Abstract
Background
Direct brain stimulation via electrodes implanted for intracranial electroencephalography (iEEG) permits the modulation of endogenous electrical signals with significantly greater spatial and temporal specificity than non-invasive approaches. It also allows, for the stimulation of deep brain structures important to memory, such as the hippocampus, that, are difficult, if not impossible, to target non-invasively. Direct stimulation studies of these deep, memory structures, though, have produced mixed results, with some reporting improvement, some impairment, and others, no consistent changes.
Objective/Hypothesis
We hypothesize that to modulate cognitive function using brain stimulation, it is essential to modulate connected nodes comprising a network, rather than just alter local activity.
Methods
iEEG data collected while patients performed a spatiotemporal memory retrieval task were used to map frequency-specific, coherent oscillatory activity between different brain regions associated with successful memory retrieval. We used these to identify two target nodes that exhibited selectively stronger coupling for spatial vs. temporal retrieval. In a subsequent session, electrical stimulation - theta-bursts with a fixed phase-lag (0° or 180°) – was applied between the two target regions while patients performed spatiotemporal retrieval.
Results
Stimulation selectively impaired spatial retrieval while not affecting temporal retrieval, and this selective impairment was associated with theta decoupling of the spatial retrieval network.
Conclusion
These findings suggest that stimulating tightly connected nodes in a functional network at the appropriate phase-lag may effectively modulate the network function, and while in this case it impaired memory processes, it sets a foundation for further network-based perturbation studies.
Keywords: direct brain stimulation, memory retrieval, intracranial EEG, ECoG, theta burst
Introduction
The medial temporal lobes (MTL) are important structures for episodic memory processes [1] as evidenced by lesions that impair memory for recently acquired information and events [2–6]. In addition, transient, direct electrical stimulation of the MTL (hippocampus, entorhinal cortex), disrupts encoding and retrieval of recently learned word pairs and episodic information [7–9]. Together, these studies highlight the central importance of the MTL, and in particular, the hippocampus and entorhinal cortex, to episodic memory.
Numerous studies, have also shown both critical and necessary roles for structures outside of the MTL to memory. These structures together are termed the “core recollection network” [10]. Specifically, the posterior parietal cortex, parts of prefrontal cortex, retrosplenial/posterior cingulate cortex, and interactions between these areas are critical for the formation and retrieval of episodic memories [11–26]. Consistent with these ideas, we have previously demonstrated that during correct retrieval of spatial and temporal details, phase coherence in low-frequency oscillations (1–10 Hz) increases across numerous neocortical loci (prefrontal and parietal cortices) [26]. Collectively, these suggest that brain regions that show strong functional connectivity with other brain regions should be important to mediating different forms of memory retrieval.
Here, we aimed to target an individual patient’s memory network “hubs” by identifying brain areas that showed the highest degree of phase coherence with other regions during retrieval [27]. In other words, stimulation targets were defined purely based on the functional connectivity associated with memory retrieval, and we find this approach particularly suitable for direct brain stimulation using iEEG electrodes for a few pragmatic reasons. Electrodes are placed solely to meet the patients’ clinical needs, resulting in some variability in the exact anatomical locations of the electrodes across patients. Furthermore, potential network reorganization associated with the epilepsy pathology [28–34] may increase the anatomical individual variability (also see Discussion), which makes anatomical targeting less meaningful or reliable.
At a theoretical level, a focus on individually defined functional nodes, rather than targeting a single anatomically defined region across patients allows us to test an additional important prediction: stimulation of an individual patient’s hubs should modulate retrieval regardless of whether or not the MTL is one of these hubs. Thus, our network perspective emphasizes the contributions of specific brain hubs rather than a static set of brain regions across all subjects. In addition, Watrous et al. [26] have shown that interactions at specific frequencies might mediate retrieval of specific contextual details; correct spatial retrieval involved coherence at lower frequencies (1–4 Hz) compared to correct temporal order retrieval (7–10 Hz). This work, however, was correlational: The electrophysiological data acquired during retrieval could not reveal whether phase coherence is necessary for spatial vs. temporal order retrieval. In the present study, we took a causal approach by directly stimulating two network hubs based on their selective phase coupling and frequency during spatial retrieval. Thus, the present study sought to both establish the necessity of highly connected brain hubs to memory retrieval and at the same time, test areas outside of the MTL to determine their causal role in specific aspects of memory processing.
To test these ideas, our experimental approach involved two sessions. We first computed inter-regional theta coherence connectivity associated with successful retrieval using intracranial encephalography (iEEG) recordings (methods identical to [26]). Based on the functional connectivity defined using this coherence measure, we identified the two hubs in the resulting network that were selective to spatial compared to temporal retrieval. We then stimulated these two hubs at their preferred phase lag in the second session. We had two predictions for the expected outcomes. First, we predicted stimulating these hubs in concert should alter network connectivity and memory performance regardless of whether the targeted hubs involved the MTL, as the stimulation to these two areas could alter information flow in the network. Second, we predicted that stimulation of specific hubs would be process-specific – i.e., stimulation of spatial network nodes would modulate spatial but not temporal order retrieval. We were agnostic1 about whether stimulation would disrupt or enhance memory but did expect it to alter network connectivity of specific mnemonic processes.
Material and methods
Participants
Four patients undergoing pre-surgical evaluation for medically intractable epilepsy participated in this study. All data were collected at Memorial Hermann Hospital, and all experimental procedures were approved by the Institutional Review Board of the University of Texas Health Science Center at Houston and fully described to the patients before they consented to participate in the study.
