Abstract
Objective
To determine if sarcopenia in the presence of low bone mineral density (BMD) increases the risk of clinical fractures in women compared to women with either sarcopenia or low BMD alone.
Design
Women’s Health Initiative (WHI) Observational and Clinical trials
Setting
3 US Clinical Centers-Pittsburgh, PA, Birmingham, AL, and Phoenix/Tucson, AZ
Participants
10,937 women (mean age 63.3 ± 0.07) with BMD measurements
Measurements
Sarcopenia was defined as appendicular lean mass values correcting for height and fat mass following the Newman et al approach. Low BMD was defined as a femoral neck T-score < −1.0 based on NHANES III reference database for white women. Cox proportional hazards analysis was used to calculate hazard ratios and 95% confidence intervals. We followed women for incident fractures over a median of 15.9 years.
Results
Participants were classified into mutually exclusive groups based on their BMD and sarcopenia status: normal BMD and no sarcopenia (n=3,857, 35%),; sarcopenia alone (n=774, 7%),; low BMD alone (n=4907, 45%),; and low BMD and sarcopenia (n=1399, 13%). Women with low BMD, with or without sarcopenia, respectively, had greater risk of fracture than did women with normal BMD that remained statistically significant with adjustment for important covariates (HR=1.72; 1.44–2.06 and HR= 1.58; 1.37–1.83). Women with low BMD, with and without sarcopenia, respectively, had higher risk of hip fractures, (HR=2.78; 1.78–4.30 and HR=2.42; 1.63–3.59). Women with sarcopenia alone had similar HRs to women with normal BMD.
Conclusion
Women in their sixth decade of life with low BMD irrespective of their sarcopenia status have the highest risk of fracture compared to women with normal BMD.
Keywords: epidemiology, sarcopenia, low bone mineral density/osteoporosis, fracture
Introduction
Osteoporotic fractures result in increased morbidity, mortality, and healthcare cost.1 By the year 2040, the world population ≥ 65 years of age will have doubled from 506 million to 1.3 billion in 2040.2 Fracture events occur disproportionately in older adults, due in part to age-associated declines in bone mineral density (BMD).3–5 Declines in muscle mass have been associated with mobility disability and fractures.6,7 The loss of bone and alterations in the structure of the bone coupled with the increased risk of falling contribute to age related increases in fractures.
Sarcopenia, which can be defined by the loss of muscle mass, in addition to the loss of strength and physical performance, has been associated with fractures.8 Aging is associated with loss in lean mass and strength, which predicts incident fracture independent of BMD9, by influencing functional ability and fall risk.10–13
The associations of the combination of sarcopenia and BMD and fracture risk have not been well examined in women. The condition “sarco-osteopenia” may indicate a higher fracture risk than either of osteoporosis or sarcopenia alone.14 A recent study in women after hip fracture, showed that those with sarcopenia were more likely to have osteoporosis or low BMD at the lumbar spine and femoral neck.15 The prevalence of sarco-osteopenia in this population was high (45%), likely since all women had had a previous hip fracture. Other studies have reported a lower prevalence of this phenotype closer to 10%. Assessing the role of sarco-osteopenia as a risk factor for fracture in women may improve the risk assessment. In men, it has been reported that a combination of low BMD and sarcopenia resulted in almost a 4 fold risk of fracture compared to men without sarcopenia and low bone mass.8 However, women with sarcopenia and low BMD had a similar risk of fracture as women with low BMD alone.8 The addition of sarcopenia did not increase the risk in women. However this study had a much smaller sample of women than men and perhaps less statistical power.8
To further explore the combined effect of sarcopenia and low BMD in women we used data from the Women’s Health Initiative. Improved understanding of the relative contributions of each body compartment on fracture risk is needed to optimize therapeutic strategies to reduce the fracture risk in older adults. In the current report, we test the hypothesis that women with both low BMD and sarcopenia will have a greater risk of fractures than women with sarcopenia or low BMD alone and women without either condition.
