Abstract
INTRODUCTION
Post-traumatic stress disorder (PTSD) is associated with an increased risk of dementia in male veterans, but little is known in females and civilians.
METHODS
PTSD and comorbidities were abstracted from medical records from 1/1/1996–12/31/2001. Dementia incidence from 1/1/2002–12/31/2014 in 499,844 healthcare members aged 60+ over an average of 8.2 years. Cox proportional hazards models were adjusted for age, demographics and comorbidities.
RESULTS
PTSD was associated with increased risk of dementia over an average of 8 years of follow-up (Females: Hazard Ratio [HR]= 1.59, 95% Confidence Interval [CI]= 1.30–1.95; Males: HR= 1.96, 95% CI= 1.51–2.55). There was a two-fold risk of dementia in those with both PTSD and depression (Females: HR= 2.08; 95% CI= 1.66–2.59; Males: HR= 2.06; 95% CI= 1.47–2.91) versus those without.
DISCUSSION
PTSD was a risk factor for dementia in both sexes, with a heightened risk in those with comorbid depression.
Keywords: Dementia, Posttraumatic stress disorder, PTSD, Sex differences, Alzheimer’s disease
1. Background
Post-traumatic stress disorder (PTSD) is a common mental health problem with long-term health consequences. PTSD has a known etiological agent, experiencing an event that involves life threat, serious injury, or risk of death in early or late life [1]. Research suggests that the lifetime prevalence of PTSD for older adults ranges from 3% to 6% [1–3]. Studies have found that PTSD is associated with a higher risk of dementia in male veterans [4, 5]. Findings suggest that older male veterans were nearly two times as likely to develop incident dementia compared to those without PTSD, while a recent study in Taiwan found that both male and female patients with PTSD had a nearly four times higher risk of dementia [6]. Yet, there are currently no studies in the U.S. examining whether PTSD is a risk factor for dementia in older females. This is quite alarming given that PTSD and dementia are more prevalent in females.
Studies have shown that the prevalence of PTSD is more than two times higher in females compared to males [7]. Annual estimates from the National Comorbidity Survey suggest that 10% of females vs. 5% of males have PTSD [8]. Other studies have found that the prevalence of PTSD was as high as 13% in females and 6% in males [9]. In a population-based study of older adults aged 60 and older, the lifetime prevalence of PTSD was 6% for females and 3% for males [3]. While research has suggested that females may be less likely to experience certain types of traumatic events, they develop PTSD more often than males [10, 11].
Studies have linked stress and PTSD to an increased risk of memory impairment and dementia in later life [12–16]. There is also a higher incidence of chronic illnesses in individuals with PTSD, including depression, traumatic brain injury (TBI), diabetes, stroke, and heart disease [16, 17]. These chronic conditions may link PTSD to higher rates of dementia. For example, depression is associated with a 2- to 3-fold increased risk of dementia [18, 19]; and co-morbid depression among individuals with PTSD is very common, with estimates of co-occurrence ranging from 30% to 50% [20, 21].
The current study utilizes 13 years of prospective data on a large, diverse civilian population of older adult members of an integrated healthcare delivery system (n = 499,844) in Northern California. Our primary objective was to examine the association between PTSD and risk of dementia among both older male and female members. Because PTSD and dementia are associated with other medical conditions, we also explored whether the association might be explained by vascular risk factors, depression, and TBI.
2. Methods
2.1 Study population
The study population consists of members (aged 60+) from the Kaiser Permanente Northern California (KPNC) health system, which is a large, integrated healthcare delivery system that provides comprehensive medical care to over 3.9 million members in Northern California (approximately 30% of the geographic region). This includes 16% of members enrolled in Medicare and 8% enrolled in the California Medical Assistance Program (Medi-Cal) or other state subsidized health insurance program. Past research has shown that the KPNC member population is generally representative of the overall regional population, with regards to history of chronic conditions and lifestyle factors, but may underrepresent individuals at the very extreme tails of the income distribution [22, 23].
