Abstract
Late-life depression is characterized by both lower mood and poor cognitive performance, symptoms that often do not fully respond to current antidepressant medications. Nicotinic acetylcholine receptor (nAChR) agonists such as nicotine may serve as a novel therapeutic approach for this population. Both preclinical and preliminary clinical studies suggest that nAChR agonists can improve depressive behavior in animal models and improve mood in depressed individuals. Substantial literature also supports that nAChR agonists benefit cognitive performance, particularly in older populations. These potential benefits may be mediated by the effects of nAChR stimulation on neural network function and connectivity. Functional neuroimaging studies detail effects of nAChR agonists on the default mode network, central-executive network, and salience network that may oppose or reverse network changes seen in depression. We propose that, given the existent literature and the clinical presentation of late-life depression, nicotine or other nAChR agonists may have unique therapeutic benefits in this population and that clinical trials examining nicotine effects on mood, cognition, and network dynamics in late-life depression are justified.
INTRODUCTION
Late Life Depression (LLD), or major depressive disorder occurring in adults 60 years or older, is a significant public health issue. LLD has a prevalence of approximately 5% in community-dwelling older adults, and the number of individuals with LLD is expected to rise with global population aging. LLD is characterized by high healthcare costs and a high risk of suicide (Blazer, 2003; Taylor, 2014). Complicating this picture, individuals with LLD often exhibit a poor response to currently available antidepressants (Beekman et al., 2002). Systematic reviews and meta-analyses support that between five to eight patients must be treated to have one achieve treatment response (i.e. number needed to treat (NNT)) with no differences in response rates between antidepressant drug classes (Nelson et al., 2008; Taylor and Doraiswamy, 2004). Jointly, these data support the need for new, effective treatments.
The purpose of this review is to examine the hypothesis that nicotinic acetylcholine receptor (nAChR) agonists may benefit both mood and cognitive symptoms in LLD. After a brief review of cognitive symptoms in LLD, we describe the cholinergic system and how it is relevant to the etiology of depression, specifically focusing on the nAChR system. We next summarize preclinical and clinical studies examining the effects of nAChR agonists on depressive behavior, depressive symptoms, and cognitive performance. We next utilize a cognitive neuroscience framework to describe intrinsic network alterations in LLD, how nAChR agonists affect these networks, and how such effects may benefit patients with LLD. Finally, we discuss clinical trial design considerations when testing this clinical neuroscience model.
COGNITIVE IMPAIRMENT IN LATE LIFE DEPRESSION
Beyond the diagnostic affective symptoms, LLD is also associated with poorer cognitive performance. Even in the absence of dementia, individuals with LLD exhibit poor performance across multiple cognitive domains, including executive function, processing speed, episodic memory, and visuospatial ability (Sexton et al., 2012; Sheline et al., 2006). While cognitive performance can improve with depression remission, often patients do not reach performance levels seen in psychiatrically healthy elders (Bhalla et al., 2006; Butters et al., 2000; Lee et al., 2007; Taylor et al., 2002) and such deficits are associated with greater disability (Alexopoulos et al., 2002; Murphy and Alexopoulos, 2004). LLD is a strong risk factor for dementia, associated with an approximately two-fold increase in risk of all cause dementia (Diniz et al., 2013). Such cognitive deficits may be related to underlying brain pathology, however post-mortem neuro-pathologic studies found that depressive symptoms had an association with cognitive decline that was independent of underlying neuropathological findings (Wilson et al., 2014). Thus, while depression and pathologic brain aging both contribute to overall poorer cognitive performance, these factors interact to produce greater impairment than either alone.
These cognitive deficits are a significant predictor of poor antidepressant medication response. Executive function is the best studied domain, with several studies associating executive dysfunction with poor acute treatment response, lower remission rates, and increased relapse and recurrence rates (Alexopoulos et al., 2004, 2002, 2000; Kalayam and Alexopoulos, 1999; McLennan and Mathias, 2010; Pimontel et al., 2012). Other cognitive domains are similarly predictive of poor treatment response, including processing speed, episodic memory, and language processing (Sheline et al., 2010). Unfortunately, despite cognitive symptoms being common and often disabling, there is no current clinical treatment for LLD that effectively improves both the mood and cognitive symptoms of the disorder.
OVERVIEW OF THE CENTRAL ACETYLCHOLINE SYSTEM: FOCUS ON NICOTINIC RECEPTORS
While acetylcholine (ACh) in the Peripheral Nervous System serves as an excitatory neurotransmitter, in the Central Nervous System (CNS) ACh plays a widespread neuromodulatory role. ACh is synthesized in a single step from Acetyl CoA and choline by the enzyme choline acetyltransferase; it is later hydrolyzed and degraded by acetylcholinesterase. Cholinergic projection neurons originate in discrete nuclei, specifically the basal forebrain nucleus basalis of Meynert, the medial septal nucleus, and the pedunculopontine and laterodorsal tegmental nuclei of the ponto-mesencephalotegmental complex. The two primary sources of ACh in the brain include long axon projection neurons and local interneurons. Long-axon cholinergic neurons project widely and diffusely, innervating neurons throughout the CNS. Local tonically-active cholinergic interneurons are found primarily in the striatum and nucleus accumbens.
Acetylcholine receptors (AChRs) consist of two major subtypes: the metabotropic (G-protein-coupled, slow) muscarinic acetylcholine receptors (mAChR) and the ionotropic (ion channel, fast) nicotinic acetylcholine receptors (nAChRs). Both subtypes bind ACh and many non-endogenous ligands, actually being named for their respective non-endogenous ligands: mAchRs are activated by the toxin muscarine and nAChRs by nicotine (Albuquerque et al., 2009; Philip et al., 2010).
The nAChR system has a widespread neuromodulatory role. There are various nAChR subtypes, the most common being the homomeric α7 and heteromeric α4β2. These receptors are distributed in different neuronal compartments and found throughout the brain’s cortex and various subcortical regions, including the hippocampus, thalamus, hypothalamus, amygdala, basal ganglia, and substantia nigra. Changes in nAChR activity modulate the synaptic release of acetylcholine itself, but also other neurotransmitters including dopamine (DA), norepinephrine (NE), serotonin (5-hydroxytryptamine, 5-HT), glutamate (Glu), and γ-aminobutyric acid (GABA) (Jensen et al., 2005). In animal models, nicotine administration increases the extracellular concentration of DA in the hippocampus, frontal cortex, cingulate cortex, and pontine nucleus, 5-HT in the cingulate gyrus and frontal cortex, and NE in the substantia nigra, cingulate gyrus, and pontine nucleus (Toth et al., 1992).
