Abstract
The past decade has brought tremendous progress in unraveling the pathophysiology of Alzheimer’s disease (AD). While increasingly sophisticated immunotherapy targeting soluble and aggregated brain Amyloid-beta (Aβ) continues to dominate clinical research in AD, a deeper understanding of Aβ physiology has led to the recognition of distinct neuronal signaling pathways linking Aβ to synaptotoxicity and neurodegeneration, and new targets for therapeutic intervention. Identifying specific signaling pathways involving Aβ has allowed for the development of more precise therapeutic interventions targeting the most relevant molecular mechanisms leading to AD. In this invited review we highlight the discovery of Cellular Prion Protein (PrPC) as a high affinity receptor for Aβ oligomers (Aβo), and the downstream signaling pathway elucidated to date, converging on non-receptor tyrosine kinase Fyn. We discuss preclinical studies targeting Fyn as a therapeutic intervention in AD, and our recent experience with the safety, tolerability and cerebrospinal fluid (CSF) penetration of the Src family kinase inhibitor saracatinib (AZD0530) in AD patients. Fyn is an attractive target for AD therapeutics, not only based on its activation by Aβ via PrPC, but also due to its known interaction with tau, uniquely linking the two key pathologies in AD. Fyn is also a challenging target, with broad expression throughout the body and significant homology with other members of the Src family kinases, which may lead to unintended off-target effects. A phase IIa proof-of concept clinical trial in AD patients is currently underway, providing critical first data on the potential effectiveness of targeting Fyn in AD.
Keywords: Alzheimer’s disease, Fyn, Cellular Prion Protein, Saracatinib, AZD0530, Amyloid-beta
Introduction
Despite encouraging signs of a decrease in the prevalence and incidence of Alzheimer’s disease (AD), improved longevity is expected to contribute to a continued and dramatic increase in the number of individuals affected by this devastating illness (1, 2). There is still no disease-modifying therapy for AD, but groundbreaking discoveries over the past several decades have significantly improved our understanding of the mechanisms underlying this disease, along with an optimistic outlook with regards to drug development. An important element to this optimism is a diversification of therapeutic targets, complementing and moving beyond ongoing efforts to remove soluble and fibrillar Amyloid-beta (Aβ) by monoclonal antibodies (3–6). One such approach is focusing on the downstream effects of Aβ, rather than its accumulation and aggregation. Soluble assemblies of Aβ, termed Aβ oligomers (Aβo), bind to Cellular Prion Protein (PrPC) on the neuronal cell surface with high affinity, initiating a pathologic cascade converging on the non-receptor tyrosine kinase Fyn (7–9). There is growing evidence that targeting known and emerging elements of this signaling cascade, particularly Fyn, is a promising therapeutic intervention in AD.
Fyn Meets Aβ and Tau– A Brief History
The individual pieces ultimately linking Aβ to Fyn were derived from neuroscience research spanning several decades. Important early work involved the study of long-term potentiation (LTP), first described in 1973, and broadly used as a model of synaptic plasticity in the brain (10, 11). While it was known by 1991 that tyrosine kinase activation by N-methyl-D-aspartate (NMDA) receptor-mediated calcium influx was required for full LTP expression, the identity of the specific kinase(s) critical to this process was not yet determined (12, 13). An early study of LTP in mice with mutations in the non-receptor tyrosine kinases Src, Fyn, Yes, and Abl indicated an important modulatory role of Fyn in LTP induction (14). Further mechanistic work has shown that Src is likely the principal tyrosine kinase regulating both basal NMDAR transmission and LTP induction, while confirming a modulatory role of Fyn (15). Activated Fyn phosphorylates both NR2A and NR2B subunits of the NMDAR, but selectively increases NR2B trafficking and membrane stabilization resulting in increased synaptic expression and enhanced receptor transmission (16–19). In line with these findings, NR2B activation at Tyr1472 is selectively increased in rodents overexpressing Fyn (20). Fyn modulates NR2B in part via its association with the synaptic scaffolding protein postsynaptic density protein 95 (PSD-95) (16, 18). The PSD-95 is part of a protein complex anchoring numerous proteins regulating cell signaling, including kinases, phosphatases, ion channels, and cell surface receptors critical to both LTP and, central to this review, Aβ synaptotoxicity.
