports receiving grant funding from NHLBI, PCORI, and AHA, and has received consultancy fees from Novartis, Janssen, St Jude, and ZS Pharma. Dr. Go reports receiving grant funding through his institution (Kaiser Permanente Northern California Division of Research) from Astra-Zeneca, Novartis and GlaxoSmithKline, as well as from NHLBI, NIDDK, NIA and PCORI. Dr. Smith reports receiving grant funding through his institution (Center for Health Research, Kaiser Permanente Northwest) from Novartis to undertake an FDA mandated drug safety study.
Funding: The study was supported by R21 AG045320 and R24 AG045050 from the National Institute on Aging, as well as RC1 HL099395 and U19 HL91179 from the National Heart, Lung, and Blood Institute. Dr. McManus was supported by R01HL126911-01A1 and KL2RR031981.
Sponsor’s Role: The National Institute on Aging and the NIH had no role in the preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest:
The other authors report no conflicts.
Author Contributions: Study concept and design: JHG, DJM, DHS, GHT, SHS, LAA, DDM, RJG, MT, ASG. Acquisition of data: JHG, DJM, DHS, GHT, SHS, ASG. Analysis and interpretation of data: JHG, DJM, DHS, GHT, SHS, LAA, DDM, RJG, MT, ASG. Preparation of manuscript: JHG, DJM, DHS, GHT, SHS, LAA, DDM, RJG, MT, ASG.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Oct 15;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure: most patients have comorbidities that need to be addressed. BMJ. 2003;327:513–514. doi: 10.1136/bmj.327.7414.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid disease: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 4.Braunstein JD, Anderson GF, Gerstenblith G, et al. Non-cardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 5.Alter DA, Ko DT, Tu JV, et al. The average lifespan of patients discharged from hospital with heart failure. J Gen Intern Med. 2012;27:1171–1179. doi: 10.1007/s11606-012-2072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahluwalia SC, Gross CP, Chaudhry SI, et al. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med. 2011;27:513–519. doi: 10.1007/s11606-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tisminetzky M, Goldberg R, Gurwitz JH. Magnitude and impact of multimorbidity on clinical outcomes in older adults with cardiovascular disease: a literature review. Clin Geriatr Med. 2016 doi: 10.1016/j.cger.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahluwalia SC, Gross CP, Chaudhry SI, et al. Change in comorbidity prevalence with advancing age among persons with heart failure. J Gen Intern Med. 2011;26:1145–1151. doi: 10.1007/s11606-011-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusinaru D, Saaidi I, Godard S, Mahjoub H, et al. Impact of chronic obstructive pulmonary disease on long-term outcome of patients hospitalized for heart failure. Am J Cardiol. 2008;101:353–358. doi: 10.1016/j.amjcard.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Lee WH, Medina N, et al. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med. 1987;106:346–354. doi: 10.7326/0003-4819-106-3-346. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Magid DJ, Wells B, et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Lee WY, Yang J, et al. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 14.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Investigators. Ann Intern Med. 2009;150:604–612. [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003 Nov 1;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Yang J, Gurwitz JH, et al. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008 Dec 8;168(22):2415–21. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 19.Allen LA, Magid DJ, Gurwitz JH, et al. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail. 2013 Jul;6(4):635–46. doi: 10.1161/CIRCHEARTFAILURE.112.000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Thompson CA, Arah OA. Selection bias modeling using observed data augmented with imputed record-level probabilities. Ann Epidemiol. 2014;24(10):747–53. doi: 10.1016/j.annepidem.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavishi C, Chatterjee S, Ather S, et al. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20:193–201. doi: 10.1007/s10741-014-9453-8. [DOI] [PubMed] [Google Scholar]
- 23.Emdin CA, Callender T, Cao J, et al. Meta-analysis of large-scale randomized trials to determine the effectiveness of inhibition of the renin-angiotensin aldosterone system in heart failure. Am J Cardiol. 2015;116:155–161. doi: 10.1016/j.amjcard.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891–975. doi: 10.1002/ejhf.592. 2016. [DOI] [PubMed] [Google Scholar]
- 25.Setoguchi S, Gerhard T. Comparator selection AHRQ Publication No. 12(13)-EHC099. In: Velentgas P, Dreyer NA, Nourjah P, et al., editors. Developing a Protocol for Observational Comparative Effectiveness Research. Vol. 2013. Agency for Healthcare Research and Quality; Rockville, MD: pp. 59–70. [PubMed] [Google Scholar]
- 26.Yang W, Joffe MM. Subtle issues in model specification and estimation of marginal structural models. Pharmacoepidemiology and drug safety. 2012;21:241–245. doi: 10.1002/pds.2306. [DOI] [PubMed] [Google Scholar]
- 27.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherubini A, Oristrell J, Pla X, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550–556. doi: 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- 29.Gurwitz JH, Goldberg RJ. Age-based exclusions from cardiovascular clinical trials: implications for elderly individuals (and for all of us) Arch Intern Med. 2011;171:557–558. doi: 10.1001/archinternmed.2011.33. [DOI] [PubMed] [Google Scholar]
- 30.Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;123:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurray JJV, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med. 2014;370:1453–1454. doi: 10.1056/NEJMe1401231. [DOI] [PubMed] [Google Scholar]
- 32.Gurwitz JH, Magid DJ, Smith DH, et al. Contemporary prevalence and correlated of incident heart failure with preserved ejection fraction. Am J Med. 2013;126:393–400. doi: 10.1016/j.amjmed.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Assembly of cohorts with dyads of heart failure and chronic lung disease.
Supplementary Figure S2. Assembly of cohorts with dyads of heart failure and chronic kidney disease.
Supplementary Table S1. Additional baseline characteristics of patients with HF and lung disease, stratified by HFrEF or HFpEF status and use of beta-blocker therapy.
Supplementary Table S2. Baseline medication use characteristics of patients with HF and lung disease, stratified by HFrEF or HFpEF status and use of beta-blocker therapy.
Supplementary Table S3. Additional baseline characteristics of patients with HF and CKD stratified by HFrEF or HFpEF status and use of ACE inhibitors/ Angiotensin II receptor blockers (ACEi/ARB).
Supplementary Table S4. Baseline medication use characteristics of patients with HF and CKD stratified by HFrEF or HFpEF status and use of ACE inhibitors/Angiotensin II receptor blockers (ACEi/ARB).