General Procedure
iEEG signals were recorded from depth electrodes implanted for a stereo-encephalographic (SEEG) evaluation for pharmaco-resistant epilepsy while patients performed a spatiotemporal memory retrieval task (details on the SEEG implantation and electrode localization are provided in the Supplementary Material). The spatiotemporal memory retrieval paradigm has been used in our previous studies [23,26,38,39] (details also provided in Supplementary Material: Spatiotemporal Memory Retrieval Paradigm) and is comprised of two different parts: delivery (Figure 1a, 1b) followed by retrieval (Figure 1c). During the delivery phase, participants were asked to navigate to 5 stores in a virtual city and remember the spatial locations of the stores as well as the temporal order in which they visited the stores. In the retrieval phase, they were asked to report either the spatial relationship between these target locations or the order in which they visited them. Patients performed this task in both the pre-stimulation recording (Session I) and direct electrical stimulation (Session II) sessions. The network obtained from the analysis of Session I recording determined the loci for stimulation in Session II.
Figure 1. Spatiotemporal memory retrieval paradigm and stimulation protocol.

a) Delivery phase of the task. Patients navigated through a virtual city to find the passenger and then the store to deliver them to (as instructed on the screen). b) A reporting period after patients finished delivering passengers to all five stores. Patients are asked to indicate where each store was located (spatial retrieval, top) and the sequence in which they visited the stores (temporal retrieval, bottom). c) Retrieval phase of the task. Patients reported which store at the bottom was closer to the one on top - in terms of distance (spatial retrieval) or delivery order (temporal retrieval). d) Stimulation protocol. Theta-burst stimulation (TBS) was applied to two pre-selected target nodes for 2 seconds preceding the retrieval cue onset in half of the spatial and temporal trials.
iEEG Recording and Data Processing
Session I iEEG data were recorded using the Neuroport NSP recording system (Blackrock Neuromed, Utah), referenced to the signal from a single channel in white matter. Session II data were recorded using the Neurofax EEG-1200 recording system (Nihon-Kohden), referenced to an average of two neighboring channels in white matter as pre-determined by the clinicians (one of these was always the on-line reference channel in Session I). Electrodes with excessive line noise or abnormal electrophysiology were excluded from further analysis. Data in both sessions were collected at 2000 Hz, down-sampled to 1000 Hz and then re-referenced to a common average of signals from electrodes that met the following criteria: absence of excessive line noise, time-locked ERP, or interictal spikes.
Network Construction and Target Selection from Session I Data
The total number of electrodes to be included in the network construction was restricted to 20–45 electrodes to reduce computation time for the network construction. We included electrodes located in regions previously implicated in memory processes while avoiding selecting adjacent electrodes from the same probe in an effort to reduce overrepresentation of electrodes by possible volume conduction.2 Table 1 summarizes the anatomical locations of electrodes included in the analyses for each patient.
Table 1. Electrodes included in the analyses for each individual patient.
Abbreviations. SFG = superior frontal gyrus, SFS = superior frontal sulcus, MFG = middle frontal gyrus, MFS = middle frontal sulcus, IFG = inferior frontal gyrus, IFS = inferior frontal sulcus, antLF = anterior lateral fissure, SPG = superior parietal gyrus, IPG = inferior parietal gyrus, SIntermPrim = suclus intermedius primus, IPS = intraparietal sulcus, SUBP = subparietal sulcus, PO = parieto-occipital sulcus, PREC = precuneus, STG = superior temporal gyrus, STS = superior temporal sulcus, MTG = middle temporal gyrus, MTS = middle temporal sulcus, ITG = inferior temporal gyrus, ITS = inferior temporal sulcus, TP = temporal pole, TCS = transverse collateral sulcus, PHG = parahippocampal gyus, HPC = hippocampus.
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | ||
|---|---|---|---|---|---|
| Frontal | SFG/SFS | 2 | 4 | 2 | 1 |
| MFG/MFS | 4 | 4 | 9 | 2 | |
| IFG/IFS | 2 | 3 | 1 | 3 | |
| antLF | 0 | 1 | 0 | 0 | |
| Parietal | SPG | 0 | 2 | 4 | 0 |
| IPG | 0 | 4 | 3 | 1 | |
| SIntermPrim | 0 | 1 | 0 | 0 | |
| IPS | 2 | 2 | 4 | 1 | |
| SUBP | 0 | 2 | 3 | 1 | |
| PO | 0 | 0 | 5 | 0 | |
| PREC | 0 | 3 | 1 | 1 | |
| Temporal | STG/STS | 7 | 1 | 0 | 4 |
| MTG/MTS | 0 | 0 | 0 | 1 | |
| ITG/ITS | 0 | 0 | 2 | 2 | |
| TP/TCS | 0 | 0 | 4 | 2 | |
| Mediotemporal | PHG | 0 | 0 | 1 | 1 |
| HPC | 3 | 0 | 2 | 2 | |
| TOTALS | 20 | 27 | 41 | 22 |
Trial epochs were extracted for all trials from 1 s before to 2.2 s following the retrieval cue onset. Phase estimates were obtained using a fast-Fourier transform at 37 equally spaced timepoints using the EEGLab “newcrossf” function from −745 to 1943 ms relative to cue store onset and at 10 frequencies from 1 to 10 Hz. As a measure of inter-regional phase synchronization at the single trial level, we adapted pairwise phase consistency (PPC [40]), which minimizes the coherence estimate bias arising from differences in trial numbers between conditions [26,40]. This single-trial PPC (stPPC) measure is similar to traditional PPC but adapted for single trials analysis and is conceptually similar to other previously used metrics of phase coherence (e.g. [41]). Computational details of stPPC are provided in Supplementary Material: Single-trial PPC.