EFFECTS OF nAChR AGONISTS AND ANTAGONISTS ON DEPRESSIVE BEHAVIOR AND MOOD
Although hypothesized to play a role in the etiology of depression for decades, the cholinergic system has been overshadowed by the monoamine hypothesis of depression. In 1972, Janowsky and colleagues proposed the cholinergic–adrenergic theory of depression, postulating that emotional affect is governed by a balance between cholinergic and noradrenergic systems and over-activation of or hypersensitivity to the cholinergic system leads to depressive symptoms (Janowsky et al., 1972). This hypothesis derived from clinical observations demonstrating that acetylcholinesterase inhibitor drugs, such as diisopropylfluorophosphonate (insecticide) and physostigmine, both increase central ACh concentration and exacerbate depressive symptoms (Janowsky et al., 1974; Reynolds et al., 2011). However, it is largely unclear whether this effect is related to a specific ACh receptor type. Early data supporting a potential benefit of nAChR agonists came from studies of depressed smokers, who after quitting tobacco use exhibited an increased risk of depression relapse (Covey et al., 1997; Glassman et al., 2001).
Preclinical studies examining animal models of depressive behavior
Pre-clinical data demonstrates antidepressant effects for both nAChR agonists and antagonists. Differences exist between antidepressant effects of nicotine in rat versus mouse models (Andreasen and Redrobe, 2009a). In rats the more consistent finding is antidepressant-like effects for nicotine but not the nAChR antagonist mecamylamine, with nicotine being associated with decreased depressive behaviors after both acute and chronic administration of up to 2 weeks (Semba et al., 1998; Tizabi et al., 1999; Vázquez-Palacios et al., 2004). Conversely, in mice, the more consistent finding is antidepressant-like effects of mecamylamine (nAChR antagonist), with nicotine demonstrating no antidepressant-like effects except in select strains (Andreasen and Redrobe, 2009a, 2009b; Popik et al., 2003). In both rat and mouse depression models, pre-clinical data demonstrates that nicotine may augment monoamine-based antidepressant drugs. In a mouse model, nicotine had no antidepressant-like effect alone, but it significantly enhanced the antidepressant-like effect of imipramine (Popik et al., 2003). Similar findings were observed in a rat model using fluoxetine (Vázquez-Palacios et al., 2004).
Explaining discrepant results: nAChR pharmacology
Species differences aside, pharmacologic effects may explain inconsistencies in the pre-clinical data for nAChR agonists and antagonist effects on mood (Picciotto et al., 2008). One hypothesis is that with chronic administration, nAChR agonists cause desensitization of nAChR receptors, ultimately resulting in decreased nAChR activity similar to what may be seen with antagonists. As with other ligand-gated ion channels, the nAChR channel pore opens in response to the binding of an agonist (“activation”), however it can also rapidly shift to a conformational state that is refractory to further binding and activation (“desensitization”) (Fenster et al., 1997; Shytle et al., 2002). Because of nAChR desensitization, chronic exposure to nAChR agonists can lead to functional nAChR antagonism (Gentry and Lukas, 2002; Quick and Lester, 2002; Semba et al., 1998; Shytle et al., 2002). Desensitization is consistent with theories that reductions in cholinergic neurotransmission benefit depression and explain how nAChR agonists might have similar antidepressant effects as nAChR antagonists.
There are caveats to this theory. For example, a rat study found that the nAChR antagonist mecamylamine blocks the antidepressant effect of nicotine (Tizabi et al., 1999). Thus, activation of nAChRs may play a role in the antidepressant effects of nAChR agonists. This is concordant with the known pharmacology of nAChRs as even with desensitization, acute nAChR activation still occurs and nAChR expression increases in response to chronic nAChR agonist exposure (Picciotto et al., 2008). While the mechanism underlying this receptor upregulation is not fully understood, nicotine may act as a molecular chaperone for nAChRs both intracellularly and at the cell surface (Lester et al., 2009). The increased number of nAChRs may be a compensatory response to the functional decrease in nAChR activity induced by desensitization, and is comparable to observations that nAChR antagonists also increase nAChR receptor numbers (Fenster et al., 1997).
These data support that chronic use of nAChR agonists may result in a dynamic balance between desensitization and increased receptor number (Picciotto et al., 2008). This balance is influenced by multiple factors, including dose, duration, type of nAChR agonist administered, and whether the ligand binds to a specific nAChR subtype. For example, relatively low nicotine concentrations like that experienced by smokers leads to preferential desensitization in the ventral tegmental area of α4β2 nAChRs compared with α7 nAChRs. In turn, this has the functional consequence of favoring glutamatergic over GABAergic tone, inducing greater dopaminergic activity in the mesolimbic circuit (Corringer et al., 1998; Fenster et al., 1997; Mansvelder et al., 2002; Mansvelder and McGehee, 2000). Similar nAChR modulation of monoamine systems may occur in other brain regions.
Clinical studies examining antidepressant effects of drugs modulating nAChR activity
A small number of clinical trials in humans have examined the effect of nAChR agonists and antagonists in depressed populations with similarly mixed results. These inconsistencies may be explained by methodological differences in duration or timing of drug administration and differences in dosage that determine pharmacologic effect at the nAChR. The nAChR antagonist mecamylamine showed effective antidepressant augmentation in a small clinical trial (George et al., 2008) but larger-scale trials examining mecamylamine’s S-enantiomer (dexmecamylamine) failed to support antidepressant efficacy (Moller et al., 2015; Vieta et al., 2014). Varenicline, a nicotinic partial agonist at the α4β2 receptor and full agonist at α7 receptors, is FDA approved for smoking cessation but with concerns for negative effects on mood. Early case reports of varenicline in smokers suggested increased depressive and suicidal behavior (Harrison-Woolrych and Ashton, 2011; Moore et al., 2011; Williams et al., 2011), however a recent meta-analysis of placebo controlled trials concluded the evidence did not support increased risk for depression or suicide (Thomas et al., 2015).
Studies of nAChR agonists primarily focus on nicotine, reporting that nicotine benefits depressive symptoms in depressed non-smokers. In a short-term 4-day open-label trial, transdermal nicotine decreased depression symptoms and increased REM sleep time (Salin-Pascual, 2002), although in a small 8-day randomized controlled trial, the effect of transdermal nicotine on depression severity did not differ from placebo (Cox et al., 2003). In contrast, a small, randomized placebo-controlled trial of transdermal nicotine over 4 weeks demonstrated decreased depression severity after 8 days. Moreover, it showed evidence for improvement in attention measured by the Connors Continuous Performance Task (CPT) (McClernon et al., 2006). Finally, a small 8 month randomized controlled trial of transdermal nicotine demonstrated long-term antidepressant efficacy comparable to fluoxetine (Haro and Drucker-Colín, 2004). While preliminary and of insufficient duration compared with current antidepressant clinical trials, they support the hypothesis that nicotine administration may benefit depressive symptoms.
EFFECTS OF nAChR AGONISTS ON COGNITIVE PERFORMANCE
Links between the cholinergic system and cognition arose from clinical observations in the 1950s that central anticholinergic drugs, then used for conscious sedation during childbirth, produced a “dementia-like” syndrome with concomitant memory loss (Bartus et al., 1985). A series of early influential experiments demonstrated that the mAChR antagonist scopolamine induced robust memory deficits that were not reversed by stimulants such as amphetamines, but were ameliorated by the cholinesterase inhibitor physostigmine (Drachman and Leavitt, 1974). The nAChR system was implicated after reports that smokers exhibited subjective decline in cognitive performance during abstinence, with improvement in psychomotor vigilance during nicotine administration (Frankenhaeuser et al., 1971; Heimstra et al., 1967).