Methods
Study Population
The WHI included 161,808 women aged 50–79 years at baseline, free of medical conditions with less than 3 years predicted survival, and post-menopausal.16 Recruitment has been described elsewhere. Briefly, women were enrolled at 40 US clinical centers16 into one or more randomized clinical trials assessing the interventions of low-fat diets, hormone therapy, and calcium and vitamin D supplementation.16 Women who were ineligible or not interested in the clinical trials were enrolled in an Observational Study. BMD was measured in women enrolled in WHI at 3 clinic sites (Pittsburgh, PA; Birmingham, AL and Phoenix/Tucson, AZ) and form the analytic sample for this report. All women with a baseline dual energy x-ray absorptiometry scan (DXA) were examined in this report. Of the women with BMD measurements (N=11,350) we excluded women taking bisphosphonates at baseline, leaving a final analytic sample of 10,937 women. When the WHI ended on March 30, 2005, women were re-consented to participate in an extension study: 77% of surviving women agreed to participate in the first extension (2005–2010).
Sarcopenia and BMD
BMD was measured at the femoral neck and total body. Details of this measurement and densitometry procedures have been previously published.17 Briefly, all trained technicians used QDR 2000, 2000+, or 4500 machines. A standardized procedure for participant positioning and scan analysis were utilized throughout all centers.17,18 The WHI quality assurance program included machine and technician performance monitoring by reviewing phantom scans, random sampling of scans, and review of scans with specific problems. Low bone density was defined as a femoral neck T-score < −1.0 based on NHANES III reference database for white women. This is the current reference database recommended by the International Society for Clinical Densitometry.19 Lean mass was measured using whole body scans (Hologic QDR 2000, 2000+, or 4500, New Bedford, MA). Appendicular lean mass (ALM) was derived from the sum of lean mass in the upper and lower extremities while removing bone mineral content.20
The definition of sarcopenia was based on appendicular lean mass values following the Newman et al. approach.21 To correct ALM for fat mass and height, linear regression was performed to model the association between appendicular lean mass on height (meters) and fat mass (kg). The 20th percentile of the distribution of residuals from this model was used as the cut point for sarcopenia. The Residuals method was chosen because it has been shown to be a stronger predictor of mobility and disability limitations in the Health, Aging and Body Composition and Framingham studies as compared to other definitions of sarcopenia.21
Participants were classified into 4 mutually exclusive groups based on their BMD and sarcopenia status. The groups are: normal BMD and no sarcopenia (3,857 women, 35%), normal BMD and sarcopenia (774 women, 7%), low BMD and no sarcopenia (4907 women, 45%), and low BMD and sarcopenia (1399 women, 13%). In additional analyses, we calculated sarcopenia based on the Baumgartner method (ALM/height2)22, the Health ABC cutoff for sarcopenia (1.73 kg/m2)21 and the osteoporosis cutoff for BMD (T score ≤−2.5).
Other Measurements
Information on covariates was obtained from self-report questionnaires, clinic interviews, or physical measures at baseline.16 The covariates include age, race/ethnicity, clinic site, history of fracture, fall history, smoking status, alcohol consumption, hormone use, corticosteroid use, physical activity, BMI, total vitamin D and calcium intake, and rheumatoid arthritis. Smoking status was categorized as current smoker or not and alcohol consumption was measured as the average number of alcoholic drinks per week. BMI was calculated from height and weight measurements using calibrated scales and stadiometers. Fall history was quantified dichotomously as having a fall or not in the past 12 months. Recreational physical activity was from self-report and measured as metabolic equivalent task (MET) hours per week. METs were derived from literature and validated standardized questionnaires for this population.23 The RAND-36 score was used a measure of physical function, with a score of 90 or less indicating poor physical function (scale from 0–100).24,25 Corticosteroid use was based on self-report. Vitamin D and calcium intake were calculated from self-report on the Food Frequency Questionnaire.
Fractures
Information on incident fractures was obtained prospectively, annually in women enrolled in the observational study and semi-annually in women enrolled in the clinical trials. We included all self-report clinical fractures except for fingers, toes, face, and sternum/ribs, and skull. Pathological fractures were also excluded. Hip fractures were locally and centrally adjudicated26; all other fractures were self-reported. In WHI, although the agreement between self-reported fractures and adjudicated fractures was 76%27, self-report of clinical spine fractures was poor. Therefore we performed a sensitivity analysis excluding clinical spine fractures. We analyzed the risk of all fractures and hip fractures separately. The follow up time period ranged from 1993 to 2009 for a median of 15.9 years.