Meanwhile, work was well underway to understand the mechanisms of Aβ toxicity. Highly influential was the unexpected finding in 1995 that soluble rather than aggregated Aβ fibrils displayed enhanced toxicity in cell culture (21). Further work with synthetic Aβ identified what is now broadly referred to as Aβ oligomers (Aβo), which are soluble Aβ assemblies ranging from dimers to high molecular weight species (7, 22, 23). Critically, Aβo was shown to be synaptotoxic in neuronal cultures, and potent inhibitors of LTP in slices from rodent brain (22). The latter finding helped push LTP as a favorite in vitro and in vivo model of Aβ toxicity, and numerous studies have replicated the strong inhibition of LTP by a wide range of Aβ oligomer preparations, including human-derived brain homogenates, and endogenous Aβo in mouse models of AD (7, 23–25). Linking Aβo to LTP benefited tremendously from the large body of research already accumulated on LTP mechanisms. In 1998, Fyn was first reported to mediate Aβo-induced inhibition of synaptic plasticity in vitro through a mechanism that remained unknown (22). In rodents, overexpressing Fyn was found to accelerate synapse loss and the onset of cognitive impairment in the J9 (APPswe/Ind) transgenic AD mouse model, while removing Fyn expression rescued synapse loss in the J20 (APPswe/Ind) transgenic AD model (26, 27), the latter replicating and expanding previous in vitro findings.
Prior to this functional association between Aβo and Fyn, there was already emerging evidence linking Fyn to Aβ. Various Aβ preparations were shown to increase tyrosine phosphorylation in vitro (28–30), and histologic analysis of brain sections from patients with AD showed an enhanced Fyn staining pattern in neurons also containing abnormally phosphorylated tau (31). The molecular interaction between fyn and tau has since been greatly refined. Fyn phosphorylates tau at residues near the amino terminus, and this interaction has been postulated to impact AD pathogenesis (32–35). However, in terms of AD pathophysiology, the most relevant interaction between Fyn and tau is likely separate from tau phosphorylation. Tau has a profound impact on Aβ-induced toxicity, both in vitro and in vivo. Indeed, Aβ has no effect on LTP in brain slices without tau expression, and impairments in both spatial memory and synaptic function in transgenic AD mice are reversed in the absence of tau (36, 37). Interestingly, dendritic tau was recently found to function as an intracellular shuttle for Fyn to the post-synaptic density (PSD) (38). This is of major interest as the localization of Fyn to the PSD is critical for Aβo synaptotoxicity (38). Without functional tau, Fyn is uncoupled from NMDARs and other synaptic substrates, and Aβ toxicity is prevented (38). Confirming these findings in vivo, mice lacking tau expression have lower levels of synaptic Fyn compared to wildtype animals (38). More recent evidence indicates that tau phosphorylation at specific residues, and not only its presence or absence, can modulate the interaction between Fyn, PSD95, Tau and the NMDAR. Phosphorylation at T205 on Tau through activation of the p38 mitogen-activated protein (MAP) kinase prevents association of the Fyn- PSD95-Tau-NMDAR complex, and ameliorates Aβo toxicity both in cellular and mouse models of AD (39). The presumed effect of T205 phosphorylation is the functional inhibition of Fyn-mediated signaling critical to Aβo toxicity in vitro and in vivo.
Fyn and the Aβ-PrPC signaling pathway
Taken together, the accumulated data clearly show that extracellular Aβo derived from a variety of sources can disrupt LTP and cause synaptotoxicity in both in vitro and in vivo models of AD. A likely mechanism is through the disruptive action of Aβo on an intracellular signaling cascade important for normal synaptic physiology, mediated by Fyn kinase. But how can extracellular Aβo modulate an intracellular signaling cascade? The first clue to this puzzle came from observing the binding pattern of Aβo on neurons in vitro. When applied directly to cultured neurons, Aβo consistently show a specific and saturable dendritic binding pattern suggesting the presence of a cell surface receptor (7, 40). While many Aβ receptors have been proposed (41), Cellular Prion Protein (PrPC) is one of the highest affinity receptors identified, with an estimated Kd of 0.4nM, exclusively engaging oligomeric Aβ (7). PrPC is a widely expressed protein highly abundant in the central nervous system (42). Consistent with a functional role, removing PrPC expression prevents impairments in LTP by Aβo, and ameliorates deficits in spatial memory and synapse loss in transgenic AD mice (7, 25, 43). PrPC-interacting Aβo emerge at the time of cognitive impairment in several AD mouse lines, supporting a role for this specific Aβ assembly in the pathophysiology of AD in preclinical models (44). Critically, PrPC-interacting Aβ has been consistently found in human AD brain homogenates (9, 25, 45), strongly suggesting that the signaling cascade characterized in preclinical models of AD may also be present in human disease.
