Abstract
Traumatic brain injury (TBI) is associated with acute cerebral metabolic crisis (ACMC). ACMC-related atrophy appears to be prominent in frontal and temporal lobes following moderate-to-severe TBI. This atrophy is correlated with poorer cognitive outcomes in TBI. The current study investigated ability of acute glucose and lactate metabolism to predict long-term recovery of frontal-temporal cognitive function in participants with moderate-to-severe TBI. Cerebral metabolic rate of glucose and lactate were measured by the Kety-Schmidt method on days 0–7 post-injury. Indices of frontal-temporal cognitive processing were calculated for 6 months post-injury, 12 months post-injury, and recovery (the difference between the 6 and 12 month scores). Glucose and lactate metabolism were included in separate regression models, as they were highly intercorrelated. Also, glucose and lactate values were centered and averaged and included in a final regression model. Models for the prediction frontal-temporal cognition at 6 and 12 months post-injury were not significant. However, average glucose and lactate metabolism predicted recovery of frontal-temporal cognition, accounting for 23% and 22% of the variance, respectively. Also, maximum glucose metabolism, but not maximum lactate metabolism, was an inverse predictor recovery of frontal-temporal cognition, accounting for 23% of the variance. Finally, the average of glucose and lactate metabolism predicted frontal-temporal cognitive recovery, accounting for 22% of the variance. These data indicate that acute glucose and lactate metabolism both support cognitive recovery from TBI. Also, our data suggest that control of endogenous fuels and/or supplementation with exogenous fuels may have therapeutic potential for cognitive recovery from TBI.
Keywords: Traumatic brain injury, brain metabolism, cognition, neuropsychology, glucose, lactate
Graphical Abstract

INTRODUCTION
Decreases in oxygen metabolism and increases in glucose metabolism, as well as elevated lactate levels, commonly occur days after TBI (Glenn et al., 2003; Vespa et al. 2005). This abnormal increase in glucose metabolism is followed by a period of metabolic depression. Elevated lactate levels, the product of anaerobic glycolysis, occur due a decrease in cerebral oxygen (Jalloh et al., 2013). Recent studies demonstrated that when the brain’s glucose levels are low, lactate may be utilized as an alternative fuel (Bouzat et al., 2014; Jalloh et al., 2013).
Research indicates that early metabolic dysfunction due to TBI varies greatly by brain region and time since injury (Hattori et al., 2003; Marcoux et al., 2008; Vespa et al., 2004; Vespa et al., 2007; Xu et al., 2010). This acute cerebral metabolic crisis (ACMC) is most related to chronic atrophy in the frontal and temporal lobes (Xu et al., 2010) and extends beyond pericontusional tissue. Also, this atrophy is not due to ischemia and appears distinct from diffuse axonal injury (DAI; Hattori et al., 2003; Xu et al., 2010).
Chronic impairments in attention, psychomotor speed, memory, and executive function are routinely observed following moderate-to-severe TBI. This cognitive profile has often been characterized as ‘frontal’ or ‘frontal-temporal’ (Stuss and Gow, 1992; Lezak et al., 2004; Vakil, 2005). Numerous studies have determined that the presence of DAI and focal lesions, frequently located in the frontal and temporal lobes, are associated with poorer cognitive outcomes following TBI (Auerbach, 1986; Bigler, 2001; Fork et al. 2005; Kraus et al., 2007; Lehtonen et al., 2005; Levine et al., 2002; Wallesch et al., 2001; Williamson et al., 1996). Interestingly, our group found a relationship between long-term cognitive outcomes and frontal and temporal lobe atrophy associated ACMC following TBI (Wright et al., 2013). Specifically, ACMC-related brain atrophy correlated with attention, executive function, and psychomotor speed at 12 months post-TBI.
In the current study, we assessed neuropsychological function at 6 and 12 months post-injury in a sample of TBI participants who exhibited non-ischemic ACMC. We investigated glucose and lactate metabolism directly via Kety-Schmidt jugular bulb catheterization. Given the frontal-temporal cognitive signature of TBI and the impact of acute metabolic crisis on the frontal and temporal lobes, our goal was to determine if early markers of global glucose and lactate metabolism predict indices of cognition that require interaction between the frontal and temporal lobes.