Preclinical studies examining animal models of cognition
Pre-clinical data demonstrate that nAChR agonists improve memory, learning, and attention in both normal and cognitively impaired rodents. Across studies in cognitively normal animals, nicotine improves working memory without any diminishment in effect when comparing acute to chronic administration (Arendash et al., 1995; Levin, 2013; Levin et al., 2006). Beyond benefits to working memory, nicotine also improves attention for some rodent strains (Levin et al., 2006). Cognitively impaired rodents similarly benefit from nAChR agonists. Studies in rats with spatial working memory deficits induced by lesions to the nucleus basalis of Meynert or the medial septum cholinergic projection systems demonstrate that nicotine can reverse or attenuate such deficits (Decker et al., 1992; Levin et al., 1993; Tilson et al., 1988). Studies of aged animals demonstrates that both aged and young rats exhibit improvements in working memory after acute nicotine administration, but aged rats failed to benefit from chronically administered nicotine (Attaway et al., 1999; Levin and Torry, 1996). However, both aged and young monkeys demonstrated comparable improvement in working memory with chronic administration when the aged monkeys received a higher pre-treatment dose of nicotine (Buccafusco and Jackson, 1991).
Clinical studies examining cognition
Trials of nicotine in cognitively intact human populations show mixed results for cognition, with differences being largely dependent on what cognitive domain is being examined and how it is being measured. Whereas nicotine administration in animals improves memory and learning, most human data support that nicotine improves attentional performance. Across studies in smokers and non-smokers, nicotine administration results in improved attentional performance as measured by rapid visual information processing and continuous performance tests (File et al., 2001; Foulds et al., 1996; Harte and Kanarek, 2004; Kelemen and Fulton, 2008; Lawrence et al., 2002; Levin et al., 1998; Myers et al., 2008; Poltavski and Petros, 2006). However, other attentional tests, such as the signal detection task and attention network test, do not always demonstrate drug effects (Barr et al., 2008; Ernst et al., 2001; Heishman and Henningfield, 2000; Kleykamp et al., 2005). Studies of long term memory (File et al., 2001; Foulds et al., 1996; Kelemen and Fulton, 2008; Perkins et al., 2008) and working memory (Heishman and Henningfield, 2000; Jacobsen et al., 2006; Kleykamp et al., 2005), mostly conducted in non-smokers, also have mixed results that may be related to the tasks. For example, a blinded trial in smokers and never-smokers showed that nicotine only benefitted working memory accuracy during a distracting condition, suggesting that nicotine benefits performance but only for attentionally demanding tasks (McClernon et al., 2003).
Recent work supports a more generalized cognitive benefit. A meta-analysis by Heishman and colleagues examined studies of non-abstinent smokers and non-smokers, concluding that nicotine benefitted both attention and memory, specifically in response time (RT) (Heishman et al., 2010). They describe positive benefits of nicotine in five domains: alerting attention accuracy and RT, orienting attention RT, short-term episodic memory accuracy, and working memory RT. In contrast, domains of working memory accuracy and long term episodic memory accuracy did not show benefit across studies. It is possible that benefits to memory may be mediated primarily through improvements in attentional functioning (Newhouse et al., 2004) and explained as downstream effects of the primary optimization of attentional function. Such improvements in ‘front-end’ attentional functioning are required to achieve the basic steps of memory function including acquisition, encoding, storage and retrieval of information. Although informative, this meta-analysis was limited by few studies allowing for evaluation of nicotine’s effects on executive function or performance on higher order real-world tasks. Relevant to our focus on LLD, studies of elderly patients were excluded from this analysis.
Explaining discrepant clinical results
Two hypotheses, reviewed by Newhouse and colleagues (Newhouse et al., 2004), may explain discrepancies across individual studies. First, studies generally show that nicotine does not improve cognitive functioning or may actually impair cognition in healthy non- or never-smokers but does improve cognitive functioning in smokers or neuro-psychiatric populations (Newhouse et al., 2004). This suggests that nicotine may have more benefit in groups with altered cholinergic system function or underlying nAChR dysfunction. Second, studies in non-smokers that most consistently show benefits of nicotine utilize cognitively demanding tasks requiring sustained or focused attention despite distracting stimuli. Thus, nicotine’s effect on cognitive performance depends on both the subject’s underlying cholinergic activity and the level of effort required by the task itself. Studies suggest that nicotine’s effect on cognitive performance can be modeled as an inverted “U” shape dose-response relationship for each task, with intermediate levels of nAChR activity producing optimal performance on the task while either low or high levels of nAChR activity results in poorer performance (Buccafusco and Jackson, 1991; Perkins et al., 1993). This model has two findings (Fig. 1). First, administration of a nAChR agonist in individuals with relatively normal nAChR activity may improve performance on cognitively difficult tasks, but worsen performance on easier tasks (Fig 1a). Second, in individuals with lower inherent baseline nAChR activity such as smokers or individuals with neuropsychiatric pathology, administration of a nAChR agonist may improve performance on both cognitively difficult and less difficult tasks (Fig 1b).
FIGURE 1.
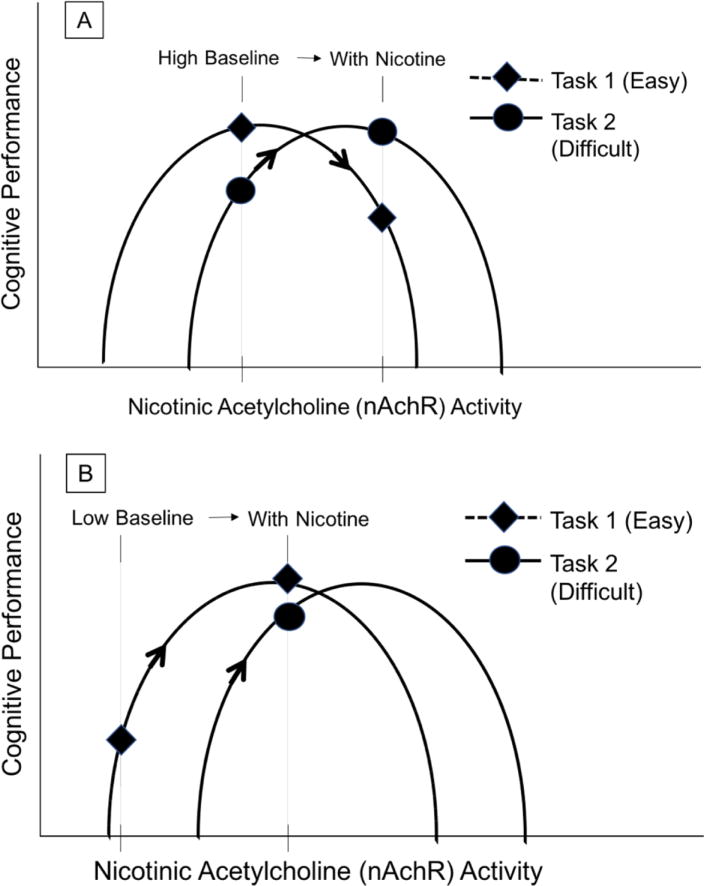
Variability in effects of nicotine on cognitive performance
Nicotine’s effect on cognitive performance can be modeled as an inverted “U” shape dose-response relationship for each cognitive task. This figure illustrates two situations in which an equivalent degree of nAChR stimulation by extrinsic nicotine administration produces opposite effects depending on both the subject’s underlying nAChR activity and the level of cognitive effort required by the task itself. (1a) Illustrates an example of a Non-smoker who has healthy relatively high baseline nAChR activity and has improvement in cognitively difficult tasks, but worsening on easier tasks. (1b) Illustrates a different scenario of a smoker or individual with neuropsychiatric pathology that has relatively lower baseline nAChR activity and has improvement both in cognitively difficult and less difficult tasks. Figure adapted from Newhouse and colleagues (Newhouse et al., 2004).