2.2 Study design
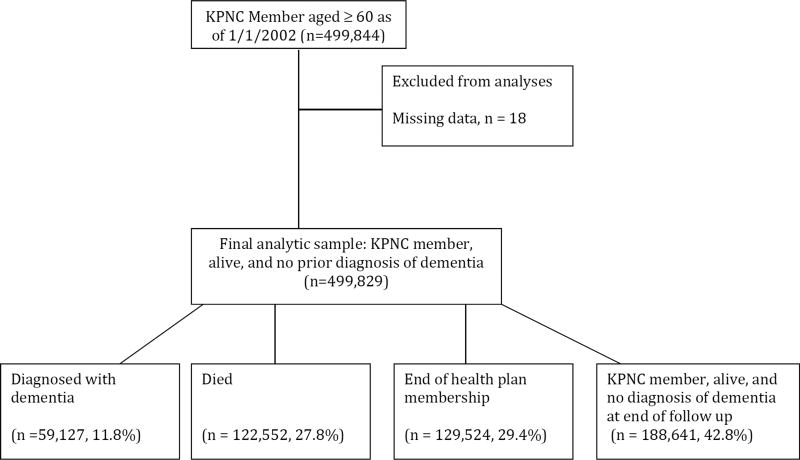
The present study includes KPNC members who were enrolled as of January 1, 1996 (date when electronic medical records were implemented). To ensure dementia diagnoses detected were incident cases, anyone with a dementia diagnosis between January 1, 1996 to December 31, 2001 was omitted from the sample.. Figure 1 provides an overview of the study flow. The present analyses include members who were still alive, KPNC members, and had no dementia diagnosis as of January 1, 2002. Cohort members were followed for incident dementia until a lapse in health plan membership (defined as a gap in health plan coverage of ≥3 months), death, or December 31, 2014 (end of study period). The Kaiser Division of Research Internal Ethics Committee approved study procedures.
Figure 1.
Flow chart of study participants. Abbreviation: KPNC, Kaiser Permanente Northern California.
2.3 Measures
Dementia diagnoses were identified from electronic medical records. Diagnoses were from inpatient and outpatient visits between from January 1, 2002 to December 31, 2014 based on International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes for Alzheimer’s disease (331.0), vascular dementia (290.4x) and other nonspecific dementia (290.0x, 290.1x, 290.2x, 290.3x, 294.1x, 294.2x). PTSD diagnoses were determined from electronic medical records, which includes diagnoses from all inpatient and outpatient encounters at KPNC. PTSD was coded using ICD-9 code 309.81, which was based on a physician’s diagnosis between January 1, 1996 and December 31, 2001.
Other measures included demographics, healthcare utilization, and medical comorbidities. Race/ethnicity was self-reported as White, Black, Latino, Native American, Pacific Islander, Asian or other and was included in analyses as a potential confounder. Healthcare utilization was defined as the average number of visits during follow-up. Diagnoses of diabetes (249.x, 250.x, and 357.2 to 366.41), stroke (433.01 to 436, and 997.02), heart disease (410.xx to 414.9), depression (296.2x, 296.3x, and 311), and TBI (850.0x to 859.9x) were also extracted from the electronic medical records and based on a physician’s diagnosis from January 1, 1996 to December 31, 2001.
2.4 Statistical analysis
Age-adjusted dementia incidence rates by sex were estimated by standardizing to the 2000 United States Census population. Follow-up began on January 1, 2002 and ended on the date of dementia diagnosis, death, gap in health plan coverage of ≥3 months, or end of the study period (December 31, 2014), whichever came first. The Kaplan-Meier method was used to estimate the distribution of dementia-free survival, overall, by sex and by depression diagnosis. We estimated cumulative incidence of dementia (at 5-year intervals from 5 to 35 years) by PTSD diagnosis and sex conditional on surviving dementia-free to age 60 using the Practical Incidence Estimator macro [24]. The macro uses information on age-specific rates to estimate cumulative incidence and incorporates information on death rates by assuming that individuals who die without a dementia diagnosis never develop dementia. Cox proportional hazards models, overall and stratified by sex, were used to evaluate associations between PTSD and dementia risk providing of unadjusted and covariate-adjusted estimates of hazard ratios and 95% confidence (CI). Age was the time scale in all Cox regression analyses. Adjusted models included race/ethnicity, vascular factors (diabetes, stroke, and heart disease), TBI, and depression at baseline (January 1, 1996 to December 31, 2001). Analyses of PTSD and dementia were stratified by sex, depression, and TBI to test for effect modification. Heterogeneity in the strength of association between PTSD and risk of dementia by depression and TBI status was assessed by inclusion of appropriate cross-product (interaction) terms in regression analyses. Sensitivity analyses were also conducted to examine the impact of healthcare utilization while adjusting for sex and race/ethnicity with age time scale. SAS 9.4 was used for all data analyses.