When extracellular Aβ is applied to a neuronal culture, intracellular Fyn is phosphorylated at p416 (p419 in human Fyn) in a PrPC-dependent fashion (9, 46). Under these conditions other members of the Src family kinases, including Src, are not activated, suggesting a unique pathway involving Fyn (9). Since PrPC does not cross the plasma membrane, it requires an interaction with the transmembrane, G-protein-coupled metabotropic glutamate receptor 5 (mGluR5) which functionally bridges extracellular Aβo with the intracellular milieu (45, 47, 48). Application of the mGluR5 antagonist 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) prevents Aβo-dependent activation of Fyn, and more recent evidence has shown a genetic interaction between the genes for PrPC (PRNP) and mGluR5 (GRM5) in that removing a single allele in both genes, but neither in isolation, prevents both Fyn activation by Aβo and subsequent synaptotoxicity (45, 47). Fyn activation by Aβo leads to an increase in pY1472 NR2B expression, a process that is also dependent on PrPC (9). Acute Aβo exposure also phosphorylates the eukaryotic elongation factor 2 (eEF2) at T56 (45, 47). eEF2 is phosphorylated by eEF2 kinase (eEF2K) which is known to both associate with mGluR5, and be released upon receptor activation (49). This is of interest as Aβo-induced impairment of LTP is dependent on eEF2 phosphorylation at T56, and eEF2 phosphorylation is increased in brain from both AD transgenic mice and AD patient autopsies (45, 50). Further, inhibiting eEF2K either genetically or pharmacologically prevents Aβo synaptotoxicity in vitro (51). Interestingly, genetic removal of either PRNP or GRM5 prevents Aβo-induced phosphorylation of eEF2 at T56, which is also seen when inhibiting Fyn, either genetically or pharmacologically in vitro (45, 47). Finally, protein tyrosine kinase 2 beta (Pyk2), encoded by the AD risk gene PTK2B, is known to regulate synaptic plasticity, and is phosphorylated and activated by Fyn (52–54). Given its synergistic relationship with Fyn, the anticipated response to acute exposure to extracellular Aβo is an increase in Pyk2 activation, and this is indeed the case (55). Pharmacologic inhibition of Fyn in vitro reverses Pyk2 activation, further implicating this kinase in the Aβo-PrPC-Fyn signaling cascade in AD (55).
In summary, acute exposure to Aβo activates a pathologic intracellular signaling cascade dependent on the expression of PRNP, GRM5, and intracellular activation of Fyn. Based on these findings, Fyn activity might be expected to be elevated in AD brain or during chronic exposure to endogenous Aβo in transgenic rodent models. However, despite evidence of accumulation of total Fyn in AD, more recent work in preclinical models indicates that Fyn activation is suppressed with chronic exposure to Aβo, both in vitro and in vivo (9, 27). Shortly after acute activation by extracellular Aβo in vitro, Fyn returns to baseline and NR2B phosphorylation is decreased. These changes are associated with an increase in STEP (striatal-enriched protein tyrosine phosphatase) which is known to dephosphorylate Fyn and internalize glutamate receptors (9, 56). Under the same conditions, both eEF2 and Pyk2 remain activated in vitro, with likely pathologic significance. In aged transgenic AD mice, a similar, albeit less complete pattern is observed. Fyn activation is suppressed AD transgenic mice (J9) (27), but both eEF2 and Pyk2 remain activated in APP/PS1 AD mice compared to non-transgenic littermates (45, 55). Importantly, genetic removal of either PRNP or GRM5, or a single allele of both, prevents chronic activation of both eEF2 and Pyk2 in vivo (45), and chronic pharmacologic inhibition of Fyn normalizes Pyk2 activation in transgenic AD mice (55).
Whether the same signaling pathway is operational in human AD is not yet fully known. Similar to preclinical studies, Pyk2 decouples from the PrPC protein complex in human AD brain (45). Changes in total and activated Fyn in post-mortem human AD brain are less clear, with conflicting results from several recent studies (46, 57, 58), but the accumulated data thus far strongly support targeting Fyn in AD whether reducing Fyn overactivation directly, or modifying pathologic Fyn-mediated changes in downstream substrates, such as Pyk2.
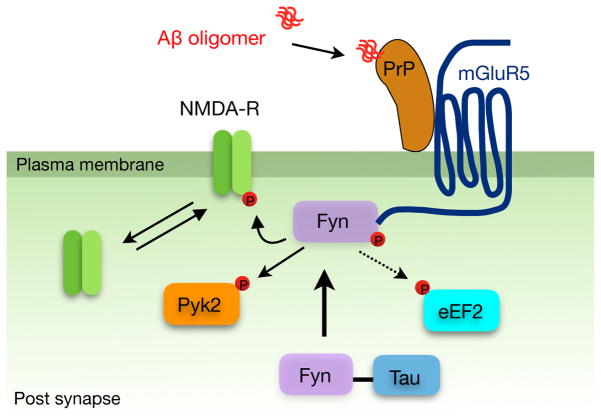
The proposed Aβo signaling cascade is summarized in Figure 1. Acute exposure to Aβo activates a neuronal signaling pathway dependent on PrPC, mGluR5, and Fyn. Due to compensatory mechanisms, NMDA activation is suppressed and Fyn phosphorylation is normalized or depressed with chronic exposure to Aβo, but downstream Fyn substrates, such as Pyk2, remain dysregulated in both rodent and human AD brain Based on these findings, we and others have proposed targeting Fyn as a promising therapeutic strategy in AD (59, 60).