Clinical studies in older populations
Evidence supports a potential benefit of nicotine in age-related neuropsychiatric conditions including Alzheimer’s dementia (AD) (Newhouse et al., 1988), Mild Cognitive Impairment (MCI) (Newhouse et al., 2012), and Age Associated Memory Impairment (AAMI) (White and Levin, 2004), but also schizophrenia (Harris et al., 2004) and attention-deficit hyperactivity disorder (ADHD) (Levin et al., 2001). Altered cholinergic function, including altered nAChR activity, may contribute to the cognitive symptoms in these neuropsychiatric disorders (Beane and Marrocco, 2004; Court et al., 2001; Freedman et al., 2000; Picciotto and Zoli, 2002; Sabbagh et al., 2006). Cholinergic dysfunction is best studied in AD, a disorder characterized by decreased cortical and striatal α4β2 nAChRs (Court et al., 2001) where the current primary treatments are acetylcholinesterase inhibitors. Newhouse and colleagues first used intravenous nicotine in AD, demonstrating evidence of improved cognitive functioning characterized as decreased intrusion errors on an immediate recall task, interpreted as an improvement in attentional focus (Newhouse et al., 1988). Subsequent clinical studies of nicotine in AD demonstrated improvements in rapid visual information processing (Sahakian et al., 1989) and continuous performance tests (White and Levin, 1999). Importantly for AD subjects, attentional improvement did not diminish over four weeks of transdermal nicotine patch administration, reinforcing the notion that nicotine’s cognitive effects are not diminished with chronic administration (White and Levin, 1999).
Nicotine also benefits cognitive performance in other older populations, including individuals with MCI or AAMI. Newhouse et al. (Newhouse et al., 2012) and White et al.(White and Levin, 2004) hypothesized that MCI and AAMI, respectively, may show greater response to nicotine administration compared with AD as the nAChR system is relatively more intact (Sabbagh et al., 2006) and thus able to respond to stimulation by nicotine. In a recent 6-month double blind RCT of subjects with amnestic MCI, compared with placebo, participants receiving transdermal nicotine showed greater improvements in attentional performance and episodic memory (Newhouse et al., 2012). In AAMI, White et al. showed similar results in a 4-week double-blind RCT of transdermal nicotine, observing not only improvement in attention, but also that participants endorsed a subjective improvement in memory (White and Levin, 2004).
NEURAL NETWORK MODELS OF nAChR AGONIST ACTIVITY AND APPLICABILITY TO DEPRESSION
Functional neuroimaging provides an opportunity to move beyond behavioral or cognitive measures into an assessment of how nicotine or other nAChR agonists affect brain activity and functional connectedness of neural networks. Initial work examining nicotine effects on brain function utilized task-based functional MRI, examining how nicotine changes brain activity during cognitive tasks (for review, see (Newhouse et al., 2011)). More recent work incorporates our understanding of the brain’s functional organization and the identification of multiple intrinsic functional networks. These functional networks represent brain regions exhibiting synchronous coupling of spontaneous fluctuations in activity during both active tasks and during rest. This coupling is referred to as “functional connectivity” and represents the temporal coherence of the MRI BOLD signal within or between regions (Friston, 2011; Menon, 2011). While there are several intrinsic networks discussed in the literature, we focus on key networks implicated in MDD (Hamilton et al., 2013; Kaiser et al., 2015; Mulders et al., 2015) that also appear to be affected by nAChR agonists.
Overview of Key Intrinsic Functional Networks
The Default Mode Network (DMN, initially referred to as the “task-negative network”) is a set of brain regions where activity decreases during externally-driven task performance and increases at rest (Raichle et al., 2001). The DMN consists of interconnected subsystems that converge on two key subnetwork “hubs,” specifically the anterior medial prefrontal cortex hub and a posterior hub centering on the posterior cingulate cortex and precuneus cortex (Buckner et al., 2008). The DMN is important in internally-directed and self-referential mental activity (Buckner et al., 2008), with the posterior hub also being related to memory processing (Andrews-Hanna et al., 2014, 2010).
The Central-Executive Network (CEN, also referred to as the “cognitive control network” or “executive control network”) is a set of regions more active during external cognitive tasks. CEN regions are primarily located in the frontoparietal cortices with network hubs in the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (Seeley et al., 2007). The CEN is hypothesized to be involved in attention, working memory, and executive functions such as decision making and conflict resolution (Seeley et al., 2007).
During normal brain functioning there exists an antagonistic relationship between the DMN and CEN observed both during tasks and at rest (Fox et al., 2005). Typically activity increases in the CEN and decreases in the DMN during externally focused tasks, with the pattern reversed during rest or self-reflection. This balance is believed to be a basis for the normal cognitive ability to alternate effectively between introspective or internally oriented information processing and information processing of external stimuli (Fox et al., 2005; Fransson, 2006, 2005; Sonuga-Barke and Castellanos, 2007).
Switching between the DMN and CEN is mediated by the Salience Network (SN) which has the role of monitoring and responding to environmental stimuli (Goulden et al., 2014). The primary hub of the SN is the insula cortex and includes the amygdala, other frontotemporal areas, and the anterior cingulate cortex (ACC) (Seeley et al., 2007). It is activated in response to salient environmental stimuli including acute stressors, serving to provide awareness of and response to relevant environmental stimuli. It also plays a role in emotional control, although potentially at the expense of CEN function (Hermans et al., 2014).
Effects of nAChR agonists on intrinsic network function
Numerous studies, primarily focusing on smokers with a smaller number examining nonsmokers, have examined the effect of nicotine and other nAChR agonists on brain function (Newhouse et al., 2011). Cumulatively, by linking imaging findings with cognitive task performance, this body of work led to hypotheses (Bentley et al., 2011; Newhouse et al., 2004; Sutherland et al., 2015) that nAChR agonists may benefit cognition by: 1) decreasing activity in regions involved in task-irrelevant information processing; 2) increasing activity in regions involved in task-related information processing; and/or 3) decreasing activity in some task-related regions, possibly reflecting improved efficiency. This implies that nAChR agonists may decrease activity in DMN (task-negative) regions while increasing activity or improving efficiency in CEN (task-positive) regions.