3. Results
Of the 499,844 KPNC members who were aged ≥ 60 years as of January 1, 2002, only 18 were excluded due to missing information about sex or race/ethnicity (Figure 1). At baseline, the mean age was 71.1 (Standard deviation [SD] = 7.9; range = 60 to 102) and 54.7% were females. Among the participants, a total of 1,147 members were diagnosed with PTSD (Table 1), which was more prevalent for females, Whites, and younger adults. Individuals with PTSD were also more likely to have been diagnosed with depression and a TBI.
Table 1.
Baseline characteristics of the sample by PTSD diagnosis
| Characteristics | Non-PTSD (n = 498,697) |
PTSD (n = 1,147) |
P-value |
|---|---|---|---|
| Age (y), mean (SD) | 71.1 (7.9) | 67.7 (6.9) | <0.001 |
| Females, % | 272,550 (54.7) | 780 (68.0) | <0.001 |
| Race\Ethnicity, % | <0.001 | ||
| White | 340,538 (68.3%) | 770 (67.1%) | |
| Black | 31,556 (6.3%) | 82 (7.1%) | |
| Latino | 36,971 (7.4%) | 105 (9.2%) | |
| Native American | 2,163 (0.4%) | 9 (0.8%) | |
| Pacific Islander | 1,790 (0.4%) | 1 (0.1%) | |
| Asian | 47,411 (9.5%) | 78 (6.8%) | |
| Other | 20,703 (4.2%) | 91 (7.9%) | |
| Diabetes, % | 85,285 (17.1%) | 205 (17.9%) | 0.49 |
| Stroke, % | 41,625 (8.3%) | 104 (9.1%) | 0.38 |
| Heart disease, % | 168,115 (33.7%) | 400 (34.9%) | 0.41 |
| Traumatic brain injury, % | 6,852 (1.4%) | 66 (5.8%) | <0.001 |
| Depression, % | 42,117 (8.4%) | 813 (70.9%) | <0.001 |
Note: PTSD: post-traumatic stress disorder.
Over an average of 8.0 years of follow up (SD = 4.6), 11.8% (59,127) were diagnosed with dementia, 27.8% died (122,552), and 29.4% (129,524) were lost to follow-up (lapse in KPNC membership). The remaining 42.8% (188,641) were still alive, KPNC members, and did not have a diagnosis of dementia at the end of study period. The age-adjusted incidence rates for dementia were higher among individuals with PTSD (17.53 per 1,000 person years) compared to those without PTSD (11.11 per 1,000 person years). Table 2 shows the overall age-adjusted incidence rate for dementia among individuals with and without a PTSD diagnosis. In both older males and females, the ageadjusted incidence rate of dementia for individuals with PTSD was higher than the rate among their counterparts without PTSD. For each 5-year increment, the cumulative incidence of dementia conditional on survival to age 60 without dementia was greater among individuals with PTSD then their counterparts without PTSD (Supplemental Table A1). For example, the 25-year cumulative incidence of dementia was 23.58% (95% CI: 16.98–28.68) for females with PTSD while it was 15.14% (95% CI: 14.89–15.34) for females without PTSD.
Table 2.