Based on our previous work, we hypothesized that greater glucose and lactate metabolism would be associated with better recovery in frontal-temporal cognitive processing. In addition, we hypothesized that greater rates of glucose and lactate metabolism would be predictive of improved performance on indices of cognition at 6 months and 12 months post-injury.
MATERIALS AND METHODS
Participants
The current study was approved by the institutional review board of the University of California, Los Angeles and all participants consented directly or by proxy to voluntary participation. Eligible participants consisted of patients with moderate to severe head injuries who were admitted to UCLA Medical Center within 24 hours of injury between 09/06/1999 and 03/12/2006. The participant inclusion criteria comprised a Glasgow Coma Score (GCS) of 8 or less, or a GCS between 9 and 15 if an initial computerized tomography (CT) brain scan showed evidence of intracranial bleeding, brain metabolism assessment via the Kety-Schmidt method, and completion of a brief neuropsychological test battery at approximately 6 and 12 months post-injury. The current study included 27 participants.
On average, the sample was in their late twenties/early thirties (M =29.08, SD = 11.22; Mdn = 27.00, R = 42.00), completed some college (M = 13.39 years of education; SD = 3.07), primarily male (81.48%; N = 22) and Caucasian (55.56%). Closed head injures (55.56%) were most common and the majority of injuries were due to acceleration-deceleration events [40.74% motor vehicle accident (MVA), 22.22% long fall, 14.81% MVA versus pedestrian, 11.11% bicycle, 7.40% blunt force trauma, and 3.70% gunshot). The average participant ER GCS score was 7.87 (SD = 3.28; Mdn = 7.00, R = 11), which generally falls within the severe TBI range.
Measures
Neuropsychological instruments
We employed F-A-S (phonemic fluency) from the Controlled Oral Word Association Test (F-A-S; Benton, Hemsher, Varney, and Spreen, 1983), and the 6-trial version of the Buschke Selective Reminding Test (SRT-6; Hannay and Levin, 1985).
Phonemic fluency has been associated with frontal and temporal lobe function (Martin, Loring, Meador, and Lee, 1990; Meyers and Rohling, 2009), as it assesses rule governed generation of words that requires both frontal-executive skills and temporal-language abilities. Specifically, this test requires speedy production of words beginning with a specific letter, given a set of restrictions (i.e., no proper nouns or repeating words with different suffixes).
The SRT-6 is a test of verbal memory and learning. Unlike other list-learning tasks, on each trial after the first, the examinees are only presented with the words that they did not recall during previous trials. The SRT-6 long-term storage (LTS) score is the number of words that have been recalled on two consecutive trials and thus are assumed to have entered long-term storage; the items are thought to have been successfully encoded and stored, engaging both the frontal and temporal lobes (Wright et al., 2010). The SRT-6 LTS score was included because it is a measure of memory acquisition (encoding and storage). Encoding and storage have been shown to involve frontal and temporal lobe structures (Meyers and Rohling, 2009; Habib, Nyberg, and Tulving, 2003; Bernard et al., 2001). Additionally, the degree of long-term memory impairment as measured by the SRT one year after a severe TBI corresponds to the overall level of disability in TBI survivors (Levin et al., 1979).
Procedures
Acute care protocol
After initial stabilization, all participants were admitted to a neurointensive care unit. Hematomas and intracranial mass lesions were evacuated by craniotomy. Intracranial pressure (ICP) was maintained below 20mmHg by means of a standardized stepwise treatment protocol that included head of bed elevation to 30 degrees, mild hyperventilation (PaCO2 = 30–35mmHg), external ventricular drainage, low doses of propofol for moderate sedation, maintenance of normal blood sugar (80 to 120mg/dL), and maintenance of mildly elevated sodium level (sodium = 140 to 145 mmol/L). If these measures did not control ICP, pentobarbital was used to induce burst suppression coma. Cerebral perfusion pressure was maintained above 60mmHg using volume repletion and anti-hypertensive medication. Jugular venous oxygen saturation was continually monitored and maintained between 60% and 70% by adjusting cerebral perfusion pressure. Continuous electroencephalography was used to monitor for possible seizure activity and barbiturate effects. Acute rehabilitation was provided to all participants.