Figure 1. Aβ oligomers (Aβo) bind Cellular Prion Protein (PrPC) to activate Fyn kinase at Tyr416.
Extracellular binding initiates a pathologic intracellular signaling cascade, leading to acute changes in NMDA receptor trafficking, and persistent activation of Pyk2 and eEF2. Fyn plays a central role in the proposed signaling cascade, and can be inhibited in vivo by the orally available Src family kinase inhibitor saracatinib.
Inhibiting Fyn kinase in AD
Fyn is one of 9 members of the Src family of non-receptor tyrosine kinases (SFKs), which also includes Src, Lck, Hck, Blk, Lyn, Fgr, Yes, and Yrk (61, 62). The prototype member of the family is Src kinase, so named for the critical role of viral-Src (vSrc) in cancer transformation (63). The Fyn gene is located on chromosome 6q21, and has 3 known splice variants (64). The known active forms are FynT and FynB, with only the latter as well as SFK members Src, Lck, Lyn, and Yes being highly expressed in the central nervous system (64–66). The structure and regulation of Fyn are critical to current and emerging efforts to target this kinase with high specificity. All SFKs share a short C-terminal tail, which contains the autoinhibitory phosphorylation site Tyr527 (Tyr530 in human Fyn) (61, 62). Tyr527 phosphorylation stabilizes a conformation promoting molecular interactions between the Src Homology domains (SH) 2 and 3, inhibiting kinase activity. Fyn activation is achieved by phosphorylation at Tyr416 within the activation loop of the kinase domain, leading to a conformational change exposing the catalytic site.
As with any drug target, successfully engaging Fyn as a therapeutic approach relies on target specificity and minimizing off-target effects. Most Fyn inhibitors developed thus far bind the catalytic site and act as competitive ATP inhibitors, blocking transfer of the terminal phosphate of ATP to Tyr416 (67). While strict homology in the kinase domain across Src-family kinases generally precludes exclusive specificity for Fyn, several known compounds have reasonable differential specificity for various Src family members, including Fyn. We have focused on the orally bioavailable SFK inhibitor saracatinib (AZD0530), originally developed to target pathogenic signaling pathways in cancer. It is based on quinazoline, an organic compound with a bicyclic base structure consisting of fused benzene and pyrimidine rings, which was successively modified to produce saracatinib (67, 68). Saracatinib acts through reversible ATP inhibition, and in enzymatic assays inhibits both Fyn isoforms with an IC50 value of 8–10nM (55, 69). It also inhibits several other SFKs expressed in the brain, including Src (IC50 2.7nM), Yes (IC50 4 nM), and Lyn (IC50 5nM), but these kinases are not activated by Aβo in vitro (9). Saracatinib also shows activity against Abl, a kinase related to SFKs (IC50 30nM) (68, 69). Using Stat5 phosphorylation as a measure of Abl activity, saracatinib was found to have an IC50 of 156nM against Abl, and plasma from human subjects taking once daily doses up to 125mg of saracatinib does not alter Abl kinase activity (70).
While saracatinib is the first effort to target Fyn by a specific SFK inhibitor in AD, other tyrosine kinase inhibitors have been used in both preclinical AD models, and clinical trials. Masitinib, an orally available tyrosine kinase inhibitor was assessed in a small Phase II clinical trial in AD, showing a trend towards improvements across measures of cognitive function and activities of daily living (71). A Phase III trial (NCT01872598) of masitinib in AD was expected to read out in 2016, but its current status is unclear. Masitinib is selective for colony stimulating factor 1 receptor (CSF-1R) (IC50 90nM), c-Kit (IC50 200nM), platelet-derived growth factor (PDGF; IC50 540nM), Lyn (IC50 500nM), Src (IC50 1.87 uM, and Fyn (IC50 240nM) (72–74). Its promise in AD has been speculated to involve, in part, Fyn inhibition, although this has not been demonstrated experimentally in preclinical AD models or clinical AD (75). Publicly available data are currently insufficient to fully determine whether a masitinib dose of 200mg twice daily, used in the Phase II study in AD, will achieve a plasma concentration equal to or greater than the IC50 of masitinib against Fyn (71, 76). Moreover, the ability of masitinib to penetrate the blood-brain barrier in AD is not known, and thus the extent to which the drug may engage its intended targets in the brain is unclear.