To construct the network associated with a given retrieval condition, we considered an “edge” (i.e., connection) to exist between electrodes if there was a significant stPPC value at a given time and frequency. Significance was assessed by creating a surrogate distribution of stPPC values that were calculated by shuffling trial labels (n = 500) within time bin, frequency, and condition (i.e. space, or time, only correct trials). stPPC values that fell in the top 5% of the distribution were labeled as significant. This process resulted in a network for every dimension (time bin, frequency, condition, and trial). To obtain a subject’s retrieval network, we summed edge values across time, frequency, and trials, which then provided a weight for every edge. Conceptually, a larger edge weight indicates that a significant functional connection between two regions occurs more frequently across a range of time and frequencies. Because our stimulation protocol implemented 4 Hz theta burst stimulation, we only utilized the summed network from 3 to 5 Hz for the entire 1943 ms post-cue retrieval time window for all trials within a condition. The edges were thresholded to isolate the top 20th percentile, and the node degree was defined as the sum of edge weights of all connections that the node has.
We determined stimulation targets as the two electrodes that 1) exhibited the highest difference in node degree between spatial and temporal selective networks and that 2) were in distinct anatomical regions. In cases where test stimulation triggered overt sensory or other behavioral changes in the patient or the neurosurgeon (NT) had concerns about stimulating the predetermined target region, the next best electrode that met the same criteria was chosen as an alternative. Figure 2 shows an example of target selection (Patient 4).
Figure 2. Stimulation target selection based on the spatial and temporal retrieval networks: Patient 4.
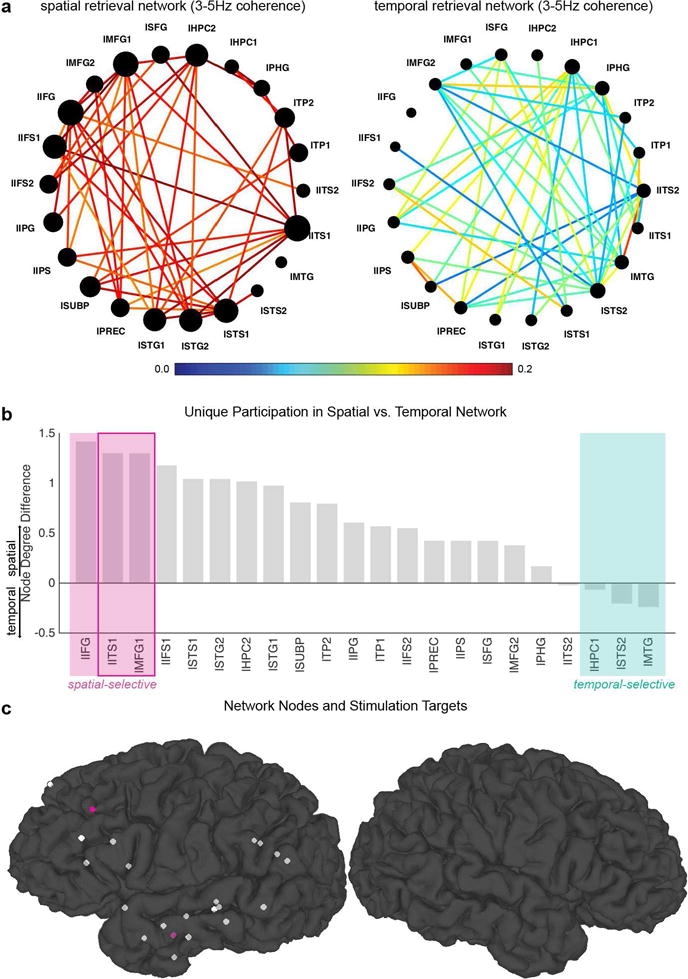
a) Theta (3–5Hz) phase coherence (connectivity) networks selective during spatial (left) and temporal (right) retrieval. The edge color represents the edge weight (the color bar), and the node size represents each node’s connectivity with the rest of the network (node degree). b) Node degree differences (spatial – temporal) sorted in a descending order. This difference values can be interpreted as an index of the node’s unique participation in the spatial vs. temporal retrieval networks. Nodes with positive values (on the left end) are more selective to ‘spatial’ retrieval whereas nodes with negative values (on the right end) are more selective to ‘temporal’ retrieval. As examples, three extreme cases are marked with pink (spatial-selective) and green (temporal-selective) shades. Stimulation targets are selected from the nodes that are most selective to spatial retrieval (pink outlined box, lITS1 and lMFG1). c) Nodes of the network and stimulation targets. Each electrode included in the network construction is depicted as a small sphere. The two nodes selected as stimulation targets (lITS1, lMFG1) are shown in pink. See Supplementary Material for other patients (Supplementary Figures 1–3).
Stimulation Protocol
A Grass Technologies S88X dual output square pulse stimulator (Natus Medical Inc.) was used for cortical stimulation. The dual output ports on this stimulator were connected to selected intracranial electrodes via independent bi-phasic stimulus isolation units (Astro-Med, Inc.). Stimulation was delivered in a form of theta-burst to the two target nodes, with either 0 or 180 ° angle phase lag between the nodes. The phase lag to be used was determined by the average phase lag in the theta oscillations between the two node channels during successful spatial retrieval based on Session I data. 180° phase lag was used for Patient 1, and 0° for Patients 2–4 (this affected the datasets available for some analyses; see Supplementary Material: Trial boundaries in Session I and Session II for more details). For each target node, a neighboring channel on the same probe that resides in the same anatomical region was used for bipolar current injection (inter-electrode distance between neighboring channels: 3 – 3.75 mm).