This proposal is supported by a meta-analysis analyzing MRI and PET studies that examined task-independent effects of nAChR agonists (Sutherland et al., 2015). The authors found that nAChR agonist administration resulted in two patterns of convergent activity changes that did not substantially differ based on smoking status. First, nAChR agonists decreased activity across multiple regions. These changes reflected enhanced deactivation in the ventromedial prefrontal cortex, posterior cingulate cortex, and parahippocampus with reduced activation in the subgenual ACC, bilateral insula, and parietal and precentral cortices. Second, nAChR agonists increased activity in the dorsomedial prefrontal cortex, dorsal ACC, thalamus, and lateral prefrontal and parietal cortices. The authors concluded that nAChR agonists have the general neuropharmacological effects of decreasing activity in DMN regions and increasing activity in CEN regions (Sutherland et al., 2015). Although the authors did not specifically discuss the SN, they did observe that nAChR agonists decreased activity in the anterior insula, the primary hub of that network.
Importantly, there is significant overlap in regions where brain activity changes following nAChR agonist administration and regions of nAChR distribution, such as the prefrontal cortex, thalamus, and medial temporal lobe. However, this does not mean that all observed changes in brain activity are directly related to agonist activity at nAChRs. The nAChR system modulates the activities of several other neurotransmitters including dopamine and serotonin, so some of the observed functional effects may be related to downstream changes in activity of other neurotransmitters.
Intrinsic Network Alterations in Depression
Depressed populations typically exhibit increased DMN activity, both when engaging in the assessment of external stimuli (when the DMN should deactivate) (Sheline et al., 2009) and during maladaptive ruminative self-focus (Cooney et al., 2010). In depressed populations the DMN is also broader, including regions of the thalamus and subgenual ACC (Greicius et al., 2007; Zhou et al., 2010), a region not included in the DMN in nondepressed samples. Studies also associate depression with differences in functional connectivity between DMN regions. Specifically, depression is associated with increased connectivity within each anterior and posterior hub of the DMN (Greicius et al., 2007; Kaiser et al., 2015), however there may also be decreased connectivity between the anterior and posterior hubs (van Tol et al., 2013).
The CEN tends to exhibit reduced activity in MDD. Most work in depression focuses on the dlPFC which exhibits decreased activity both at rest and in response to negative stimuli (Pizzagalli et al., 2009; Strigo et al., 2008). Other CEN regions also exhibit differences in activation during executive function tasks such as the Stroop task and the Tower of London task, where in depressed groups the dorsal ACC exhibits less activation (Elliott et al., 1997; George et al., 1997). Functional connectivity studies using the dlPFC as a seed region associate depression with reduced connectivity between the dlPFC and other CEN regions in both younger adult populations (Kaiser et al., 2015; van Tol et al., 2013) and in LLD (Alexopoulos et al., 2012).
In MDD the SN exhibits increased activity, particularly in response to negative stimuli (Hamilton et al., 2013). This negativity bias may be characterized by negatively valenced stimuli evoking a more pronounced and rapid response than neutral or positive stimuli. A number of studies of MDD report heightened activity in the amygdala with anticipation of receiving negative stimuli (Abler et al., 2007; Pizzagalli et al., 2009; Strigo et al., 2008), while the insula may exhibit increased activity during the receipt of negative stimuli (Craig, 2009). SN regions in MDD are generally over-responsive to affective challenges (Suslow et al., 2010), particularly negative stimuli. MDD is also associated with differences in SN functional connectivity, although the direction of the difference differs by SN region. Specifically, in MDD the amygdala exhibits decreased connectivity with frontal regions (Yue et al., 2013) and with other SN regions including the insula (Manoliu et al., 2013; Veer et al., 2010). In contrast, in MDD the insula exhibits increased connectivity with frontal and ACC regions, including DMN and CEN regions (Avery et al., 2014; Horn et al., 2010; Li et al., 2017).
Nicotine effects on intrinsic networks may reverse network alterations seen in depression
The effects of nAChR agonists on these intrinsic networks support a potential therapeutic role for nicotine. Broadly, the effects of nAChR agonists on intrinsic network activity appear to antagonize the alterations observed during depressive episodes (Table 1). Considering core features of depression, we refined a previous model (Zurkovsky et al., 2013) detailing how modulation of key networks by nAChR agonists may benefit specific symptoms of LLD (Figure 2). These theories are based on observed relationships between network function and clinical symptoms and follow the hypothesis that normalization or reversal of these network alterations may ameliorate depressive symptoms or improve critical cognitive processes. For example, maladaptive rumination is associated with increased activity in the DMN as well as altered connectivity between the DMN and the subgenual prefrontal cortex (Hamilton et al., 2011, 2015). Similarly, the negativity bias seen in depression involves frontal, cingulate, and temporal regions and is associated with increased activity in some SN regions but decreased CEN activity (Gollan et al., 2015; Hamilton et al., 2013; Jung et al., 2006). Nicotine’s effect of decreasing DMN and SN activity while enhancing CEN activity may improve these core symptoms of MDD and reduce depression severity.
TABLE 1.
Contrast in the effects of depression and nicotinic acetylcholine receptor agonists on intrinsic functional network activity
| Activity in Depression | Effect of nAChR agonists | |
|---|---|---|
| Default Mode Network (DMN) | ↑ Activity | ↓ Activity |
| Central-Executive Network (CEN) | ↓ Activity | ↑ Activity |
| Salience Network (SN) | ↑ Activity | ↓ Activity (Anterior insula) |
The table describes a summary of activity of brain regions within each network across tasks and does not reflect resting state functional connectivity. Note that there are less data on the effects of nAChR agonists on salience network activity. Although a meta-analysis reported that nAChR agonists decrease activity in the anterior insula,(Sutherland et al., 2015) they did not find evidence for an effect on other SN regions such as the amygdala.
FIGURE 2.
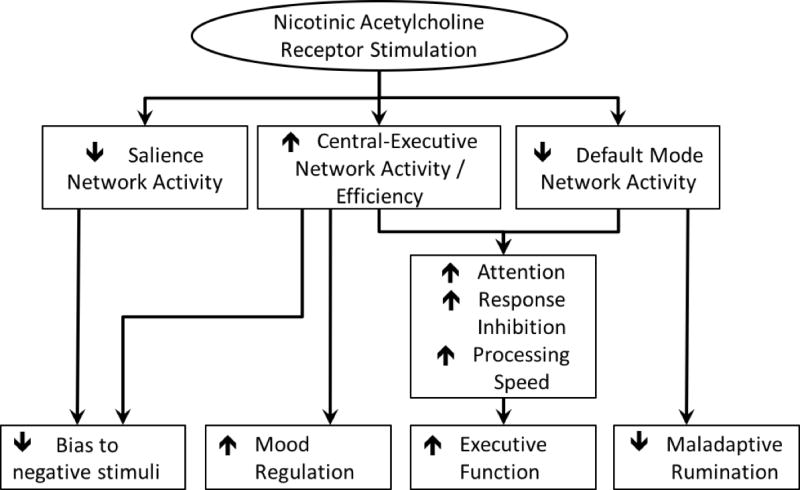
Model of nicotinic acetylcholine receptor agonist effects on intrinsic network activity, cognition, and depressive behaviors
The figure details how nAChR agonists appear to influence key intrinsic networks. In turn, it then builds on how such network effects may influence cognitive performance, core depressive behaviors (negativity bias, rumination), and the ability to modulate mood. In turn, these changes would be expected to result in overall reduced depression severity. Notably but not shown in the figure, it is also possible that improvement in cognition may reduce depressive symptoms or improve mood regulation abilities.