Assessment of glucose and lactate cerebral metabolic rates
The Kety-Schmidt jugular bulb catheterization method, widely recognized as the most accurate technique for the evaluation of global cerebral metabolism (Nybo et al., 2002), was utilized for the current study because it provides continuous monitoring of brain metabolism. The catheter is inserted into the jugular bulb, which is the preferred location for sampling blood from the brain. The jugular bulb is the dilated or enlarged portion of the internal jugular vein that is situated just below the base of the skull (Schell and Cole, 2000). The blood in the jugular bulb originates from both cerebral hemispheres (approximately 70% ipsilateral and 30% contralateral); however, it is commonly accepted that there is a dominant side of venous drainage, generally the right, in the majority of participants.
In the current study, all participants underwent intubation and jugular bulb catheterization via the Kety-Schmidt method (Glenn et al., 2003) and their cerebral metabolic rates of glucose (CMRglc) and lactate (CMRlac) were assessed on days 0–7 post-injury via sampling from the radial artery. These samples were taken either in the morning (53% of the time; between 09:00 & 11:00: M = 10:35, SD = 0:27) or afternoon (47% of the time; between 12:00 & 15:00: M = 14:26, SD = 1:22). Participants had a jugular bulb catheter inserted as soon as possible following admission to allow serial measurements of cerebral blood flow (CBF), and the differences in arterial and venous glucose (AVDglc), lactate (AVDlac), and O2 concentrations (AVDO2). The catheter was inserted in the dominant jugular vein, which was selected based on the CT scan performed when the participant was admitted. In accordance with standard techniques, the catheter was inserted in the vein until resistance was encountered, approximately 15 cm, and then placement was verified by lateral skull x-ray. The catheter was calibrated in vivo and calibration was conducted every 12 hours. Arterial and venous samples were collected every 12 hours for the first 7 days post-injury while the bedside CBF was scheduled every 12 hours for the first 48 hours and then daily on days 2–7 post-injury.
CBF was assessed via daily measurements that were performed using the intravenous 133Xe clearance technique using a portable apparatus (Cerebrograph Cortexplorer 16a) from Ceretronix, Randers, Denmark as previously described (Glenn et al., 2003). ~20 to 30 mCi of gaseous 133Xe dissolved in a saline solution was injected intravenously for each study. The CBF unit estimates cerebral perfusion by analyzing 133Xe wash-out curves, which were measured over 11 minutes from 8 extracranial sodium iodide detectors located over the cerebral hemispheres (over the middle cerebral artery territory). Computer software was used to calculate via a two-compartment model the clearance decay curve to 15 minutes; this has been shown to be very stable in patients with reduced CBF (Obrist and Marion, 1996). Clearance curve analysis considers the arterial input function using monitoring of 133Xe in the end-tidal expired air. A derived height-over-area equation was used to calculate the global CBF15 from the sixteen detectors (Obrist and Wilkinson, 1990). The CBF15 values reported are are not corrected for pCO2. Participants underwent serial CBF measurements typically starting between 12 and 24 hours after admission and following the established Brain Injury Research Center protocol (on post-injury days 0–6, 8, and 10 or as needed clinically).
The CMRglc was calculated as the product of CBF15 and the difference in concentration of glucose between AVDglc, and the CMRlac was the product of CBF15 and the difference in concentration of lactate between AVDlac. CBF15 is a cerebral blood flow parameter that was calculated utilizing a two-component mathematical model. CBF15 represents the mean blood flow of the fast clearing (gray matter) and slow clearing (white matter) compartments of the brain (Obrist et al., 1984). CBF15 was used to calculate CMRglc and CMRlac because it has been shown to be very stable in participants with reduced blood flow (Glenn et al., 2003). For this study, the CMRglc and CMRlac values were averaged over days 0–7 days post-injury; for participants where CMRglc and CMRlac were not collected for all of the days, their three or more days (over days 0–7) of metabolism assessments were averaged. Overall, CMRglc tends to be depressed following TBI and CMRlac is often more positive during the first 48 hours in contrast to healthy persons (Glenn et al., 2003). That said, cerebral metabolic rate of oxygen appears to be generally depressed during acute recovery (Glenn et al., 2003).