Theta-burst stimulation (TBS) was applied for 2 s during the inter-trial-interval (ITI) and terminated at the onset of the retrieval cue (Figure 1d). Theta-burst was designed as trains of high frequency pulses repeating four times in a second, spaced to start every 250 ms (i.e., 4Hz). Specifically, each train comprised of three short pulses (balanced square-wave pulses with a width of 500 μs) delivered at 50Hz, therefore was 60ms long. The stimulation current was determined as the maximum that did not elicit epileptiform after-discharges or any changes that patients could feel or report (4–5Hz, see table 2 for the current intensity used for individual patients). Twenty out of the 40 trials in each retrieval type (spatial, temporal) were randomly assigned (double-blind) to the stimulation condition, and the rest of the trials were non-stimulation trials. A neurologist or neurosurgeon specializing in the management of epilepsy monitored the stimulation session.
Table 2.
Patient demographics, clinical characteristics, behavioral performance, and stimulation parameters. HPC = hippocampus, SFG = superior frontal gyrus
| demographics | clinical characteristics | stimulation parameter | behavioral performance | |||||
|---|---|---|---|---|---|---|---|---|
| patient | gender | age | seizure onset zone | previous resection | current amplitude | Session I accuracy | Session II accuracy | accuracy % change |
| 1 | F | 21 | HPC | none | 4 mA | 91.3 | 81.3 | −10 |
| 2 | M | 18 | cingulate cortex, SFG | none | 4 mA | 71.7 | 72.5 | 0.8 |
| 3 | F | 19 | orbital lobe, insula | none | 4 mA | 68.8 | 61.3 | −7.5 |
| 4 | M | 33 | HPC | none | 5 mA | 85.0 | 61.3 | −23.7 |
Statistical Analysis of Behavioral Data
Statistical analysis of behavioral data was conducted using R statistics toolbox (v3.1.3, https://www.r-project.org/). A mixed-model logistic regression model was used to test the effects of our categorical variables (session, retrieval type, and/or stimulation on/off) and relevant interaction terms on accuracy (a binary measure for each trial), with patient ID included as a random intercept.
Comparisons of Theta Coherence in Sessions I and Session II
As the main behavioral effects were observed in spatial retrieval across sessions, we further examined whether there were associated changes in theta coherence in spatial trials. We evaluated theta-coherence changes induced by stimulation to answer three critical questions: 1) Does TBS modulate coupling/decoupling of the target nodes in the network? 2) Does TBS induce global theta coupling or decoupling across the broader memory network? 3) How prolonged are the effects of TBS - i.e., do they carry over from stimulation “on” to stimulation ‘off’ trials?
To answer these questions, we computed stPPC values separately for Sessions I and II and obtained the network representing the theta coherence changes across the sessions (see Supplementary Material: Spectral Decomposition for Theta Coherence Comparisons for details on the spectral decomposition). Welch’s t’s were obtained as a standardized measure of the stPPC difference between sessions (independent two-samples t-tests). These t-values were then converted to z-scores using a permutation t-distribution generated by shuffling the session labels (number of permutations = 500). This yielded a set of connectivity z-scores that correspond to each frequency and time point. Coherence changes were considered significant when absolute z-scores were greater than 1.96 (p = .05, two-tailed). Significant coherence changes with positive z-scores were counted into the theta ‘coupling’ connections and significant coherence changes with negative z-scores into the ‘decoupling’ connections. Network metrics (edge weight, node degree, and threshold) were defined using the same approach as before (see Material and methods: Network Construction and Target Selection from Session I Data).
Results
Overall retrieval accuracy was well above chance level (50%) in Session I (M = 79.2, SD = 10.72, range = 68.8–91.3, Table 2) as well as in Session II (M = 69.1, SD = 9.70, range = 61.3–81.3).
The mixed-model logistic regression revealed a significant interaction of experiment session (pre-stimulation vs. stimulation) and retrieval type (spatial vs. temporal; logit coefficient (SE) = 1.14 (0.46), 95% CI = [0.25 2.14]; OR = 3.13 (95% CI = [1.28 7.67]); p = 0.01). This suggested that direct stimulation of hubs determined based on the theta phase coherence during Session I impaired retrieval more for one type of information over the other (i.e., spatial > temporal), concordant with the fact that we were stimulating the nodes localized for the functionally determined “spatial” network. The regression model further revealed that the likelihood of correct retrieval was comparable for spatial and temporal information during the pre-stimulation session (Session I, p = 0.61), but was lower in stimulation than pre-stimulation session for ‘spatial’ information (logit coefficient (SE) = −0.91 (0.30), 95% CI = [−1.50, −0.32], OR = 0.40 (95% CI = [0.22 0.73]), p = 0.003).
During Session II, there were trials in which stimulation was applied (stimulation ‘on’) and trials in which stimulation was not applied (stimulation ‘off’). We had designed our experiment to assess whether TBS enhanced or disrupted retrieval on specific trials consistent with some past work [7,37], and this design also allowed assessing if there was a residual or persistent effect of stimulation. We conducted an additional logistic regression model to test whether there was spatial-selective retrieval impairment specific to stimulation ‘on’ trials. This model revealed no significant interaction of retrieval type and stimulation condition (p > 0.1). This result, coupled with findings of condition × session interaction, suggested that the effects of stimulation were not specific to trials on which stimulation was applied, but rather persisted, or “spilled over” across all trials during the stimulation session.