Statistical Approach
Baseline characteristics were compared across the 4 groups using analysis of variance for continuous variables and chi-square tests for categorical variables. Multiple comparisons were calculated for baseline characteristics of participants.
Incidence rates of total fracture were estimated using a Poisson model for each of the four groups adjusted for age and race.
Using Cox proportional hazards models, the base and multi-variable adjusted hazard ratios and 95% confidence intervals were calculated. Base models were adjusted for age and race. The multi-variable adjusted model was adjusted for established risk factors for fracture: age, race, clinic site, random clinical trial assignment, self-report fall history, fracture history, menopausal hormone therapy use over time, alcohol consumption, smoking status, total vitamin D and calcium intake, and physical activity. Participants with normal bone mineral density and no sarcopenia formed the reference group.
Results
Women with low BMD only and low BMD and sarcopenia tended to be older than the referent group of women (No sarcopenia or low BMD). (Table 1) Women with sarcopenia only, low BMD only, and low BMD and sarcopenia were more likely to be white compared with the referent group as well. Women who had normal BMD and no sarcopenia were less likely to be current smokers than those with sarcopenia and those with low BMD and sarcopenia combined. Physical activity was lowest in the groups with sarcopenia both with and without low BMD. Lumbar spine and femoral neck BMD was lowest in both low BMD groups with no difference by sarcopenia status.
Table 1.
Baseline Characteristics according to Bone Mineral Density and Body Composition; adjusted for multiple comparisons;
| Baseline Characteristics | Normal BMD, No Sarcopenia n=3857 |
Sarcopenia n=774 |
Low BMD n=4907 |
Low BMD, Sarcopenia n=1399 |
P-value |
|---|---|---|---|---|---|
| Mean (SD) or n(%) | Mean (SD) or n(%) | Mean (SD) or n(%) | Mean (SD) or n(%) | ||
| Age | 60.8 (7.0) | 62.0 (7.2) | 65.0 (7.1) | 65.3 (7.0) | a,b,c,d,f |
| Race/White | 2465 (64.1) | 633 (82.0) | 4249 (87.0) | 1271 (91.1) | a,b,c,d,e,f |
| Education | a | ||||
| <high school | 421 (11.3) | 78 (10.4) | 405(8.7) | 125 (9.0) | |
| High School/Vocational School | 2299 (59.6) | 470 (60.7) | 2938 (59.9) | 858 (61.5) | |
| College + | 1124 (29.1) | 224 (28.9) | 1543 (31.4) | 413 (29.5) | |
| Total Appendicular Skeletal Mass Kg | 16.6 (2.9) | 13.0 (1.9) | 14.4 (2.2) | 12.0 (1.6) | a,b,c,d,e,f |
| BMI kg/m2 | 31.0 (6.3) | 29.2 (5.8) | 26.5 (4.9) | 26.2 (4.3) | a,b,c,d,e,f |
| Baseline BMD g/cm2 (femoral neck) | .84 (.09) | .82 (.08) | .64 (.07) | .64 (.07) | a,b,c,d,f |
| Current Smoker | 314 (8.3) | 49(6.5) | 399 (8.3) | 104 (7.5) | b,c,d |
| Alcohol Use (servings/week) | 1.6 (4) | 1.7 (4.1) | 1.9 (4.3) | 1.6 (3.8) | a,e |
| Fall history >1 fall | 914 (29.3) | 185 (27.7) | 1264 (28.7) | 421 (32.5) | c,e,f |
| History of fracture | 1038(30.8) | 191 (28.9) | 1831(42.8) | 543 (44.2) | a,c,d,f |
| Use of hormones | 2070 (53.9) | 513 (66.5) | 2346 (48.0) | 811 (58.1) | a,b,c,d,e,f |
| Physical Activity MET-hours/week | 10.9 (14.1) | 9.0 (11.4) | 12.9 (14.5) | 9.4 (11.9) | a,b,c,d,e |
| RAND-36 Score ≥90 | 2690 (69.7) | 602 (31.8) | 3036 (68.2) | 987 (68.2) | a,b,c,d,e,f |
normal versus low BMD,
normal versus sarcopenia,
normal versus low BMD +sarcopenia,
low BMD versus sarcopenia,
low BMD versus low BMD+sarcopenia,
sarcopenia versus low BMD+sarcopenia.