Neuropsychological assessment
Neuropsychological testing was conducted by a clinical neuropsychologist using a modified version of a test battery designed for TBI clinical trials (Clifton et al., 1992). All tests were administered and scored in accordance with standard instructions. The assessments were conducted ~ 6 and 12 months post-injury. Test scores were normed by age and education for group comparisons. This was accomplished by calculating T-scores (M = 50, SD = 10) using common normative datasets.
The F-A-S score and SRT-6 long-term storage (LTS) score were chosen to represent frontal-temporal cognitive processing. The F-A-S scores were converted to T-scores via metanorms (Mitrushina et al., 2005), and the SRT-6 scores were converted to T-scores via the normative dataset provided by Larrabee et al. (2000). After the long-term storage score from the SRT-6 and the total F-A-S score were converted to T-scores, they were averaged for both the 6 and 12 month performances to produce indices of frontal-temporal cognitive processing. The 6 month index score was subtracted from the 12 month index score to provide a recovery index of frontal-temporal cognition [((6-months post-injury: F-A-S T-score + LTS T-score)/2) – ((12 months-post-injury: F-A-S T-score + LTS T-score)/2) = frontal-temporal recovery].
Statistical Analysis
All statistical analyses were conducted in Statistical Package for Social Sciences (SPSS), version 23. A threshold of p < .05 for statistical significance was set for all analyses. Simple linear regressions were utilized to predict cognitive outcomes from average and maximum glucose and lactate metabolism at 6 months post-injury, 12 months post-injury, and cognitive recovery between 6 and 12 months post-injury. As expected, glucose and lactate metabolic rates were highly intercorrelated (r= .93; VIF= 6.97). Accordingly, separate regressions were carried out for glucose and lactate metabolism. Also, the glucose and lactate values were centered and averaged for a final regression model.
RESULTS
We found higher average CMRglc and maximum CMRglc did not result in significantly better frontal-temporal cognitive processing at 6 and 12 months post-injury [b= −.22, t(26)= −1.12, p= .27, F(1,26)= 1.25, R2= .05; b= .01, t(26)= .06, p= .96; F(1,26)= 0.00, R2= .00; & b= −.22, t(26)= −1.91, p= .07, F(1,26)= 3.65, R2= .11; b= −.4.84, t(26)= −.32, p= .75; F(1,26)= 0.10, R2= .00, respectively]. Also, higher average CMRlac and maximum CMRlac did not result in significantly better frontal-temporal processing at 6 and 12 months post-injury [b= −.25, t(26)= −1.30, p= .21, F(1,26)= 1.68, R2= .06; b= −.03, t(26)= .08, p= .88, F(1,26)= 0.02, R2= .00; & b= −4.60, t(26)= −.34, p= .73, F(1,26)= 0.12, R2= .00; b= −13.33, t(26)= −.95, p= .35, F(1,26)= .91, R2= .03, respectively]. Finally, the average of the centered values for CMRglc and CMRlac did not result in significantly better frontal-temporal cognition scores at 6 and 12 months post-injury [b= −.239, t(26)= −1.230, p= .23, F(1,26)= 1.51, R2= .06; b= −.01, t(26)= −.05, p= .96, F(1,26)= 0.00, R2= .00, respectively].
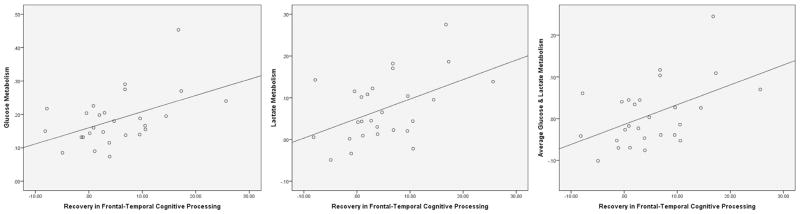
That said, average CMRglc and maximum CMRglc were predictive of participant recovery of frontal-temporal cognition [b= .48, t(26)= 2.74, p= .01, F(1,26)= 7.53, R2= .23 & b= .42, t(26)= −2.76, p= .01, F(1,26)= 7.62, R2= .23, respectively]. That said, average CMRglc was a positive predictor of recovery, while maximum CMRglc was a negative predictor. Moreover, most of the maximum CMRglc scores (56%) occurred earlier during acute recovery (days 0–3). Average CMRlac was also predictive of participant recovery of frontal-temporal cognition, but maximum CMRlac was not [b= .468, t(26)= 2.65, p= .01, F(1,26)= 7.01, R2= .22 & b= 8.98, t(26)= 2.65, p= .13, F(1,26)= 2.46, R2= .09]. Finally, average CMRglc and CMRlac over days 0–7 was predictive of recovery frontal-temporal cognition in participants [b= .48, t(26)= 2.76, p= .01, F(1,26)= 7.63, R2= .23] (see Figure 1).