Next, theta phase coherence changes induced by stimulation were examined – first from the ‘target node’ perspective and then from the ‘whole network’ perspective. Figure 4 illustrates the temporal evolution of the target nodes’ coupling and decoupling strength during stimulation ‘on’ and ‘off’ trials. In all stimulation target nodes, a very prominent but brief increases in theta coupling was noted in stimulation ‘on’ trials (red solid lines) – starting at the end of the stimulation and quickly dropping off around 400 ms. Mirroring this drop-off in theta coupling, theta decoupling increased over this time window (i.e., ~ 400ms; blue solid lines). After 500 ms, there was no clear or consistent relationship between the coupling and decoupling strength during the stimulation ‘on’ trials (i.e., no clear pattern distinguishing the solid red and blue lines). During stimulation ‘off’ trials (dashed lines), the target nodes exhibited stronger decoupling than coupling for the most time during the trial in two patients (Patients 3 & 4; blue dashed lines running above red dashed lines).
Figure 4. Stimulation-induced theta phase connectivity changes in the stimulation target nodes.
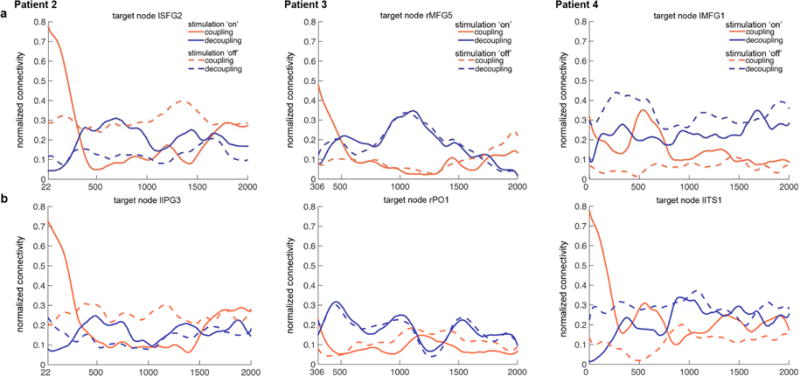
The coupling (red lines) and decoupling (blue lines) strength change over time is shown separately for stimulation ‘on’ (solid lines) and ‘off’ (dashed lines). Upper panel (a) and bottom panel (b) correspond to the two stimulation targets in each patient.
Then, the modulation of the network’s ‘global’ connectivity was assessed. Figure 5a illustrates temporal evolution of the coupling (red) and decoupling (blue) density over the whole spatial retrieval network. During stimulation ‘on’ trials (solid lines), the spatial retrieval network exhibited very strong theta coupling immediately following the stimulation, but this coupling weakened with a steep slope within the first 500 ms of trials (red). On the other hand, more and more node pairs exhibited significant theta-phase coherence decreases compared to Session I (decoupling) at the beginning of stimulation trials, as reflected by the ‘rise’ in blue solid lines within the first 500 ms following trial onsets. This decoupling became greater than coupling and remained sustained. In stimulation ‘off’ trials (dashed lines), the network density for coupling and decoupling remained at levels similar to the density at the end of stimulation ‘on’ trials (solid lines). To summarize, spatial retrieval networks were strongly connected in theta phase coherence immediately after stimulation, but quickly became decoupled. Importantly, this state (i.e., dropped coupling and increased decoupling) remained during stimulation ‘off’ trials, which suggested stimulation-related disruption of memory effects tended to “persist” through the network even when no stimulation was explicitly applied.
Figure 5. Global density of spatial retrieval network.
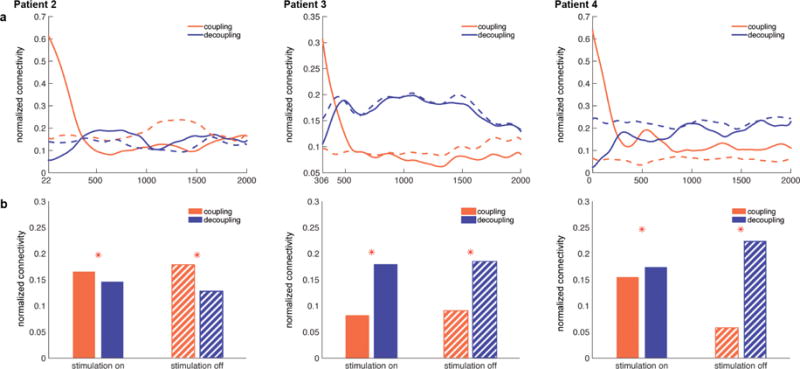
a) The theta coupling (red) and decoupling (blue) strength change over time in the spatial retrieval network during stimulation ‘on’ (solid lines) and ‘off’ (dashed lines) trials. During stimulation ‘on’ trials (solid lines), spatial retrieval network initially exhibits strong theta coupling that rapidly dissipates (red). At the same time, theta decoupling (blue) gradually increases remains sustained, remaining greater than theta coupling strength (red). During stimulation ‘off’ trials (dashed lines), the network exhibits coupling and decoupling strength similar to the levels during the later phase of stimulation ‘on’ trials. b) Total network density for the coupling and decoupling networks. Network density was greater for theta decoupling than coupling during both stimulation ‘on’ (solid fill) and ‘off’ (striped fill) trials in patients 3 & 4. * < .001
Figure 5b illustrates total coupling and decoupling density of the spatial memory retrieval network as a whole. In stimulation ‘on’ trials (solid fill), the network exhibited greater theta decoupling than coupling in 2 patients (b & c, Patients 3 & 4, ‘stimulation on’). Importantly, in these two patients that exhibited greater decoupling than coupling of spatial retrieval network during stimulation ‘on’ trials, network decoupling was greater than coupling during stimulation ‘off’ trials as well (striped fill, ‘stimulation off’). In other words, in these two patients, stimulation reduced the theta phase coherence between the nodes of spatial memory retrieval network, and this effect was carried over to stimulation ‘off’ trials (a persistence effect). Importantly, these two patients showed the largest behavioral impairment effects by stimulation (Figure 3).