Dasatinib (Bristol Myers Squibb) is a selective and potent SFK inhibitor with a Fyn IC50 of 0.2nM (67, 77). It also inhibits Lck and Src with similar potency (IC50 0.4–0.5 nM). Dasatinib is approved by the US FDA, Health Canada, and the European Medicines Agency for the treatment of chronic myeloid leukemia (CML), and has shown some promise in modulating microglial activation and improving memory function in transgenic AD mice (78). There are no ongoing studies with dasatinib in human AD, but it may be a good drug candidate in AD. Also noteworthy for high Fyn specificity are the pyrazolo [3,4-d]pyrimidines PP1 and PP2 (Pfizer), both with IC50 values of 4–6nM for Fyn and Lck (67, 79). The same compounds have lower affinities for Src (IC50 170nM) and thus a somewhat higher specificity for Fyn compared to other SFK inhibitors. Bosutinib (Pfizer) and ponatinib (ARIAD) are potent, but relatively nonspecific kinase inhibitors approved for clinical use in CML, with IC50 values against Fyn of 0.36–1.8 nM (80–82).
Saracatinib shows high specificity for SFKs, but ubiquitous expression in a variety of tissues highlights the potential for off-target effects. The degree of kinase inhibition sought for the treatment of AD is approximately 50%, thus avoiding many of the issues encountered in drug development programs where nearly complete target blockage is required. Nevertheless, the involvement of Src family kinases in several important physiologic functions has required careful monitoring of key parameters in clinical trials, both related to short-term and long term exposure. Fyn is involved in a number of cellular processes, including cellular proliferation and metastasis (83, 84), T-cell function and the humoral immune response (85–88), central nervous system myelination (89–91), platelet function (92, 93), bone physiology (94).
Fyn inhibition increases bleeding time in rodents, presumably by its interaction with the glycoprotein IIb–IIIa on platelets, and decreases osteoclast activity (92, 94). While episodes of bleeding have been reported in cancer trials, increased bleeding time has not been demonstrated in human subjects treated with saracatinib. In contrast, osteoclast activity is clearly reduced both in healthy volunteers and in patients with AD treated with saracatinib. In the latter group, 100–125mg of saracatinib decreased serum cross-linked C-telopeptide of type 1 collagen (sCTX), a marker of osteoclast activity, by 83–85%, although the effect on bone mass in this population was not tested (70). It is not known whether the possible antiplatelet action of saracatinib might afford some cardiovascular protection in non-cancer patients, or whether the drug has any potential as a therapy for osteoporosis, but both should be considered in chronic studies alongside possible adverse effects.
Arguably, the most relevant off-target effects from a clinical monitoring perspective involve the role of Fyn in the development of the host immune responses. Thus far, there is no clear evidence of opportunistic infections as a consequence of chronic SFK inhibition, but a clinical study of dasatinib in patients with CML showed evidence of cytomegalovirus (CMV) reactivation with a clonal expansion of CMV-specific CD8+ T-cells (95). The precise mechanism for this reactivation is not clear, but is thought to be related to the immunosuppressive action of dasatinib (95). Similar findings have not been reported with saracatinib, possibly due to unique interactions with CD8+ T-cells relative to dasatinib (96), but the available data clearly support continued monitoring of immune function with chronic exposure to saracatinib and other SFK inhibitors.
As is evident by the continued development of AZD0530 in several Phase 2 clinical trials in cancer, saracatinib has excellent pharmacokinetic properties. Its oral bioavailability is >90%, and a half-life of approximately 40 hours allows once daily dosing (97). Steady state is reached after 10–17 days. In mice, the brain half-life of saracatinib is 16 hours, with a plasma:CSF ratio of 3:1 (55). Chronic oral dosing in mice of 5mg/kg/day divided in twice daily dosing yields a trough CSF level of 3.1–7.7ng/ml (5.8–14nM), and a trough brain level of 10–25ng/g (19–46nM) (55). Thus, CSF drug level after oral dosing in mice is approximately 35% of brain level. Considering the IC50 of saracatinib of 10nM, 5mg/kg/day achieves at least 50% of kinase inhibition in the mouse brain.
For preclinical efficacy, we tested several concentrations of saracatinib in two rodent models of AD (APP/PS1 and 3XTg-AD), the latter harboring a mutant tau transgene in addition to APP and PS1 transgenes (55, 98, 99). Chronic oral dosing (4–6 weeks) of 5mg/kg/day of saracatinib fully rescued spatial memory impairments and synaptic loss in APP/PS1 mice, with a reduction in brain microgliosis (55). Interestingly, short-term dosing was not effective, suggesting that the therapeutic effect of saracatinib requires chronic cellular changes that remain to be fully elucidated. In 3XTg-AD mice, a similar dosing regimen reduced insoluble p-tau and total tau by 50%, without altering soluble protein. The mechanisms underlying this effect are not yet fully known, but are likely in part related to known interaction between Fyn and tau. Importantly, 50% reduction of brain Fyn was sufficient to reverse AD-like phenotypes in rodent models, and this formed the bases for determining the dose used in human studies.