Dementia incidence rates by PTSD diagnosis and sex, 2002–2014
| PTSD diagnosis by sex | Events | Person-years | Age adjusted incidence rates per 1000 person-years (95% CI) |
|---|---|---|---|
| Total | |||
| No PTSD | 58,978 | 3,993,284 | 11.11 (11.02. 11.20) |
| PTSD | 149 | 9,217 | 17.53 (14.40, 20.65) |
| Female | |||
| No PTSD | 35,965 | 2,228,678 | 11.47 (11.35, 11.59) |
| PTSD | 93 | 6,486 | 17.03 (13.21,20.85) |
| Male | |||
| No PTSD | 23,013 | 1,764,606 | 10.54 (10.40, 10.68) |
| PTSD | 56 | 2,731 | 18.73 (13.25, 24.20) |
Note: PTSD: post-traumatic stress disorder; CI: confidence interval
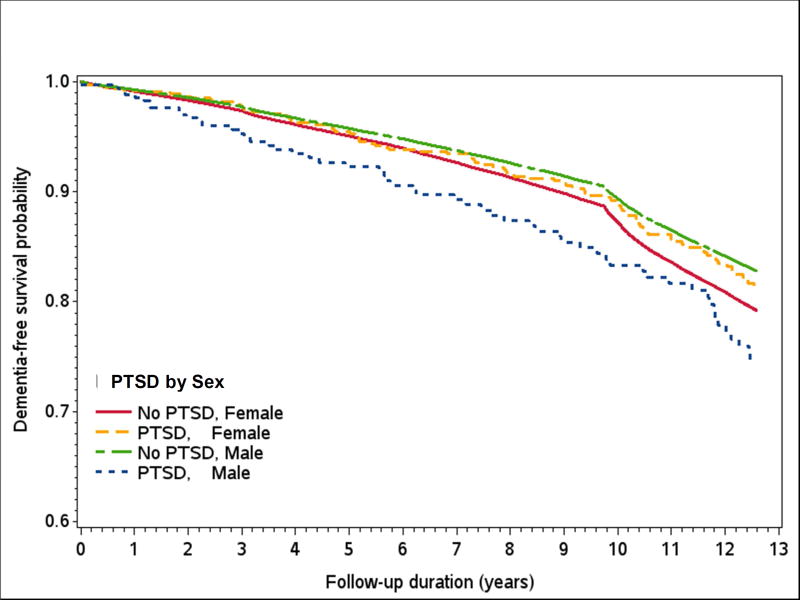
In Cox proportional hazard models adjusted for demographic variables (model 2; Table 3 and Supplemental Table A2), individuals with PTSD had a 73% increase in risk of dementia (HR: 1.73, 95% CI=1.47, 2.02). Male with PTSD had a two-fold increase in risk of dementia, and females with PTSD had a 60% increase (Females: HR = 1.60, 95% CI=1.31, 1.97; Males: HR = 2.03, 95% CI=1.54, 2.61). While the magnitude of risk was larger in males, the interaction by sex was not significant (p = .16 for PTSD by Sex). Further adjustment for individual vascular factors (stroke, diabetes, and heart disease) and all three combined did not attenuate the association (Females: HR = 1.59, 95% CI = 1.30, 1.95; Males: HR = 1.96, 95% CI = 1.51, 2.55). Adjustment for TBI did not impact the association between PTSD and dementia (Females: HR = 1.57, 95% CI = 1.28, 1.94; Males: HR = 1.92, 95% CI = 1.48, 2.50). After addition of depression to the models, males with PTSD had a 35% increase in risk (HR = 1.35; 95% CI = 1.04, 1.76), and for females, the association was decreased and no longer significant (HR = 1.12; 95% CI = 0.91, 1.37). A sensitivity analysis building on the base model (including sex, race/ethnicity and with age as the time scale) to account for healthcare utilization resulted in minimal attenuation of the PTSD hazard ratio (HR: 1.61, 95% CI=1.37, 1.89). The Kaplan-Meier depicting the probability of remaining free of dementia over 13 years of follow-up for those with and without PTSD diagnosis by sex are shown in Figure 2.
Table 3.