Figure 1.
Scatter plots with regression lines depicting the relationship between acute brain metabolism and recovery in frontal-temporal cognitive processing in TBI participants. Linear regression revealed that greater acute glucose, lactate, and average glucose and lactate metabolism predicted recovery of frontal-temporal cognitive processing (R2s = .22–23). Acute glucose, lactate, and average glucose and lactate metabolism values are displayed in average mg/100g/min. Frontal-temporal processing scores represent the difference between T-scores (M =50, SD =10) for neuropsychological performances at 6 months and 12 months post-injury.
DISCUSSION
While greater acute glucose and lactate metabolism (assessed by the Kety-Schmidt jugular bulb catheterization method) did not significantly predict frontal-temporal cognitive processing at 6 or 12 months post-TBI, both did predict recovery in frontal-temporal processing. More specifically, CMRglc and CMRlac, and the average of centered values for CMRglc and CMRlac predicted recovery in between 6 and 12 months-post injury, accounting for 22–23% of the variance. Also, maximum CMRglc was found to be a negative predictor of recovery in frontal-temporal processing; suggesting that maximum CMRglc, which tended to occur earlier in recovery, was related to hyperglycolysis and may be indicative of increased injury severity. Overall, our results suggest that sustained, greater acute glucose and lactate metabolism benefit cognitive recovery from TBI. While the current study was novel in that it investigated the ability of direct measures of acute glucose and lactate metabolism to predict recovery of frontal-temporal cognitive processing in TBI participants, the results are based on data from a relatively small sample. This reduced our ability to examine the full complexity of the relationship between acute brain metabolism and cognitive outcomes following TBI (e.g., potential moderators, variations in metabolism over time, etc.). Also, as this was a human study of TBI, we utilized a sample of convenience. Additionally, our measures of glucose and lactate metabolism were general, they were not specific to any given brain region. Nevertheless, our data corroborate our previous work with greater spatial resolution (utilizing positron emission tomography and magnetic resonance imaging) that suggested that ACMC results in frontal and temporal lobe atrophy and poorer cognitive outcomes following TBI (Wright et al., 2013). Moreover, while the current study made use of global measures of cerebral metabolism, we also utilized neuropsychological measures that are somewhat specific to frontal and temporal lobe processing (Martin, Loring, Meador, and Lee, 1990; Bernard et al., 2001; Habib, Nyberg, and Tulving, 2003; Meyers and Rohling, 2009).
In conclusion, the present findings further confirm that ACMC is related to cognitive outcomes following TBI and suggest that it may play a significant role in the ‘frontal-temporal’ cognitive profile associated with TBI. Moreover, these data are consistent the notion that both glucose and lactate are utilized as fuels sources following TBI (Glenn et al., 2003; Glenn et al., 2015). In addition, our data, like other studies (e.g., Meng et al., 2009; Quintard et al., 2016), suggests that control of endogenous fuels and/or supplementation with exogenous fuels (e.g., infusion of lactate) may improve outcomes from TBI.
SIGNIFICANCE/LAY SUMMARY.
Traumatic brain injury (TBI) results in neuropathology via primary and secondary mechanisms (Werner and Engelhard, 2007). The current study is concerned with a secondary injury mechanism, acute cerebral metabolic crisis (ACMC). ACMC has been associated with neuropathology (Xu et al., 2010), cognitive deficits (Wright et al., 2013), and functional decline (Glenn et al., 2003) following TBI. We show that both greater glucose and lactate metabolism days after injury are related to improved cognitive recovery from TBI. These findings support the potential therapeutic benefit of interventions focused on controlling and/or supplementing fuel sources during acute recovery from TBI.