The goal in human AD subjects was to achieve an estimated brain saracatinib level approximating 10nM, using an acceptable daily dose of drug. Prior studies in cancer have suggested that 125mg of saracatinib once daily may be the upper limit of what could be well tolerated for chronic dosing in older individuals. In a 1-month phase Ib multiple ascending dose study in patients with AD, 100 and 125mg of once daily oral saracatinib was shown to yield a CSF trough level of 1.1–4.5 ng/mL (2.1–8.3 nM), and 1.4–7.6 ng/ml (2.5–14 nM), respectively (70). Assuming a 1:3 CSF to brain ratio, both 100mg and 125mg of once daily saracatinib are predicted to inhibit brain Fyn >50%, with concentrations estimated to be 7–27nM and 8–46nM, respectively. Thus, in humans, 100–125mg of once daily saracatinib yields a CSF and estimated brain concentrations closely overlapping with the dose and Central Nervous System (CNS) concentrations reversing multiple AD phenotypes rodent models.
AD subjects treated with saracatinib as part of the Phase 1b study were closely monitored for the safety and tolerability. 24 subjects with mild-moderate AD (Mini-Mental State Examination score of 18–26) were enrolled and exposed to 50–125mg of once daily, oral saracatinib for 1 month (70). Each dose group consisted of 8 patients, with a drug:placebo ratio of 3:1. Overall, saracatinib was well tolerated across doses, with all adverse events of either mild or moderate severity. The most common adverse events were diarrhea, headache, fatigue, and nausea, with no statistically difference between placebo and treatment groups. Considering the relatively small cohort size, there was a trend towards more significant adverse events in the 125mg group, which will be further defined in a larger, ongoing study of saracatinib in patients with AD. Exploratory outcome measures, including 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging, Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog), and Clinical Dementia Rating Scale (CDR) did not significantly change with treatment (70). Based on the Phase Ib data, a larger 12-month Phase IIa study of saracatinib for AD was launched in 2015, aiming to treat 152 patients with either 100–125mg of saracatinib, or placebo (NCT02167256). Enrolment for the trial closed in November 2016, and study outcomes are expected to be reported in the first half of 2018. The primary outcome measure for the trial, aside from safety and tolerability, is an improvement in the decline of brain glucose metabolism, measured by 18F-FDG PET.
Conclusion
In summary, there is mounting and compelling evidence implicating the Src family kinase Fyn in the pathophysiology of AD. The major question is whether the same molecular pathways linking Aβo to Fyn in preclinical models are both present and critical in human disease, and whether targeting Fyn has a disease-modifying effect in AD. Saracatinib inhibits Fyn at a nanomolar concentration, crosses the human blood-brain barrier, and oral, once daily dosing achieves a CNS concentration expected to inhibit Fyn by approximately 50%. Notwithstanding important issues related to the optimal timing of a therapeutic intervention in AD and the selection of the most appropriate outcome measures, the ongoing Phase IIa trial of Saracatinib in AD may provide important proof-of-concept that this overall approach has potential as a treatment strategy in AD. Moreover, further work delineating the full molecular components of the Aβo-PrPC-mGluR5 signaling pathway is likely to identify additional targets amendable to pharmacologic intervention in AD.
Acknowledgments
H.B.N. is supported by the US National Institutes of Health Grant UH3TR000967. H.B.N. is a Michael Smith Foundation for Health Research Scholar.
Footnotes
Financial Disclosures: Dr. Nygaard reports no biomedial financial interests or potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. The New England journal of medicine. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Nygaard HB. Current and emerging therapies for Alzheimer's disease. Clinical therapeutics. 2013;35:1480–1489. doi: 10.1016/j.clinthera.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. The New England journal of medicine. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 5.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. The New England journal of medicine. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 7.Laurén J, Gimbel D, Nygaard H, Gilbert J, Strittmatter S. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygaard HB, Strittmatter SM. Cellular prion protein mediates the toxicity of beta-amyloid oligomers: implications for Alzheimer disease. Arch Neurol. 2009;66:1325–1328. doi: 10.1001/archneurol.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, et al. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 12.O'Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- 13.Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- 14.Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 15.Yu XM, Askalan R, Keil GJ, 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 18.Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. The FEBS journal. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 20.Xia D, Gotz J. Premature lethality, hyperactivity, and aberrant phosphorylation in transgenic mice expressing a constitutively active form of Fyn. Front Mol Neurosci. 2014;7:40. doi: 10.3389/fnmol.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, et al. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1–42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 22.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 25.Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, et al. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo YQ, Hirashima N, Li YH, Alkon DL, Sunderland T, Etcheberrigaray R, et al. Physiological levels of beta-amyloid increase tyrosine phosphorylation and cytosolic calcium. Brain Res. 1995;681:65–74. doi: 10.1016/0006-8993(95)00282-u. [DOI] [PubMed] [Google Scholar]