Figure 3. Stimulation-related accuracy changes in spatial and temporal retrieval.
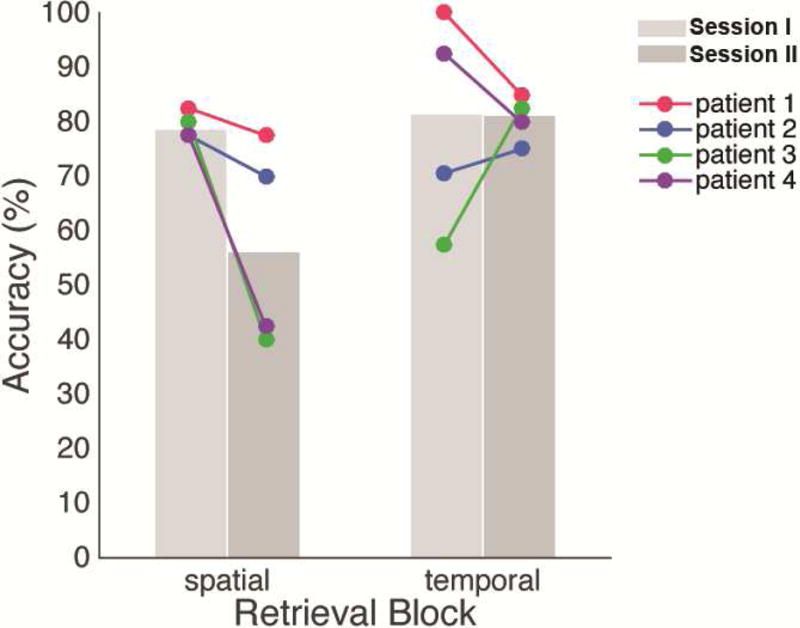
Spatial retrieval accuracy was lower in Session I (light gray) compared to Session II (dark gray). This pattern was consistent in all patients. Mean temporal retrieval accuracy was comparable across the two sessions and although the pattern was not consistent across patients.
One possible concern with our analysis, which is focused on the modulation of PPC between interacting nodes, was that it would not capture local activity changes induced by stimulation, if any. In other words, it might be that our stimulation changed patterns locally rather than, or in addition to, how connected hubs interacted. To address this issue, we analyzed theta power modulation at individual electrodes (Supplementary Material: Theta Power Modulation Analysis). This revealed no consistent effects across regions, subjects, or hubs (Supplementary Figure 7, Supplementary Material: Theta Power Modulation Results). In addition, a post-hoc analysis revealed that our experimental paradigm did not involve a full counterbalance. To address this issue, we ran a behavioral pilot in which we tested spatial and temporal blocks in a completely counterbalanced fashion. We found no effect of testing order on our behavioral results (Supplementary Material: Behavioral Pilot on Task-order Effects), suggesting that any order effects were unlikely to contribute to our pattern of results.
Discussion
In the present study, we used direct brain stimulation via iEEG electrodes to investigate the necessity and selectivity of theta phase coherence in memory retrieval. We first identified hubs of a functional network based on the phase coherence during retrieval from a pre-stimulation recording session. TBS to the two nodes that exhibited strong theta coherence with other nodes in the network most selectively during ‘spatial’ compared to ‘temporal’ retrieval impaired spatial retrieval while not affecting temporal retrieval. This behavioral impairment was accompanied by theta decoupling of the spatial retrieval network that persisted over the duration of stimulation trials. Therefore, our findings additionally provide causal evidence for distinct spatial and temporal networks. The frequency-specificity of network connectivity during spatiotemporal memory retrieval shown in our previous work [26] could not tell us if such phase coherence was necessary for spatial vs. temporal order retrieval. Via stimulating the network regions that exhibit distinct connectivity during spatial as opposed to temporal retrieval, we selectively impaired spatial rather than temporal retrieval accuracy by perturbing theta coupling of the network.
Although the MTL serves an essential role in the formation and retrieval of episodic memories [1], empirical evidence suggests that memory processing may be better conceived of as a network phenomenon where a system of coordinated regions contributes to cognition rather than an single region working in relative isolation [11–26]. In the present study, we tested this idea by stimulating regions of the memory retrieval network that lie outside of the MTL. By targeting regions belonging to a spatial retrieval network – but outside MTL –, we were able to selectively modulate memory performance while patients completed a spatiotemporal contextual retrieval task.
Of note, these stimulation targets were determined based on the low-frequency oscillatory coherence patterns the regions exhibit during particular type of memory retrieval. It is thought that such coherent oscillation mediates the long-range communication between brain regions that are many synapses away [42–44]. Therefore, while it is certainly possible that the selected target regions were also anatomically well-connected, our approach does not assume such connection must exist for the stimulation to those targets to induce changes in the network.
Although we were agnostic as to the direction of the stimulation effects (improvement vs. impairment), literature implicating theta oscillations in human episodic memory processes [11,12,26,45–53] provides supports for ‘enhancement’ prediction. In addition, previous studies showed that stimulation targeting the ‘network’ is likely to improve memory function. Specifically, memory was improved by invasive stimulation to the ‘connecting’ pathways [35–37, 54] and noninvasive stimulation to the hippocampal network [55, 56]. Nevertheless, there was a possibility that our approach would disrupt network process and consequently impair memory retrieval. For example, phase entrainment induced by stimulation may perturb the endogenous theta rhythm and result in disruption of the ongoing process. Indeed we found that theta-burst stimulation reduced the theta-phase coupling within the network, and this theta decoupling was most pronounced in the two patients that exhibited largest behavioral impairments by stimulation. This suggests that it may be very difficult to purely augment the ongoing, endogenous oscillations without perturbing them. Various factors can result in the perturbation, and we speculate that the precise phase of the endogenous oscillation at which the stimulation was applied may be particularly important. Similar to this idea, both invasive and non-invasive studies have suggested that modulatory effects of stimulation are dependent on the endogenous oscillation states [57, 58].