There were 1,648 women who experienced a fracture (379 (10%) normal, 7.1 fractures per 1000 person-years; 78 (11%) sarcopenia only, 6.6 fractures per 1000 person-years; 903 (19%) low BMD only,11.2 fractures per 1000 person-years; 288 (20%) sarcopenia + low BMD, 12.9 fractures per 1000 person-years). The log rank test is significant for difference in survival curves across all 4 groups with adjustment for multiple comparisons. (Figure 1)
Figure 1. Survival Curve in years by BMD and Body Composition Group.
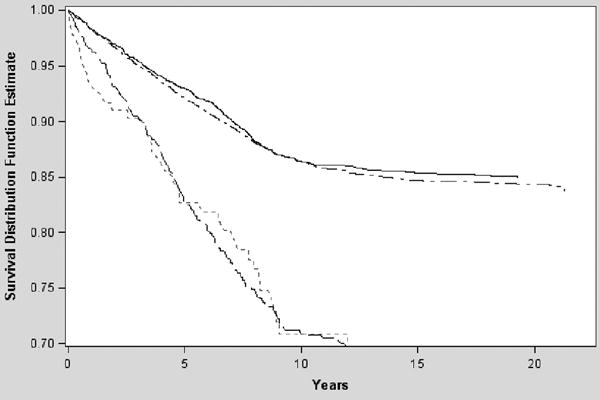
Solid Line = Referent
Small Dash Line = Sarcopenia Only
Dotted Line = Low BMD Only
Large Dash Line = Combination of low BMD & Sarcopenia
Women with low BMD, with or without sarcopenia, respectively, had greater risk of fracture than did women with normal BMD (HR= 1.76, 95% CI= 1.50–2.07) and HR= 1.57, 95% CI=1.38–1.78) that remained statistically significant even with adjustment for important covariates (HR=1.72, 95% CI=1.44–2.06 and HR= 1.58, 95% CI= 1.37–1.83). The risk of hip fractures was the highest for low bone density and sarcopenia (HR= 2.78, 95% CI = 1.78–4.34) and low bone density alone (HR=2.42, 95% CI = 1.63–3.59) groups. Hazard ratios were highest in both low bone density groups for all types of fracture. (Table 2) Women with sarcopenia alone had similar risk of fracture compared to normal women in both models. Additional analyses were performed with various definitions of sarcopenia and low bone density. The results remained consistent with our reported findings and can be found in supplemental tables.
Table 2.
Risk of Fracture by BMD and Body Composition Group
| Hazard Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Any Fracture Models | Hip Fracture Models | |||
| Group | Base* | Multi-variable** | Base* | Multi-variable** |
| Referent | 1.0 | 1.0 | 1.0 | 1.0 |
| Sarcopenia | 0.92 (0.72–1.17) | 0.85 (0.64–1.12) | 0.84 (0.39–1.81) | 0.58 (0.23–1.49) |
| Low BMD | 1.57(1.38–1.78) | 1.59(1.38–1.84) | 2.71 (1.90–3.87) | 2.42(1.63–3.59) |
| Combinationˆ | 1.76 (1.50–2.07) | 1.72 (1.44–2.06) | 3.27 (2.18–4.91) | 2.78 (1.78–4.34) |
Combination group is the low BMD + sarcopenia
Base Model adjusted for age, clinic, and race
Multivariate model adjusted for age, race, study assignment, physical function, history of fracture, history of self-report falls in past year, hormone use, physical activity, alcohol consumption, smoking status, corticosteroid use, BMI, dietary calcium intake, dietary vitamin D intake.
Overall there were 323 hip fracture events (38 normal, 8 sarcopenia, 207 low BMD, 70 low BMD + sarcopenia). Women with both low bone density and sarcopenia had a 20% increased risk of hip fracture compared to women with low BMD alone (HR= 2.78, 95% CI = 1.78–4.34) and (HR=2.42, 95% CI = 1.63–3.59), respectively. The interaction between sarcopenia and low BMD was not statistically significant (p=0.15).