- 29.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grace EA, Busciglio J. Aberrant activation of focal adhesion proteins mediates fibrillar amyloid beta-induced neuronal dystrophy. J Neurosci. 2003;23:493–502. doi: 10.1523/JNEUROSCI.23-02-00493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirazi SK, Wood JG. The protein tyrosine kinase, fyn, in Alzheimer's disease pathology. Neuroreport. 1993;4:435–437. doi: 10.1097/00001756-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Bhaskar K, Hobbs GA, Yen SH, Lee G. Tyrosine phosphorylation of tau accompanies disease progression in transgenic mouse models of tauopathy. Neuropathol Appl Neurobiol. 2010;36:462–477. doi: 10.1111/j.1365-2990.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem. 2005;280:35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- 34.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, et al. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 36.Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 38.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Ittner A, Chua SW, Bertz J, Volkerling A, van der Hoven J, Gladbach A, et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer's mice. Science. 2016;354:904–908. doi: 10.1126/science.aah6205. [DOI] [PubMed] [Google Scholar]
- 40.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarosz-Griffiths HH, Noble E, Rushworth JV, Hooper NM. Amyloid-beta Receptors: The Good, the Bad, and the Prion Protein. J Biol Chem. 2016;291:3174–3183. doi: 10.1074/jbc.R115.702704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris DA, Lele P, Snider WD. Localization of the mRNA for a chicken prion protein by in situ hybridization. Proc Natl Acad Sci U S A. 1993;90:4309–4313. doi: 10.1073/pnas.90.9.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, et al. Prion-Protein-interacting Amyloid-beta Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. J Biol Chem. 2015;290:17415–17438. doi: 10.1074/jbc.M115.643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC, Strittmatter SM. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain : a journal of neurology. 2016;139:526–546. doi: 10.1093/brain/awv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, et al. The complex PrP(c)-Fyn couples human oligomeric Abeta with pathological tau changes in Alzheimer's disease. J Neurosci. 2012;32:16857–16871a. doi: 10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas LT, Strittmatter SM. Oligomers of Amyloid beta Prevent Physiological Activation of the Cellular Prion Protein-Metabotropic Glutamate Receptor 5 Complex by Glutamate in Alzheimer Disease. J Biol Chem. 2016;291:17112–17121. doi: 10.1074/jbc.M116.720664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, et al. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci. 2014;34:12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jan A, Jansonius B, Delaidelli A, Somasekharan SP, Bhanshali F, Vandal M, et al. eEF2K inhibition blocks Abeta42 neurotoxicity by promoting an NRF2 antioxidant response. Acta Neuropathol. 2017;133:101–119. doi: 10.1007/s00401-016-1634-1. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, et al. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- 53.Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J Leukoc Biol. 2010;87:691–701. doi: 10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- 54.Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Annals of neurology. 2015 doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 57.Lau DH, Hogseth M, Phillips EC, O'Neill MJ, Pooler AM, Noble W, et al. Critical residues involved in tau binding to fyn: implications for tau phosphorylation in Alzheimer's disease. Acta Neuropathol Commun. 2016;4:49. doi: 10.1186/s40478-016-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zahratka JA, Shao Y, Shaw M, Todd K, Formica SV, Khrestian M, et al. Regulatory region genetic variation is associated with FYN expression in Alzheimer's disease. Neurobiol Aging. 2017;51:43–53. doi: 10.1016/j.neurobiolaging.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang K, Belrose J, Trepanier CH, Lei G, Jackson MF, MacDonald JF. Fyn, a potential target for Alzheimer's disease. J Alzheimers Dis. 2011;27:243–252. doi: 10.3233/JAD-2011-110353. [DOI] [PubMed] [Google Scholar]
- 60.Nygaard HB, van Dyck CH, Strittmatter SM. Fyn kinase inhibition as a novel therapy for Alzheimer's disease. Alzheimer's research & therapy. 2014;6:8. doi: 10.1186/alzrt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 62.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 63.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 64.Goldsmith JF, Hall CG, Atkinson TP. Identification of an alternatively spliced isoform of the fyn tyrosine kinase. Biochem Biophys Res Commun. 2002;298:501–504. doi: 10.1016/s0006-291x(02)02510-x. [DOI] [PubMed] [Google Scholar]
- 65.Cooke MP, Perlmutter RM. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. The New biologist. 1989;1:66–74. [PubMed] [Google Scholar]
- 66.Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends in neurosciences. 2011;34:629–637. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr Med Chem. 2011;18:2921–2942. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