Acknowledgments
Grant support: This research was supported by NS049471, NS02089, and the California State Neurotrauma Initiative.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ROLE OF AUTHORS
MJW, TCG, and CM designed the study. TCG, PV, MJW, and DLM collected all data. MJW and CM conducted the analyses. MJW, CM, TCG, PMV, DLM, DAH, and JDV drafted the manuscript. Grant support for the study was obtained by DAH and PMV.
References
- Auerbach SH. Neuroanatomical correlates of attention and memory in traumatic brain injury: an application of neurobehavioral subtypes. Journal of Head Trauma Rehabilitation. 1986;1:1–12. [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment. New York, NY: Oxford University Press; 1983. [Google Scholar]
- Bernard F, Desgranges B, Platel H, Baron J, Eustache F. Contributions of frontal and medial temporal regions to verbal episodic memory: A PET study. Neuroreport. 2001;12:1737–1741. doi: 10.1097/00001756-200106130-00044. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bouzat P, Sala N, Suys T, ZJ-B, Marques-Vidal P, Feihl F, Bloch J, Messerer M, Levivier M, Meuli R, Magistretti PJ, Oddo M. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Medicine. 2014;40:412–421. doi: 10.1007/s00134-013-3203-6. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Hayes RL, Levin HS, Michel ME, Choi SC. Outcome measures for clinical trials involving traumatically brain-injured patients: report of a conference. Neurosurgery. 1992;31:975–978. doi: 10.1227/00006123-199211000-00028. [DOI] [PubMed] [Google Scholar]
- Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch C. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Injury. 2005;19:101–108. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. Journal of Cerebral Blood Flow Metabolism. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Martin NA, McArthur DL, Hovda DA, Vespa P, Johnson ML, Horning MA, Brooks GA. Endogenous nutritive support after traumatic brain injury: Peripheral lactate production for glucose supply via gluconeogenesis. Journal of Neurotrauma. 2015;32:811–819. doi: 10.1089/neu.2014.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: The HERA model revisited. Trends in Cognitive Sciences. 2003;7:241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Levin HS. Selective reminding test: an examination of the equivalence of four forms. Journal of Clinical Experimental Neuropsychology. 1985;7:251–263. doi: 10.1080/01688638508401258. [DOI] [PubMed] [Google Scholar]
- Hattori N, Hung SC, Wu HM, Yeh E, Glenn TC, Vespa PM, McArthur D, Phelps ME, Hovda DA, Bergsneider M. Correlation of regional metabolic rates of glucose with Glasgow Coma Scale after traumatic brain injury. Journal of Nuclear Medicine. 2003;44:1709–1716. [PubMed] [Google Scholar]
- Jalloh I, Helmy A, Shannon RJ, Gallagher CN, Menon DK, Carpenter KL, Hutchinson PJ. Lactate uptake by the injured human brain: evidence from an arteriovenous gradient and cerebral microdialysis study. Journal of Neurotrauma. 2013;15:2031–2037. doi: 10.1089/neu.2013.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrabee GJ, Trahan DE, Levin HS. Normative data for a six-trial administration of the verbal selective reminding test. Clinical Neuropsychology. 2000;14:110–118. doi: 10.1076/1385-4046(200002)14:1;1-8;FT110. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Stringer AY, Millis S, Boake C, Englander J, Hart T, High W, Macciocchi S, Meythaler J, Novack T, Whyte J. Neuropsychological outcome and community re-integration following traumatic brain injury: the impact of frontal and non-frontal lesions. Brain Injury. 2005;19:239–256. doi: 10.1080/0269905040004310. [DOI] [PubMed] [Google Scholar]
- Levin HS, Grossman RG, Rose JE, Teasdale G. Long-term neuropsychological outcome of closed head injury. Journal of Neurosurgery. 1979;50:412–422. doi: 10.3171/jns.1979.50.4.0412. [DOI] [PubMed] [Google Scholar]
- Levine B, Cabeza R, McIntosh AR, Black SE, Grady CL, Stuss DT. Functional reorganization of memory after traumatic brain injury: a study with H2 15O positron emission topography. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, Hovda DA, Alger JR, Vespa PM. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Critical Care Medicine. 2008;36:2871–2877. doi: 10.1097/CCM.0b013e318186a4a0. [DOI] [PubMed] [Google Scholar]