PTSD and risk of dementia from Cox proportional hazards models with age as the time scale and adjusted for comorbidities
| Overall HR (95% CI) |
|
|---|---|
| Model 1: Unadjusted, age as time scale | 1.76 (1.49, 2.06) |
| Model 2: Model 1 plus sex and race/ethnicity | 1.73 (1.47, 2.02) |
| Model 3: Model 2 plus diabetes, heart disease, and stroke | 1.70 (1.45, 2.00) |
| Model 4: Model 3 plus traumatic brain injury and depression | 1.20 (1.02, 1.41) |
Note: PTSD: post-traumatic stress disorder; HR: hazard ratio; CI: confidence interval
Fig. 2.
The Kaplan-Meier survival curves depicting the probability of remaining free of dementia by PTSD and sex. Note: PTSD: post-traumatic stress disorder.
There was a significant three-way interaction between depression, PTSD, and sex (p = 0.006 for PTSD*Depression*Sex). Females and males with both PTSD and depression had over a two-fold risk of dementia (Females: HR = 2.08; 95% CI = 1.66, 2.59; Males: HR = 2.06; 95% CI = 1.46, 2.91) compared to those with no PTSD without depression (Table 4). Among those without depression, males with PTSD had nearly a two-fold risk of dementia (HR = 1.98; 95% CI = 1.32, 2.95), which was similar to males with depression, while females with PTSD without depression did not have an increased risk of dementia (HR = 0.87; 95% CI = 0.52, 1.44). However, the number of females with PTSD without depression (26%; n = 202) was smaller compared to females with both PTSD and depression (74%; n = 578).
Table 4.
Joint effects of PTSD and depression on risk of dementia by sex from Cox proportional hazard models using age as the time scale
| Total HR (95% CI) |
Female HR (95% CI) |
Male HR (95% CI) |
|
|---|---|---|---|
| No PTSD without depression | 1.00 | 1.00 | 1.00 |
| No PTSD with depression | 1.78 (1.74, 1.83) | 1.74 (1.69, 1.79) | 1.92 (1.84, 2.01) |
| PTSD without depression | 1.32 (0.96, 1.81) | 0.87 (0.52, 1.44) | 1.98 (1.32, 2.95) |
| PTSD with depression | 2.06 (1.71, 2.49) | 2.08 (1.66, 2.59) | 2.06 (1.46, 2.91) |
Note: Models adjusted for age (as the time scale), demographics (race/ethnicity) and comorbidities (diabetes, heart disease, stroke, and traumatic brain injury); Three-way interaction (PTSD*Depression*Sex; p = 0.006). PTSD: post-traumatic stress disorder; HR: hazard ratio; CI: confidence interval
4. Discussion
This is the first study of PTSD and dementia in civilians and of both males and females in the U.S. Our results suggest that PTSD is associated with a significantly higher risk of dementia for both females and males from a large population-based sample of members of an integrated healthcare system in Northern California over 13 years. Adjustment for demographics and vascular factors did not substantially explain differences in dementia risk for females and males with PTSD. Overall, older adults with PTSD had approximately 70% higher risk of dementia, with males having a two-fold risk and females having a 60% higher risk, compared to those without PTSD. Hypothesized explanatory factors, including diabetes, stroke, heart disease, and TBI, did not modify the magnitude of the risk of dementia associated with PTSD. However, there was nearly a two-fold increase in risk for both females and males with PTSD and depression. Interestingly, females with PTSD without comorbid depression did not have an elevated risk, while the two-fold increase in risk of dementia remained for males.
Prior research has shown a relationship between PTSD and dementia risk among male veterans and prisoners of war, and in a population-based study of females and males from Taiwan [4–6, 25]. In these past studies, older adults with PTSD had a two- to fourfold increase in risk of dementia compared to those without PTSD. However, very few studies have been conducted in civilian populations or among older females. This is the first study in the U.S. to provide evidence for PTSD being a risk factor for dementia in both civilian females and males in later life.
Comparing the current findings to the recent study in the Taiwan National Health Insurance program, the magnitude of the association between PTSD and dementia was smaller in our study compared to the Taiwan study [6]. These differences may have been due to greater access to and use of healthcare. Importantly, the study in Taiwan employed case-control matching and the control group excluded those with mental disorders, including depression, a major difference to our study design. This may have affected the size of the risk estimates for PTSD, and especially if the control group was exceptionally healthy and had a much lower risk of dementia.