Network effects of nAChR agonists may also enhance beneficial cognitive processes. For example, emotion regulation, or cognitive attempts to regulate emotional responses, requires CEN activity (Zilverstand et al., 2016). Individuals with MDD exhibit reduced activity of CEN regions when attempting to control their emotional experience (Rive et al., 2013), although regions involved depend on the regulation strategy used (Smoski et al., 2014). Thus enhanced CEN activity may lead to increased effectiveness of emotional regulation strategies. Similarly, the effects of nAChR agonists to increase CEN activity and improve DMN deactivation during external tasks would be expected to improve cognitive task performance (Giessing et al., 2007; Hahn et al., 2007).
CONCLUSIONS, CAVEATS, AND NEXT STEPS
Both preclinical and clinical studies support that nicotine and other nAChR agonists can improve depressive behavior, mood, and cognitive performance. nAChR agonists also demonstrate neuropharmacologic effects that oppose the intrinsic network alterations reported in MDD (Table 1). Through modulation of intrinsic functional networks, nAChR agonists may reduce depressive symptoms, enhance emotional regulation ability, and improve cognitive deficits common in LLD (Figure 2). For these reasons, we propose nAChR agonists as a potential novel treatment for the mood and cognitive symptoms of LLD.
If this proposal is supported by future research, it would be a significant advance as current therapeutic options for depressed older adults do not benefit cognitive performance. Past approaches combined antidepressant medications with drugs with cognitive enhancing effects. Unfortunately, reports do not show clear benefits for cognition. For example, an augmentation study of the acetylcholinesterase inhibitor donepezil during 2 years of maintenance therapy found no overall benefit to mood or cognition. However, depressed elders who also met criteria for MCI exhibited modest benefits to cognitive performance but also increased rates of depression relapse (Reynolds et al., 2011). Donepezil’s role in LLD is being further investigated in an ongoing multisite trial (Pelton et al., 2014). Memantine, another commonly used drug for AD, also did not show benefit for LLD (Lenze et al., 2012). Methylphenidate, a stimulant used for attention-deficit disorder, benefits mood in LLD but its effects on cognitive performance are inconsistent (Lavretsky et al., 2015). Alternatively, non-pharmacological approaches may be of benefit. Several small studies of physical exercise programs show modest benefit for cognition in depressed and non-depressed older adults (Bherer et al., 2013; Khatri et al., 2001; Langlois et al., 2013). Similarly, preliminary studies of Computerized Cognitive Remediation (CCR) show promise in treating LLD with executive dysfunction (Morimoto et al., 2016).
The primary caveat to our hypothesis that nAChR agonists can be an effective therapy for LLD is the possibility that nAChR agonists could worsen depressive symptoms. This concern is raised by studies associating depression with increased acetylcholine activity (Janowsky et al., 1972) and highlighted by the trial of donepezil, that found an increased risk of depression relapse (Reynolds et al., 2011). The mechanism underlying this risk is unclear as acetylcholinesterase inhibitors have broad affects across muscarinic and nicotinic receptors, so the effect may be related to activity at muscarinic AChRs. This possibility is supported by clinical trials where mAChR antagonists improve depression (Drevets and Furey, 2010; Furey and Drevets, 2006). However, nAChRs may also play a role as depressed adults exhibited reduced availability of β2-nAChR binding (Saricicek et al., 2012). In that study, binding was lower in acutely depressed than recovered depressed subjects and a postmortem sample did not exhibit differences in β2-nAChR number. The authors concluded their findings may represent higher levels of endogenous acetylcholine and greater binding at nAChRs, resulting in lower receptor availability for the exogenous ligand.
Several questions need to be addressed. First, would nicotine or a nAChR agonist be best examined as monotherapy or used to augment a traditional antidepressant? Animal models suggest that nicotine enhances antidepressant effects (Popik et al., 2003; Vázquez-Palacios et al., 2004). Nicotine’s pharmacological target is distinct from traditional antidepressants other than bupropion, which has limited nAChR antagonism but primarily works through noradrenergic and dopaminergic mechanisms. Second, within the LLD population, what is the best group to study? Nicotine could be beneficial broadly, but individuals with cognitive impairment, and thus more impaired cholinergic system functioning, could have a better or worse response. Third, could nAChR agonists be most useful for treating depression in specific populations, such as depression with AD, a population where traditional antidepressants have limited efficacy? Fourth, would agents targeting specific nAChR receptor subtypes have greater efficacy? Some have proposed that limiting signaling through α4β2 nAChRs while increasing signaling at α7 nAChRs may benefit mood, so an agent or mix of agents with differing effects at heteromeric and homomeric nAChRs may be particularly helpful (Picciotto et al., 2015). Finally, given its effects on attention and the CEN, could nAChR agonists augment psychotherapeutic or cognitive remediation treatments?
The next step is a randomized, blinded, placebo-controlled clinical trial of transdermal nicotine in nonsmokers with LLD. This trial should have dual outcomes, examining both depression severity and cognition, assessing attentional performance, episodic and working memory, and executive function. To better elucidate the relationship between clinical measures and brain function, such a trial should be optimally combined with biomarkers assessing brain function, such as repeat functional MRI. This would allow us to test our model and determine whether nicotine affects the neural networks as hypothesized, and if so, whether those changes map onto changes in depressive symptoms and cognitive performance. The advantage of this approach is that even if nicotine did not improve clinical outcomes, the study would provide important novel data about both nicotine’s effects on the brain and the role of neural networks in depression.
HIGHLIGHTS.
Nicotine improves cognitive performance in clinical and preclinical studies.
Nicotine may also benefit depressive symptoms and depressive behavior.
Cognitive and mood benefits may be mediated by nicotinic effect on neural networks.
Nicotine’s effects on networks may reverse network changes seen in depression.
Improvement to mood and cognition may particularly benefit older depressed adults.
Acknowledgments
Supported by NIH grants K24 MH110598 and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences.