- 68.Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 69.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, et al. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. 2009;3:248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease. Alzheimer's research & therapy. 2015;7:35. doi: 10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piette F, Belmin J, Vincent H, Schmidt N, Pariel S, Verny M, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer's disease: a randomised, placebo-controlled phase 2 trial. Alzheimer's research & therapy. 2011;3:16. doi: 10.1186/alzrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PloS one. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hermine O, Moussy A, Mansfield C, Dubreuil P. Masitinib for the treatment of severe systemic mastocytosis: Pooled phase 2 study simulation of phase 3 population and response criteria. 7th EMBRN International Mast Cell and Basophil Meeting; Marseille, France. 2015. [Google Scholar]
- 74.Trias E, Ibarburu S, Barreto-Nunez R, Babdor J, Maciel TT, Guillo M, et al. Post-paralysis tyrosine kinase inhibition with masitinib abrogates neuroinflammation and slows disease progression in inherited amyotrophic lateral sclerosis. Journal of neuroinflammation. 2016;13:177. doi: 10.1186/s12974-016-0620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Folch J, Petrov D, Ettcheto M, Pedros I, Abad S, Beas-Zarate C, et al. Masitinib for the treatment of mild to moderate Alzheimer's disease. Expert Rev Neurother. 2015;15:587–596. doi: 10.1586/14737175.2015.1045419. [DOI] [PubMed] [Google Scholar]
- 76.EMA. Assessment report, Masiviera (Masitinib) European Medicines Agency; 2014. EMEA/H/C/002659/0000. [Google Scholar]
- 77.Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, et al. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49:6819–6832. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- 78.Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer's disease. Journal of neuroinflammation. 2012;9:117. doi: 10.1186/1742-2094-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 80.Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, et al. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem. 2001;44:3965–3977. doi: 10.1021/jm0102250. [DOI] [PubMed] [Google Scholar]
- 81.Boschelli F, Arndt K, Gambacorti-Passerini C. Bosutinib: a review of preclinical studies in chronic myelogenous leukaemia. Eur J Cancer. 2010;46:1781–1789. doi: 10.1016/j.ejca.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 82.O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, Yang Y, Hu Y, Dang D, Regezi J, Schmidt BL, et al. Alphavbeta6-Fyn signaling promotes oral cancer progression. J Biol Chem. 2003;278:41646–41653. doi: 10.1074/jbc.M306274200. [DOI] [PubMed] [Google Scholar]
- 84.Fresno Vara JA, Caceres MA, Silva A, Martin-Perez J. Src family kinases are required for prolactin induction of cell proliferation. Mol Biol Cell. 2001;12:2171–2183. doi: 10.1091/mbc.12.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 86.Tamura T, Igarashi O, Hino A, Yamane H, Aizawa S, Kato T, et al. Impairment in the expression and activity of Fyn during differentiation of naive CD4+ T cells into the Th2 subset. Journal of immunology. 2001;167:1962–1969. doi: 10.4049/jimmunol.167.4.1962. [DOI] [PubMed] [Google Scholar]
- 87.Sugie K, Kawakami T, Maeda Y, Kawabe T, Uchida A, Yodoi J. Fyn tyrosine kinase associated with Fc epsilon RII/CD23: possible multiple roles in lymphocyte activation. Proc Natl Acad Sci U S A. 1991;88:9132–9135. doi: 10.1073/pnas.88.20.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugie K, Jeon MS, Grey HM. Activation of naive CD4 T cells by anti-CD3 reveals an important role for Fyn in Lck-mediated signaling. Proc Natl Acad Sci U S A. 2004;101:14859–14864. doi: 10.1073/pnas.0406168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 90.Sperber BR, McMorris FA. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. Journal of neuroscience research. 2001;63:303–312. doi: 10.1002/1097-4547(20010215)63:4<303::AID-JNR1024>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 91.Laursen LS, Chan CW ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy KB, Smith DM, Plow EF. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J Cell Sci. 2008;121:1641–1648. doi: 10.1242/jcs.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horak ID, Corcoran ML, Thompson PA, Wahl LM, Bolen JB. Expression of p60fyn in human platelets. Oncogene. 1990;5:597–602. [PubMed] [Google Scholar]
- 94.Hannon RA, Clack G, Rimmer M, Swaisland A, Lockton JA, Finkelman RD, et al. Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double-blind, placebo-controlled, multiple-ascending-dose phase I trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:463–471. doi: 10.1359/jbmr.090830. [DOI] [PubMed] [Google Scholar]
- 95.Kreutzman A, Ladell K, Koechel C, Gostick E, Ekblom M, Stenke L, et al. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia. 2011;25:1587–1597. doi: 10.1038/leu.2011.135. [DOI] [PubMed] [Google Scholar]
- 96.Takai S, Sabzevari H, Farsaci B, Schlom J, Greiner JW. Distinct effects of saracatinib on memory CD8+ T cell differentiation. Journal of immunology. 2012;188:4323–4333. doi: 10.4049/jimmunol.1101439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baselga J, Cervantes A, Martinelli E, Chirivella I, Hoekman K, Hurwitz HI, et al. Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin Cancer Res. 2010;16:4876–4883. doi: 10.1158/1078-0432.CCR-10-0748. [DOI] [PubMed] [Google Scholar]
- 98.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Human molecular genetics. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 99.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]