One of the compelling findings from this study involves the two-fold increase in risk for dementia among males and females with both PTSD and depression. It has been suggested that rates of comorbid PTSD and depression may be as high as 50% [21, 26]. Several recent studies have found that adults with PTSD were two to threes more likely to have comorbid psychiatric disorders, including major depression, mood disorders, anxiety disorders, and drug abuse/dependence [1, 3]. While the causal mechanism remains unclear, it is plausible that PTSD is either a causal risk factor for depression, there is a bidirectional association, or the comorbidity may reflect more severe PTSD symptoms, including greater overall distress and physical and social impairments. Studies have also found sex differences in PTSD symptoms and associated risk factors, including higher rates of depression in females [10, 27]. The higher rates of both PTSD and depression among females may be due to differential PTSD risk and severity or differences in reporting. Future research should consider potential sex differences in reporting of PTSD and symptom severity.
Several studies have examined the longitudinal relationship between PTSD and dementia. Yet, very little is known about the causal mechanisms underlying this relationship. One possible mechanism involves the physiological consequences of acute and chronic stress. For instance, studies have suggested that damage to the hypothalamic pituitary adrenal (HPA) cortical axis due to PTSD may result in an increased risk of dementia in later life [16]. Additionally, PTSD may also increase susceptibility to oxidative stress, such as increasing proinflammatory cytokines, increasing levels of C- reactive protein, altering of homocysteine levels, increased inflammation, and elevated levels of β-amyloid [4, 16]. Neuroimaging studies have found a link between PTSD and decreased hippocampal gray matter volume [16, 28]. Moreover, several neuroimaging studies have found that individuals with PTSD have a smaller gray matter volume in the hippocampus, amygdala and anterior cingulate, which are brain regions known to be implicated in memory [29–31]. PTSD has also been linked to worse cognitive functioning and impairment in older populations [32, 33].
This study have several important strengths including being the first study to examine PTSD as a risk factor in females and among civilian older adults in the U.S. In addition, this was diverse sample of members of integrated health system in Northern California with up to 13 years of follow up on dementia diagnosis. We also had a breadth of information on chronic conditions.
There are several limitations of this study that should be considered by future research. First, it is possible that the sample includes individuals with prodromal symptoms of dementia that were not captured between January 1, 1996 and December 31, 2001. Also in our large cohort there was likely an under diagnosis of clinical PTSD and there was an inability to examine subclinical cases of PTSD. However, this suggests a bias towards the null hypothesis and likely this study may underestimate the size of the effect of PTSD on risk of dementia in later life. Future studies should consider whether PTSD in earlier life is also a risk factor for dementia. In addition, it is possible that individuals with PTSD may be more likely to use healthcare services and have a greater chance of being diagnosed with dementia. This may lead to bias in the estimates of risk for dementia among individuals with PTSD. However, individuals with PTSD were more likely to be censored due to a lapse in health plan membership. This is in line with past studies that have shown that individuals with PTSD were more likely to experience social vulnerabilities and occupational challenges, which may result in loss of health insurance [17, 33]. If PTSD is associated with dementia risk, this would result in an underestimation of dementia risk.
Another limitation of this study involved relying on a clinical diagnosis of PTSD, dementia, and other comorbidities based on ICD-9-CM codes, a less sensitive method compared to structured diagnostic interviews, which may lead to underestimating the prevalence of PTSD, dementia, and other comorbidities. This also limits our ability to look at varying levels of severity and subclinical cases of PTSD and their relationship with dementia. However, this approach has been used successfully in the past [35–38], and this strategy has been shown to have a sensitivity of 77% and specificity of 95% when compares to consensus diagnosis of dementia using neuropsychiatric battery, physical exam, structured interview with informants, and review of medical record [39]. A past study in older male veterans found that PTSD was associated with risk of dementia, including different subtypes (i.e., Alzheimer’s Disease, Frontotemporal, Senile, Vascular, and Lewy Body Dementia) [4]. Future research should determine whether there are sex differences in association between PTSD and risk of dementia subtypes. Finally, a diagnosis of PTSD is typically based on previous exposure to a stressor or a traumatic event (e.g., combat, rape, brutal assault)[40]. However, it is possible that the observed comorbidity between PTSD and depression in this study may be due to either symptom overlap or an inability to differentiate between the two disorders in clinical settings [26]. It is also possible that these disorders may share common mechanisms or may be prodromal symptoms of dementia. Future research is needed to distinguish between these mental health disorders and better understand the mechanisms that may increase risk for dementia.