Of note, we observed persistence effect of stimulation; Network decoupling was observed in stimulation ‘off’ trials as well as ‘on’ trials in these patients, which may explain the lack of trial-specific stimulation effects on retrieval accuracy. This is consistent with recent findings that invasive high frequency stimulation to the middle temporal gyrus during encoding of word lists impaired encoding at the word list level rather than individual word item level [59]. Non-invasive stimulation studies also showed that stimulation-induced entrainment often outlasts stimulation [57, 60]. Importantly, our behavioral pilot results suggest that the impaired spatial retrieval effects cannot be explained by a simple lack of counterbalancing.
One caveat of the present study is that patients with epilepsy may have impairments in memory processes [61–63]. This memory impairment is attributed to aberrant neural processing related to epileptiform activity ([64] for review) and/or tissue damage resulting from epilepsy. We address the former issue by excluding trials with interictal spikes, and an additional analysis to confirm that inclusion of the spike trials did not alter the accuracy in our data (Figure 3 vs. Supplementary Figure 4). The latter issue brings concerns as pathological changes in the tissue may lead to the network reorganization of varying degrees. However, in our study, we identified the retrieval networks and core nodes based on the pre-stimulation recording data of individual patients. The resulting networks and stimulation targets varied across patients, which may be explained by reorganized network in some cases. Importantly, our approach assures that long-term network reorganization associated with the pathological changes in patient brains was taken into consideration.
Conclusions
While selectively modulating behavior through causal manipulations has proven thus far to be nuanced, our approach offers unique insights into invasive stimulation approaches: 1) By targeting principal hubs in a network, specific aspects of cognition can be modulated. 2) Taking into account an individual’s own network organization might be more productive than a “one region fits all” approach for neuromodulation. 3) Stimulating regions beyond the MTL could be effective in altering specific memory behaviors. Thus, we believe that our approach can help providing a basis for the developing field of altering cognition through network stimulation, and hope that aspects of these techniques could be used to improve rather than inhibit memory processes, especially in patient populations.
Supplementary Material
Highlights.
A network selective to spatial retrieval is identified using theta phase coherence.
Theta-burst stimulation to this network’s hubs selectively impairs spatial retrieval.
This behavioral impairment is associated with theta decoupling of the network.
Acknowledgments
The authors thank all patients for their time commitment. We thank Bart Moore, Cihan Kadipasaoglu, and Eleonora Bartoli for helpful discussions and inputs for the project. Funding: This work was supported by the National Institutes of Health [grant numbers NS087527 (R21), NS076856 (R01)].
Abbreviations
- stPPC
single trial pairwise phase consistency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because past work on neurostimulation has produced mixed results, we remain agnostic in predicting the behavioral outcome of stimulation. Indeed, reasonable predictions could be made for either direction (i.e., improvement / impairment; also see Discussion). For example, recent findings suggest that stimulation of pathways connecting nodes/hubs – as opposed to one core structure such as hippocampus – may elicit improvements in performance [35–37]. A central premise of our approach – by targeting network hubs, we should be able to modulate overall network function - was indeed motivated by those past findings. However, the gap between past approaches in the literature (targeting anatomical regions that are mostly connecting tissues or thought to be critical for certain memory functions) and our approach (targeting regions that show functionally distinct connectivity) is substantial enough that it would be somewhat of a logical leap to predict improvement. On the other hand, one may think our ‘one frequency fits all’ stimulation is likely to be disruptive to the endogenous signals, thus it would result in impairment. However, that holds true to the past studies that in fact showed memory improvement [35–37]. For these reasons, we remain agnostic about the predicted effect of stimulation of functionally connected brain hubs in our experiment.