Footnotes
No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol. 2009a;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Nicotine, but not mecamylamine, enhances antidepressant-like effects of citalopram and reboxetine in the mouse forced swim and tail suspension tests. Behav Brain Res. 2009b;197:150–156. doi: 10.1016/j.bbr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- Attaway CM, Compton DM, Turner MD. The effects of nicotine on learning and memory: A neuropsychological assessment in young and senescent Fischer 344 rats. Physiol Behav. 1999;67:421–431. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Avery Ja, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major Depressive Disorder Is Associated with Abnormal Interoceptive Activity and Functional Connectivity in the Insula. Biol Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A Single Dose of Nicotine Enhances Reward Responsiveness in Nonsmokers: Implications for Development of Dependence. Biol Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Pontecorvo MJ, Flicker C. The cholinergic hypothesis: A historical overview, current perspective, and future directions. Ann N Y Acad Sci. 1985;444:332–358. doi: 10.1111/j.1749-6632.1985.tb37600.x. [DOI] [PubMed] [Google Scholar]
- Beane M, Marrocco RT. Norepinephrine and acetylcholine mediation of the components of reflexive attention: Implications for attention deficit disorders. Prog Neurobiol. 2004;74:167–181. doi: 10.1016/j.pneurobio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Beekman ATF, Geerlings S, Deeg D, Smit J, Schoever R, De Beurs E, Braam A, Penninx BWJH, van Tilburg W. The Natural History of Late-Life Depression. Arch Gen Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: Insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–388. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, Butters Ma, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG. Depression in Late Life: Review and Commentary. Journals Gerontol Ser A Biol Sci Med Sci. 2003;58:M249–M265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991;12:233–238. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF. Changes in Cognitive Functioning Following Treatment of Late-Life Depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Bertrand S, Bohler S, Edelstein SJ, Changeux JP, Bertrand D. Critical elements determining diversity in agonist binding and desensitization of neuronal nicotinic acetylcholine receptors. J Neurosci. 1998;18:648–657. doi: 10.1523/JNEUROSCI.18-02-00648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer’s disease. Biol Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major Depression Following Smoking Cessation. Am J PsychiatryAm J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Cox LS, Patten CA, Krahn LE, Hurt RD, Croghan IT, Wolter TD, Schroeder DR, Tri D, Offord KP. The effect of nicotine patch therapy on depression in nonsmokers: a preliminary study. J Addict Dis. 2003;22:75–85. doi: 10.1300/j069v22n04_07. [DOI] [PubMed] [Google Scholar]
- Craig aDB. How do you feel--now? The anterior insula and human awareness Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ, Anderson DJ. Effects of nicotine on spatial memory deficits in rats with septal lesions. Brain Res. 1992;572:281–285. doi: 10.1016/0006-8993(92)90485-r. [DOI] [PubMed] [Google Scholar]
- Diniz BSS, Butters MAA, Albert SMM, Dew MAA, Reynolds CFF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of Scopolamine’s Antidepressant Efficacy in Major Depressive Disorder: A Randomized, Placebo-Controlled Clinical Trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O’Leary Da, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman S, Spurgeon L, London E. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester Ra. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. Int J Neuropsychopharmacol. 2001;4:371–376. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser M, Myrsten aL, Post B, Johansson G. Behavioural and physiological effects of cigarette smoking in a monotonous situation. Psychopharmacologia. 1971;22:1–7. doi: 10.1007/BF00401461. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The a7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) J Neuropsychiatry Clin Neurosci. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Giessing C, Fink GR, Rösler F, Thiel CM. fMRI data predict individual differences of behavioral effects of nicotine: a partial least square analysis. J Cogn Neurosci. 2007;19:658–670. doi: 10.1162/jocn.2007.19.4.658. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: A follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Connolly M, Buchanan A, Hoxha D, Rosebrock L, Cacioppo J, Csernansky J, Wang X. Neural substrates of negativity bias in women with and without major depression. Biol Psychol. 2015;109:184–191. doi: 10.1016/j.biopsycho.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R, Drucker-Colín R. Effects of long-term administration of nicotine and fluoxetine on sleep in depressed patients. Arch Med Res. 2004;35:499–506. doi: 10.1016/j.arcmed.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Harrison-Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: An intensive postmarketing prospective cohort study in New Zealand. Drug Saf. 2011;34:763–772. doi: 10.2165/11594450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Harte CB, Kanarek RB. The effects of nicotine and sucrose on spatial memory and attention. Nutr Neurosci. 2004;7:121–125. doi: 10.1080/10284150410001704543. [DOI] [PubMed] [Google Scholar]
- Heimstra NW, Bancroft NR, DeKock AR. EFFECTS OF SMOKING UPON SUSTAINED PERFORMANCE IN A SIMULATED DRIVING TASK. Ann N Y Acad Sci. 1967;142:295–307. [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses, in nonsmokers. Psychopharmacology (Berl) 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- Heishman SJJ, Kleykamp BAa, Singleton EGG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJAG, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology (Berl) 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]
- Janowsky D, Davis J, El-Yousef MK, Sekerke HJ. A CHOLINERGIC-ADRENERGIC HYPOTHESIS OF MANIA AND DEPRESSION. Lancet. 1972;300:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, El-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Jung YC, An SK, Seok JH, Kim JS, Oh SJ, Moon DH, Kim JJ. Neural substrates associated with evaluative processing during co-activation of positivity and negativity: A PET investigation. Biol Psychol. 2006;73:253–261. doi: 10.1016/j.biopsycho.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal Dysfunction and Treatment Response in Geriatric Depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Kelemen WL, Fulton EK. Cigarette abstinence impairs memory and metacognition despite administration of 2 mg nicotine gum. Exp Clin Psychopharmacol. 2008;16:521–531. doi: 10.1037/a0014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Blumenthal JA, Babyak MA, Craighead EW, Herman S, Baldewicz T, Madden DJ, Doraiswamy M, Waugh R, Krishnan RK. Effects of Exercise Training on Cognitive Functioning Among Depressed Older Men and Women. J Aging Phys Act. 2001;9:43–57. [Google Scholar]
- Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychol Addict Behav. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- Langlois F, Vu TTM, Chasse K, Dupuis G, Kergoat MJ, Bherer L. Benefits of Physical Exercise Training on Cognition and Quality of Life in Frail Older Adults. Journals Gerontol Ser B Psychol Sci Soc Sci. 2013;68:400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, Methylphenidate, or Their Combination in Geriatric Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Am J Psychiatry. 2015;172:561–569. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer Ka, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: A 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27:974–980. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery AAPS J. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharmacol. 2013;86:1145–1152. doi: 10.1016/j.bcp.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Channelle Christopher N, Briggs SJ, Rose JE. Chronic nicotine reverses working memory deficits caused by lesions of the fimbria or medial basalocortical projection. Cogn Brain Res. 1993;1:137–143. doi: 10.1016/0926-6410(93)90021-v. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacol. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Psychopharmacology. Springer-Verlag; 2006. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization; pp. 523–539. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Ward BD, Antuono PG, Li SJ, Goveas JS. Intrinsic internetwork brain dysfunction correlates with symptom dimensions in late-life depression. J Psychiatr Res. 2017;87:71–80. doi: 10.1016/j.jpsychires.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthöffer D, Zimmer C, Förstl H, Bäuml J, Riedl V, Wohlschläger AM, Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905 9–9. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-Term Potentiation of Excitatory Inputs to Brain Reward Areas by Nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Gilbert DG, Radtke R. Effects of transdermal nicotine on lateralized identification and memory interference. Hum Psychopharmacol. 2003;18:339–343. doi: 10.1002/hup.488. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25:933–944. doi: 10.1002/gps.2431. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moller HJ, Demyttenaere K, Olausson B, Szamosi J, Wilson E, Hosford D, Dunbar G, Tummala R, Eriksson H. Two Phase III randomised double-blind studies of fixed-dose TC-5214 (dexmecamylamine) adjunct to ongoing antidepressant therapy in patients with major depressive disorder and an inadequate response to prior antidepressant therapy. World J Biol Psychiatry. 2015;16:1–19. doi: 10.3109/15622975.2014.989261. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto SS, Gunning FM, Wexler BE, Hu W, Ilieva I, Liu J, Nitis J, Alexopoulos GS. Executive Dysfunction Predicts Treatment Response to Neuroplasticity-Based Computerized Cognitive Remediation (nCCR-GD) in Elderly Patients with Major Depression. Am J Geriatr Psychiatry. 2016;24:816–820. doi: 10.1016/j.jagp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2004;12:50–6. [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-Related Enhancement of Mood and Cognition in Smokers Administered Nicotine Nasal Spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16:558–567. doi: 10.1097/JGP.0b013e3181693288. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter AS, Dumas JA, Thiel CM. Functional brain imaging of nicotinic effects on higher cognitive processes. Biochemical Pharmacology. 2011:943–951. doi: 10.1016/j.bcp.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, Murphy DL. Intravenous nicotine in Alzheimer’s disease: a pilot study. Psychopharmacology (Berl) 1988;95:171–175. doi: 10.1007/BF00174504. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Kellar K, Aisen P, White H, Wesnes K. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology. 2012;78:91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton GH, Andrews H, Roose SP, Marcus SM, D’Antonio K, Husn H, Petrella JR, Zannas AS, Doraiswamy PM, Devanand DP. Donepezil treatment of older adults with cognitive impairment and depression (DOTCODE study): Clinical rationale and design. Contemp Clin Trials. 2014;37:200–208. doi: 10.1016/j.cct.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Epstein LH, Caggiula A, Stiller RL, Jacob RG. Chronic and acute tolerance to subjective effects of nicotine. Pharmacol Biochem Behav. 1993;45:375–381. doi: 10.1016/0091-3057(93)90254-q. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: A review of the preclinical and clinical literature. Psychopharmacology (Berl) 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, Van Schalkwyk GI, Mineur YS, Van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96:235–243. doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- Pimontel MA, Culang-Reinlieb ME, Morimoto SS, Sneed JR. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.2808. [DOI] [PubMed] [Google Scholar]
- Pizzagalli Da, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Krawczyk M. Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br J Pharmacol. 2003;139:1196–1202. doi: 10.1038/sj.bjp.0705359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RAJ. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, Lenze EJ, Holm M, Rogers JC, Mazumdar S, Houck PR, Begley A, Anderson S, Karp JF, Miller MD, Whyte EM, Stack J, Gildengers A, Szanto K, Bensasi S, Kaufer DI, Kamboh MI, DeKosky ST. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies Neurosci Biobehav Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Shah F, Reid RT, Sue L, Connor DJ, Peterson LKN, Beach TG. Pathologic and nicotinic receptor binding differences between mild cognitive impairment, Alzheimer disease, and normal aging. Arch Neurol. 2006;63:1771–1776. doi: 10.1001/archneur.63.12.1771. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ. Relationship between mood improvement and sleep changes with acute nicotine administration in non-smoking major depressed patients. Rev Investig clínica; organo del Hosp Enfermedades la Nutr. 2002;54:36–40. [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Ruf BM, Chen JI, Cosgrove KP, Kerestes R, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z. Persistent Beta2-Nicotinic Acetylcholinergic Receptor Dysfunction iN Major Depressive Disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, Le Masurier M, Mackay CE, Ebmeier KP. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med. 2012;42:1195–1202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Pieper C, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D’Angelo G, Garcia KS. Support for the Vascular Depression Hypothesis in Late-Life Depression Results of a 2-Site, Prospective, Antidepressant Treatment Trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Silver aa, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Keng SL, Ji JL, Moore T, Minkel J, Dichter GS. Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Soc Cogn Affect Neurosci. 2014;10:1187–1194. doi: 10.1093/scan/nsv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig ADB, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic Mood-Congruent Amygdala Responses to Masked Facial Expressions in Major Depression. Biol Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR. Neurobiological Impact of Nicotinic Acetylcholine Receptor Agonists: An Activation Likelihood Estimation Meta-Analysis of Pharmacologic Neuroimaging Studies. Biol Psychiatry. 2015;78:711–720. doi: 10.1016/j.biopsych.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD. Clinical practice. Depression in the elderly N Engl J Med. 2014;371:1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Doraiswamy PM. A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology. 2004;29:2285–2299. doi: 10.1038/sj.npp.1300550. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Wagner HR, Steffens DC. Greater depression severity associated with less improvement in depression-associated cognitive deficits in older subjects. Am J Geriatr Psychiatry. 2002;10:632–635. [PubMed] [Google Scholar]
- Thomas KH, Martin RM, Knipe DW, Higgins JPT, Gunnell D. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109. doi: 10.1136/bmj.h1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA, McLamb RL, Shaw S, Rogers BC, Pediaditakis P, Cook L. Radial-arm maze deficits produced by colchicine administered into the area of the nucleus basalis are ameliorated by cholinergic agents. Brain Res. 1988;438:83–94. doi: 10.1016/0006-8993(88)91326-1. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Toth E, Sershen H, Hashim A, Vizi ES, Lajtha A. Effect of nicotine on extracellular levels of neurotransmitters assessed by microdialysis in various brain regions: Role of glutamic acid. Neurochem Res. 1992;17:265–271. doi: 10.1007/BF00966669. [DOI] [PubMed] [Google Scholar]
- van Tol M-J, Li M, Metzger CD, Hailla N, Horn DI, Li W, Heinze HJ, Bogerts B, Steiner J, He H, Walter M. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med. 2013:1–13. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- Vázquez-Palacios G, Bonilla-Jaime H, Velázquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine] Pharmacol Biochem Behav. 2004;78:165–169. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Veer I, Beckmann C, van Tol MJ, Ferrarini L. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Thase ME, Naber D, D’Souza B, Rancans E, Lepola U, Olausson B, Szamosi J, Wilson E, Hosford D, Dunbar G, Tummala R, Eriksson H. Efficacy and tolerability of flexibly-dosed adjunct TC-5214 (dexmecamylamine) in patients with major depressive disorder and inadequate response to prior antidepressant. Eur Neuropsychopharmacol. 2014;24:564–574. doi: 10.1016/j.euroneuro.2013.12.008. [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999;143:158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- White HKK, Levin EDD. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl) 2004;171:465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steinberg MB, Steinberg ML, Gandhi KK, Ulpe R, Foulds J. Varenicline for tobacco dependence: panacea or plight? Expert Opin Pharmacother. 2011;12:1799–1812. doi: 10.1517/14656566.2011.587121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, Nag S, Schneider JA, Arnold SE, Bennett DA. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83:702–709. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Yuan Y, Hou Z, Jiang W, Bai F, Zhang Z. Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS One. 2013;8:e75058. doi: 10.1371/journal.pone.0075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, Li K, Jiang T. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review Neuroimage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Taylor WD, Newhouse PA. Cognition as a therapeutic target in late-life depression: Potential for nicotinic therapeutics. Biochem Pharmacol. 2013;86:1133–1144. doi: 10.1016/j.bcp.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