This study found a two-fold risk of dementia in males with PTSD and nearly 60% higher risk in females with PTSD. There was also a two-fold risk of dementia in males and females members with both PTSD and depression. Future studies are needed to better understand the mechanisms linking PTSD and dementia as well as potential sex differences. A greater understanding of biological mechanism linking PTSD and dementia are needed, including the use of neuroimaging methods to further elucidated potential causal pathways. Public health interventions aimed at improving screening and treatment outcomes for PTSD are also needed in aging populations. Finally, special attention to reducing chronic stress and risk of PTSD in late life, and targeted interventions for individuals with PTSD and comorbid depression are warranted.
Supplementary Material
Research in Context.
Systematic review: In our literature review using PubMed, there were only a handful of studies that have examined whether PTSD is a risk factor for dementia. While females experience a two-fold increase in the prevalence of PTSD, the majority of studies have been in predominantly male and veteran populations. There is a need for population-based studies in the U.S. with civilians and older females.
Interpretation: We found that PTSD was associated with a higher risk of dementia in both female and male members of a healthcare system in the U.S. Those with comorbid PTSD and depression had a two-fold increase in risk of dementia, which may suggest that the association between PTSD and risk of dementia may vary by the severity of PTSD symptoms.
Future directions: Screening, improving treatments outcomes, and reducing risk for trauma and subsequent PTSD may help to decrease the incidence of dementia in both sexes.
Acknowledgments
Funding: This work was support by grant numbers RF1A6052132, P30AG015272, P30AG044281, and T32AG049663 from the National Institute on Aging, National Institutes of Health. The funding organizations played no role in the design and conduct of the study; in the management, analysis, and interpretation of the data; or in the preparation, review, or approval of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: J.D.F. and R.A.W. were responsible for the study concept and design. K.B.A. was responsible for the analysis of data. J.D.F., P.G., K.B.A., C.P.Q., and R.A.W. interpreted the data. J.D.F. drafted the article. J.D.F., P.G., K.B.A., C.P.Q., and R.A.W. revised the article. J.D.F. and R.A.W. obtained the funding. R.A.W. is the guarantor and was responsible for the study supervision.
References
- 1.Chopra MP, Zhang H, Kaiser AP, Moye JA, Llorente MD, Oslin DW, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. The American Journal of Geriatric Psychiatry. 2014;22:86–97. doi: 10.1016/j.jagp.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 2.Jayasinghe N, Sparks MA, Kato K, Wyka K, Wilbur K, Chiaramonte G, et al. Posttraumatic stress symptoms in older adults hospitalized for fall injury. General hospital psychiatry. 2014;36:669–73. doi: 10.1016/j.genhosppsych.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. The American Journal of Geriatric Psychiatry. 2012;20:380–90. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–13. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi SU, Kimbrell T, Pyne JM, Magruder KM, Hudson TJ, Petersen NJ, et al. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–33. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang T-Y, Wei H-T, Liou Y-J, Su T-P, Bai Y-M, Tsai S-J, et al. Risk for developing dementia among patients with posttraumatic stress disorder: A nationwide longitudinal study. Journal of affective disorders. 2016;205:306–10. doi: 10.1016/j.jad.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Ditlevsen DN, Elklit A. The combined effect of gender and age on post traumatic stress disorder: do men and women show differences in the lifespan distribution of the disorder? Annals of general psychiatry. 2010;9:1. doi: 10.1186/1744-859X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell KS, Mazzeo SE, Schlesinger MR, Brewerton TD, Smith BN. Comorbidity of partial and subthreshold PTSD among men and women with eating disorders in the national comorbidity survey-replication study. International Journal of Eating Disorders. 2012;45:307–15. doi: 10.1002/eat.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of traumatic stress. 2013;26:537–47. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin. 2006;132:959. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 11.Cortina LM, Kubiak SP. Gender and posttraumatic stress: sexual violence as an explanation for women's increased risk. Journal of abnormal psychology. 2006;115:753. doi: 10.1037/0021-843X.115.4.753. [DOI] [PubMed] [Google Scholar]