Sensitivity analyses were performed excluding the spine fractures from the outcome assessment of fracture. Results from the sensitivity analysis demonstrate that the hazard ratios remain the highest in the women in the low bone mineral density groups. The results also continued to demonstrate that there is similar risk in women with sarcopenia alone and women without either condition. (Supplemental table 2)
Discussion
Women with low BMD irrespective of their sarcopenia status had a higher risk of fracture compared to women with normal BMD and normal appendicular lean mass. Our results confirm that low BMD remains as a strong risk factor for fracture in women and show that the presence of sarcopenia does not increase this risk further. Our results are similar to previous work which showed that the association between sarcopenia and low BMD was similar to the association between low BMD alone in women. However, Chalhoub et al. found that men with both low BMD and sarcopenia had a 4 fold increase in the risk of fracture compared to men without either.8 This difference may suggest that there is a sex difference in the role of sarcopenia and fracture risk. A potential explanation could be that the decline of muscle strength and lean mass in women is not as swift as it is in men.13 The decline in testosterone levels impact men greater than women throughout aging and testosterone levels have been associated with a reduction in lean mass.28 There is also potential that the women in this cohort have not experienced a significant age related decline in lean mass yet. The change in overall lean mass from baseline to year 3 visit is a net increase of 0.12 kg. Adjusting for multiple comparisons, a difference in lean mass change between women in the referent group and those with sarcopenia and low BMD (2.54 kg) and between women in the low BMD and those with sarcopenia and low BMD (1.81 kg) was found. These small differences in lean mass changes are not influencing the risk of fracture.
It has been proposed that the presence of sarcopenia may increase the risk of fracture that is generally associated with aging and improve fracture risk assessment.14 Limiting fractures to those of the hip, we found that women with the combination of low BMD and sarcopenia had a higher risk of fracture as compared to other groups. Sarcopenia alone however, was not found to be an independent risk factor for fracture in women. In previous work by Cawthon et al., the consensus definitions of sarcopenia did not improve the prediction of clinical outcomes, such as fracture.29 The addition of sarcopenia to low BMD though may suggest an increased risk of hip fracture in women. This combination may imply that there is communication between muscle and bone at this site, which is commonly associated with frailty.30 This interaction may be influenced by mechanical stimuli, genetic factors, hormonal influences and body composition. Total bone mineral content has been shown to be associated more with lean tissue mass than fat tissue mass while regional BMD has been more closely associated to loss of both fat and lean tissue mass.31 Additionally, weight reduction, which can be attributed to both fat and lean tissue mass, can lead to accelerated rates of bone loss in post-menopausal women.31
In supplementary analyses we explored differing cut-points for lean mass and bone mineral density. We opted to define low bone density with a T-score of < −1 to optimize our sample sizes across the 4 groups. Using a more stringent cut point for bone density, T-score <−2.5, which is the cut off for osteoporosis, our results for all fractures yielded similar findings. Likewise, in comparison of definitions for sarcopenia, similar results were found in our analyses for all fractures. In comparison of women currently using estrogen therapy and those who are not, the risk of fracture remained similar across all groups.
Strengths of this analysis include use of a well-established cohort with validated measures of body composition and extended 15 year follow up We adjusted for important covariates. The assessment of fracture risk based on bone and body composition in women is not completely understood. However, there are several limitations. One key limitation is that the definition of sarcopenia remains controversial. For example, the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project proposes to define sarcopenia based not only on appendicular lean mass but also muscle strength. However in WHI, we were unable to use this definition because we only had a small sample with measures of grip strength. However, the Residuals definition for sarcopenia has been proven to be a better indicator of low lean mass and to be predictive of disability and mortality, particularly among women.21,32,33 Finally, the WHI cohort of women was relatively young and healthy, with those with sarcopenia accounted for only 5% of this population. Thus we had limited power to assess the association between sarcopenia alone and fracture risk. This current study adds to the existing literature examining this association in women and has found consistent results.8 The risk of fracture is highest in women with low BMD. The work previously completed by Chalhoub et al that examined this association had a small sample of women with limited power whereas we were able to examine over 10,000 women with an extended period of follow-up in this analysis.
In conclusion, women with low BMD, both with and without sarcopenia had the highest risk of fracture as compared to women with only sarcopenia and those considered normal. Results suggest that sarcopenia does not add additional risk for fracture in women.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Current research support for Rebekah Harris is NIA Aging Training Grant (PI: AB Newman) T32-AG-000181.