- Martin RC, Loring DW, Meador KJ, Lee GP. The effects of lateralized temporal lobe dysfunction on formal and semantic word fluency. Neuropsychologia. 1990;28:823–829. doi: 10.1016/0028-3932(90)90006-a. [DOI] [PubMed] [Google Scholar]
- Meng Y, Qingjie G, Xiangtong Z, Shugang S, Yaohua W, Liwei Z, Enxi H, Changyu L. Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. International Journal of Nursing Studies. 2009;46:753–758. doi: 10.1016/j.ijnurstu.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Meyers JE, Rohling ML. CT and MRI correlations with neuropsychological tests. Applied Neuropsychology. 2009;16:237–253. doi: 10.1080/09084280903098752. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Boone KB, Razani J, D'Elia LF. Handbook of normative data for neuropsychological assessment. New York, NY, US: Oxford University Press; 2005. p. 648.p. 760.p. 782.p. 969. [Google Scholar]
- Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. Journal of Applied Physiology. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Journal of Neurosurgery. 1984;61:241–253. doi: 10.3171/jns.1984.61.2.0241. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Marion DW. Xenon techniques for CBF measurementin clinical head injury. In: Narayan RK, Welberger JE Jr, Povlishock JT, editors. Neurotrauma. New York, NY: McGraw-Hill; 1996. pp. 471–485. [Google Scholar]
- Obrist WD, Wilkinson WE. Regional cerebral blood flow measurements in humans by xenon-133 clearance. Cerebrovascular & Brain Metabolism Reviews. 1990;2:283–327. [PubMed] [Google Scholar]
- Quintard H, Patet C, Zeriauth J-B, Suys T, Bouzat P, Pellerin L, Meuli R, Magistretti PJ, Oddo M. Improvement of neuroenergetics by hypertonic lactate therapy in patients with traumatic brain injury is dependent on baseline cerebral lactate/pyruvate ratio. Journal of Neurotrauma. 2016;33:681–687. doi: 10.1089/neu.2015.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell RM, Cole DJ. Cerebral monitoring: jugular venous oximetry. Anesthesia & Analgesia. 2000;90:559–566. doi: 10.1097/00000539-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gow CA. “Frontal dysfunction” after traumatic brain injury. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1992;5:272–282. [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. Journal of Clinical Experimental Neuropsychology. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. Journal of Cerebral Blood Flow Metabolism. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, McArthur D, Alger J, O’Phelan K, Glenn T, Bergsneider B, Martin NA, Hovda DA. Regional heterogeneity of brain metabolism using cerebral microdialysis: concordance with magnetic resonance spectroscopy and positron emission tomography. Brain Pathology. 2004;14:210–214. doi: 10.1111/j.1750-3639.2004.tb00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda DA. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Critical Care Medicine. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- Wallesch C, Curio N, Kutz S, Jost S, Bartels C, Synowitz H. Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Injury. 2001;15:401–412. doi: 10.1080/02699050010005959. [DOI] [PubMed] [Google Scholar]
- Werner C, Englehard K. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Williamson DJG, Scott JG, Adams RL. Traumatic brain injury. In: Adams RL, Parsons OA, Culbertson JL, Nixon SJ, editors. Neuropsychology for Clinical Practice: Etiology, Assessment, and Treatment of Common Neurological Disorders. Washington, DC: American Psychological Association; 1996. pp. 9–64. [Google Scholar]
- Wright MJ, McArthur DJ, Alger JR, Van Horn J, Irimia A, Filippou M, Glenn TC, Hovda DA, Vespa P. Early metabolic crisis-related brain atrophy and cognition in traumatic brain injury. Brain Imaging and Behavior. 2013;7:307–315. doi: 10.1007/s11682-013-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Schmitter-Edgecombe M, Woo E. Verbal memory impairment in severe closed-head injury: the role of encoding and consolidation. Journal of Clinical Experimental Neuropsychology. 2010;32:728–736. doi: 10.1080/13803390903512652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, McArthur DL, Alger JR, Etchepare M, Hovda DA, Glenn TC, Huang S, Dinov I, Vespa PM. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. Journal of Cerebral Blood Flow Metabolism. 2010;30:883–894. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]