One exception to this was Patient 3. In this patient’s Session I recording, more than half of the channels exhibited excessive number of epileptogenic spikes, making those channels not suitable to be included in the analysis. Thus, to still maximize the number of channels used in the analysis, we included all channels that did not exhibit epileptogenic spikes.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corkin HMS, Scoville WB. Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in HM. Semin In Neurol. 1984;4:249–259. [Google Scholar]
- 3.Hermann BP, Wyler AR, Somes G, Dohan FC, Berry AD, Clement L. Declarative memory following anterior temporal lobectomy in humans. Behav Neurosci. 1994;108:3–10. doi: 10.1037/0735-7044.108.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Scoville WB, Milnder B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/JNNP.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. J Cogn Neurosci. 1992;4:232–43. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- 7.Coleshill SG, Binnie CD, Morris RG, Alarcón G, van Emde Boas W, Velis DN, et al. Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. J Neurosci. 2004;24:1612–16. doi: 10.1523/JNEUROSCI.4352-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, et al. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron. 2016;92:983–90. doi: 10.1016/j.neuron.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Lacruz ME, Valentín A, Seoane JJG, Morris RG, Selway RP, Alarcón G. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience. 2010;170:623–32. doi: 10.1016/j.neuroscience.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23:255–60. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20:1604–12. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- 12.Backus AR, Schoffelen J-M, Szebényi S, Hanslmayr S, Doeller CF. Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol. 2016;26:450–7. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia FP, Benchenane K, Sirota A, Pennartz CMA, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–8. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 15.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 16.Nyberg L, Persson J, Habib R, Tulving E, McIntosh AR, Cabeza R, et al. Large scale neurocognitive networks underlying episodic memory. J Cogn Neurosci. 2000;12:163–73. doi: 10.1162/089892900561805. [DOI] [PubMed] [Google Scholar]
- 17.Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–46. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 18.Poldrack RA, Clark J, Paré-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–50. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 19.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–57. doi: 10.1037//0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 20.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu Rev Psychol. 2016;67:105–34. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–73. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition. Prog Brain Res. 2015;219:45–64. doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. doi: 10.1038/srep06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2006;17:1190–6. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- 25.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–48. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 26.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013;16:349–56. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Ekstrom AD, Tandon N. A network approach for modulating memory processes via direct and indirect brain stimulation: Toward a causal approach for the neural basis of memory. Neurobiol Learn Mem. 2016;134:162–77. doi: 10.1016/j.nlm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Brazdil M, Chlebus P, Mikl M, Pažourková M, Krupa P, Rektor I. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy–an fMRI study. Eur J Neurol. 2005;12(4):268–75. doi: 10.1111/j.1468-1331.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- 29.DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered structural connectome in temporal lobe epilepsy. Radiology. 2013;270(3):842–48. doi: 10.1148/radiol.13131044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum Brain Mapp. 2011;32(6):883–95. doi: 10.1002/hbm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32(3):438–49. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, et al. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 2010;1323:152–60. doi: 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 33.Parent JM, Timothy WY, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloviter RS. Status Epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999;40(s1):s34–9. doi: 10.1111/j.1528-1157.1999.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013;74:223–31. doi: 10.1002/ana.23915. [DOI] [PubMed] [Google Scholar]
- 36.Miller JP, Sweet JA, Bailey CM, Munyon CN, Luders HO, Fastenau PS. Visual-spatial memory may be enhanced with theta burst deep brain stimulation of the fornix: a preliminary investigation with four cases. Brain. 2015;138:1833–42. doi: 10.1093/brain/awv095. [DOI] [PubMed] [Google Scholar]
- 37.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–10. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, Ekstrom AD. Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. J Neurosci. 2014;34:6834–42. doi: 10.1523/JNEUROSCI.5341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekstrom AD, Copara MS, Isham EA, Wang W, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;56:1803–13. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 40.Vinck M, van Wingerden M, Womelsdorf T, Fries P, Pennartz CMA. The pairwise phase consistency: A bias-free measure of rhythmic neuronal synchronization. Neuroimage. 2010;51:112–22. doi: 10.1016/j.neuroimage.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 41.Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml K-H. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–73. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011 Jul 31;15(7):310–8. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004 Jun 25;304(5679):1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 44.Watrous AJ, Ekstrom AD. The spectro-contextual encoding and retrieval theory of episodic memory. Front H um Neurosci. 2014;8:75. [Google Scholar]
- 45.Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proc Natl Acad Sci USA. 2011;108:10702–7. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD, et al. Synchronous and asynchronous theta and gamma activity during episodic memory Formation. J Neurosci. 2013;33:292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke JF, Sharan AD, Sperling MR, Ramayya AG, Evans JJ, Healey MK, et al. Theta and high-frequency activity mark spontaneous recall of episodic memories. J Neurosci. 2014;34:11355–65. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–44. doi: 10.1016/S0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 49.Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/S0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- 50.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–61. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 51.Lega B, Burke J, Jacobs J, Kahana MJ. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex. 2016;26:268–78. doi: 10.1093/cercor/bhu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–7. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 53.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23(34):10809–14. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuette PJ, Tran M, Titz A, Tchemodanov N, Mankin EA, Aghajan ZM, et al. Stimulation of entorhinal white matter enhances declarative memory encoding. San Diego, CA: Society for Neuroscience; 2016. (Program No. 556.10. 2016 Neuroscience Meeting Planner). Online. [Google Scholar]
- 55.Nilakantan AS, Bridge DJ, Gagnon EP, Van Haerents SA, Voss JL. Stimulation of the posterior cortical-hippocampal network enhances precision of memory recollection. Curr Biol. 2017;27(3):465–70. doi: 10.1016/j.cub.2016.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–57. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alagapan S, Schmidt SL, Lefebvre J, Hadar E, Shin HW, Frӧhlich F. Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLoS Biol. 2016;14(3):e1002424. doi: 10.1371/journal.pbio.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezzyat Y, Kragel JE, Burke JF, Levy DF, Lyalenko A, Wanda P, et al. Direct brain stimulation modulates encoding states and memory performance in humans. Curr Biol. 2017;27:1251–8. doi: 10.1016/j.cub.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kucewicz MT, Berry BM, Ezzyat Y, Khadjevand M, Miller L, Kremen V, et al. Human memory enhancement through stimulation of middle temporal gyrus. San Diego, CA: Society for Neuroscience; 2016. (Program No. 353.26 2016 Neuroscience Meeting Planner). Online. [Google Scholar]
- 60.Fröhlich F. Experiments and models of cortical oscillations as a target for noninvasive brain stimulation. Prog Brain Res. 2015;222:41–73. doi: 10.1016/bs.pbr.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Hermann BP, Seidenberg M. Memory impairment and its cognitive context in epilepsy. In: Schachter SC, Holmes GL, Trenité DGAK-N, editors. Behavioral Aspects of Epilepsy: Principles and Practice. New York: Springer; 2008. pp. 147–53. [Google Scholar]
- 62.Kleen JK, Scott RC, Holmes GL, Lenck‐Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67(2):250–7. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81(1):18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenck-Santini PP, Scott RC. Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022772. doi: 10.1101/cshperspect.a022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