- 12.Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, et al. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133:2217–24. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- 13.Doyle C, Dunt D, Morris P. Stress and dementia. Int Psychogeriatr. 2014;26:1235–6. doi: 10.1017/S1041610214001033. [DOI] [PubMed] [Google Scholar]
- 14.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18:223–54. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 15.Rickenbach EH, Almeida DM, Seeman TE, Lachman ME. Daily stress magnifies the association between cognitive decline and everyday memory problems: An integration of longitudinal and diary methods. Psychology and aging. 2014;29:852. doi: 10.1037/a0038072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg MS, Tanev K, Marin M-F, Pitman RK. Stress, PTSD, and dementia. Alzheimer's & Dementia. 2014;10:S155–S65. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Flatt JD, Williams BA, Barnes D, Goldenson J, Ahalt C. Post-traumatic stress disorder symptoms and associated health and social vulnerabilities in older jail inmates. Aging Ment Health. 2016:1–7. doi: 10.1080/13607863.2016.1201042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69:493–8. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DG, Felker BL, Liu C-F, Yano EM, Kirchner JE, Chan D, et al. Prevalence of depression–PTSD comorbidity: Implications for clinical practice guidelines and primary care-based interventions. Journal of General Internal Medicine. 2007;22:711–8. doi: 10.1007/s11606-006-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues in clinical neuroscience. 2015;17:141. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. 2012 [Google Scholar]
- 23.Gordon NP, Kaplan GA. Some evidence refuting the HMO" favorable selection" hypothesis: the case of Kaiser Permanente. Advances in health economics and health services research. 1990;12:19–39. [PubMed] [Google Scholar]
- 24.Beiser A, D'Agostino RB, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Statistics in Medicine. 2000;19:1495–522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimer's & Dementia. 2014;10:S236–S41. doi: 10.1016/j.jalz.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Spinhoven P, Penninx BW, van Hemert AM, de Rooij M, Elzinga BM. Comorbidity of PTSD in anxiety and depressive disorders: Prevalence and shared risk factors. Child abuse & neglect. 2014;38:1320–30. doi: 10.1016/j.chiabu.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 28.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of affective disorders. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Archives of general psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20:1402–6. doi: 10.1097/WNR.0b013e3283300fbc. [DOI] [PubMed] [Google Scholar]
- 32.Green E, Fairchild JK, Kinoshita LM, Noda A, Yesavage J. Effects of posttraumatic stress disorder and metabolic syndrome on cognitive aging in veterans. The Gerontologist. 2016;56:72–81. doi: 10.1093/geront/gnv040. [DOI] [PubMed] [Google Scholar]
- 33.Schuitevoerder S, Rosen JW, Twamley EW, Ayers CR, Sones H, Lohr JB, et al. A meta-analysis of cognitive functioning in older adults with PTSD. Journal of anxiety disorders. 2013;27:550–8. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Smith MW, Schnurr PP, Rosenheck RA. Employment outcomes and PTSD symptom severity. Ment Health Serv Res. 2005;7:89–101. doi: 10.1007/s11020-005-3780-2. [DOI] [PubMed] [Google Scholar]
- 35.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37:1009–15. doi: 10.2337/dc13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 38.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 39.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25:423–9. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman MJ. Finalizing PTSD in DSM-5: Getting here from there and where to go next. Journal of Traumatic Stress. 2013;26:548–56. doi: 10.1002/jts.21840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.