Footnotes
Disclosure statement: none
Conflict of Interest: The authors have no conflicts to report.
Author Contributions: Harris, Cauley: study concept, analysis and interpretation of data, preparation of manuscript. Chang: analysis and interpretation of data. Beavers, Laddu-Patel, Bea, Johnson, LeBoff, Womack, Wallace, Li: preparation of manuscript. Crandall: study concept and preparation of manuscript.
Sponsor’s Role: none
Supplemental Tables (S1 and S2) Comparison of fracture risk by varying definitions of sarcopenia and low BMD.
References
- 1.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003 Mar;51(3):364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 2.He W, K K. An Aging World: 2008. 2009 (Issued June 2009). Accessed 01/25/2016. [Google Scholar]
- 3.Ensrud KE. Epidemiology of fracture risk with advancing age. (Series A, Biological sciences and medical sciences).The journals of gerontology. 2013 Oct;68(10):1236–1242. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein LZ, Josephson KR. Falls and their prevention in elderly people: what does the evidence show? Med Clin North Am. 2006 Sep;90(5):807–824. doi: 10.1016/j.mcna.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Medicine Io. Falls in Older Persons: Risk Factors and Prevention. Washington, DC: The National Academies Press; 1992. The Second Fifty Years: Promoting Health and Preventing Disability. [PubMed] [Google Scholar]
- 6.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. March 1, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012 Oct;31(5):652–658. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Chalhoub D, Cawthon PM, Ensrud KE, et al. Risk of Nonspine Fractures in Older Adults with Sarcopenia, Low Bone Mass, or Both. Journal of the American Geriatrics Society. 2015;63(9):1733–1740. doi: 10.1111/jgs.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003 Nov;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 10.Woo N, Kim SH. Sarcopenia influences fall-related injuries in community-dwelling older adults. Geriatr Nurs. 2014 Jul-Aug;35(4):279–282. doi: 10.1016/j.gerinurse.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and Injurious Falls in the Year Following Hip Fracture: A Prospective Study of Incidence and Risk Factors From the Sarcopenia and Hip Fracture Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(5):599–609. doi: 10.1093/gerona/glp003. May 1, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003 Mar;51(3):323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 14.Buehring B, Krueger D, Binkley N. Effect of including historical height and radius BMD measurement on sarco-osteoporosis prevalence. Journal of Cachexia, Sarcopenia and Muscle. 2013;4(1):47–54. doi: 10.1007/s13539-012-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 52(1):71–74. doi: 10.1016/j.archger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000 Jun;27(6):1513–1517. [PubMed] [Google Scholar]
- 17.Jackson RD, et al. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(19 Supplemental):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi ML, BS, Bishop NJ, et al. Official Positions of the International Society for Clinical Denistometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol. 2010;25:37–47. doi: 10.1007/s00467-009-1249-z. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr Health Aging. 2007;137(12):2775–2780. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003 Nov;51(11):1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 23.Hsia J, Wu L, Allen C, et al. Physical activity and diabetes risk in postmenopausal women. Am J Prev Med. 2005 Jan;28(1):19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 25.Cauley JA, LaCroix AZ, Wu L, et al. Serum 25-Hydroxyvitamin D Concentrations and Risk for Hip Fractures. Ann Intern Med. 2008;149(4):242–250. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins J, Aragaki AK, Kooperberg C, et al. FActors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298(20):2389–2398. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc ES, Wang PY, Lee CG, et al. Higher Testosterone Levels Are Associated with Less Loss of Lean Body Mass in Older Men. The Journal of Clinical Endocrinology & Metabolism. 2011;96(12):3855–3863. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthon PM, Blackwell TL, Cauley J, et al. Evaluation of the Usefulness of Consensus Definitions of Sarcopenia in Older Men: Results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc. 2015;63(11):2247–2259. doi: 10.1111/jgs.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013 Sep;28(9):1857–1865. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? Jan. J Bone Miner Res. 1997;12(1):144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 32.Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia Definitions Considering Body Size and Fat Mass Are Associated With Mobility Limitations: The Framingham Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(2):168–174. doi: 10.1093/gerona/gls109. 04/13 12/09/received 03/07/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. Journal of the American Geriatrics Society. 2007 May;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


