Abstract
Penicillin-binding proteins (PBPs) are enzymes involved in the assembly of the bacterial cell wall, a major target for antibiotics. These proteins are classified by mass into high molecular weight PBPs, which are transpeptidases that form peptidoglycan crosslinks, and low molecular weight PBPs, which are typically hydrolases. We report a functionally unique family of low molecular weight PBPs that act as transpeptidases rather than hydrolases, but they do not crosslink peptidoglycan. We show that these PBPs can exchange D-amino acids bearing chemical tags or affinity handles into peptidoglycan precursors, including Lipid II, enabling biochemical studies of proteins involved in cell wall assembly. We report that, in two organisms, the PBPs incorporate lysine into peptidoglycan and, further, that this linkage can occur via attack of the primary ε-amine side chain, an unprecedented reaction for a PBP.
Graphical abstract
The bacterial cell wall, composed of layers of peptidoglycan modified with other polymers and proteins, is essential for survival.1 The enzymes that make and modify the cell wall are, therefore, targets for antibiotics. In fact, two important antibiotic classes, the β-lactams and the glycopeptides, inhibit cell wall biosynthesis. The emergence of resistance to these and other cell wall-targeting drugs has made the discovery of new antibiotics a pressing imperative. In turn, the development of tools that enable biochemical studies of cell wall proteins has become a high priority.
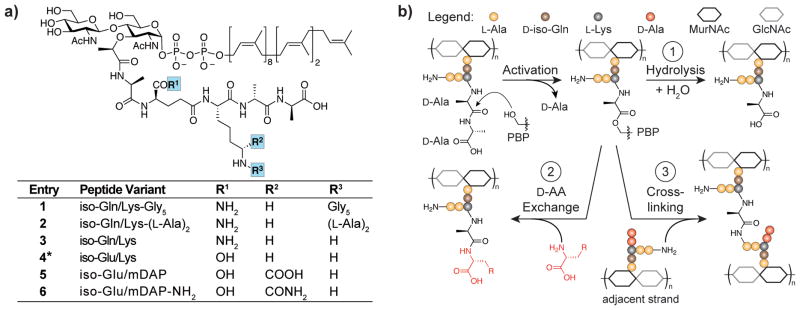
The cell wall precursor Lipid II (Figure 1a) is required to study virtually all peptidoglycan assembly processes. Lipid II contains a stem peptide that varies in structure depending on the producing organism.2 To study cell wall biosynthetic enzymes from different species, the appropriate Lipid II variant is required. We recently developed methods to isolate substantial quantities of native Lipid II in good purity from both Gram-positive and Gram-negative organisms (1, 5, and 6, Figure 1a);3 however, to make use of these substrates, strategies to incorporate labels or tags into native Lipid II are needed. One possible labeling strategy involves using penicillin-binding proteins (PBPs) to exchange the terminal D-alanine on the Lipid II stem peptide with D-amino acids bearing isotope labels, fluorophores, or functional groups that allow derivatization (Figure 1b).4 To implement this strategy, suitable enzymes must be identified.
Figure 1.
Lipid II is the immediate precursor of peptidoglycan. (a) Chemical structures of the Lipid II variants used in this study. *This synthetic Lipid II contains a heptaprenyl rather than an undecaprenyl lipid. (b) Reactions catalyzed by PBPs. Although all PBPs form the same covalent intermediate, low molecular weight PBPs are typically carboxypeptidases that catalyze stem peptide hydrolysis (reaction 1) while high molecular weight PBPs are transpeptidases that catalyze reactions 2 and 3.
PBPs are a large family of enzymes assigned by mass into subfamilies proposed to have distinct functions. High molecular weight PBPs function as transpeptidases and are responsible for crosslinking peptidoglycan strands (Figure 1b). These enzymes can exchange the terminal D-Ala in peptidoglycan stem peptides for other D-amino acids (Figure 1b);5 however they are not suitable for labeling Lipid II because they use only oligomeric peptidoglycan precursors as substrates.5a Most low molecular weight PBPs are also unsuitable for labeling because the activated intermediates are rapidly hydrolyzed (Figure 1b). The one exception is S. aureus PBP4 (SaPBP4), a low molecular weight transpeptidase that crosslinks peptidoglycan.6 This transpeptidase has the unusual ability to catalyze D-amino acid exchange into Lipid II as well as oligomeric peptidoglycan precursors.6c Unfortunately, it also crosslinks S. aureus Lipid II (1) extensively, precluding further use of the labeled material.7 Here, we report a new family of low molecular weight PBPs that have transpeptidase activity. These enzymes can be used to label Gram-positive Lipid II variants, including S. aureus Lipid II. Unlike SaPBP4, these proteins do not crosslink peptidoglycan from their native organisms, but instead have activities distinct from any previously described PBP.
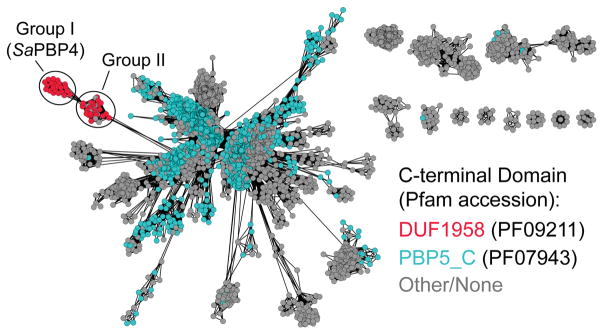
Many low molecular weight PBPs, including SaPBP4, contain a catalytic domain annotated as Peptidase S11 (Pfam PF00768). This domain is often followed by a β-strand-rich C-terminal domain and a membrane anchor (Figure S1). To identify other possible low molecular weight transpeptidases, we generated a sequence similarity network (SSN) for the Peptidase S11 family (Figures 2, S2).8 The alignment analysis was restricted to only the catalytic domain of the family members. We found that SaPBP4 and PBPs from at least 40 other Gram-positive bacteria (Table S2) clustered into two related groups that were separated from a much larger group containing known hydrolases, including E. coli PBP5 (EcPBP5), a well-characterized carboxypeptidase.9 SaPBP4 and nearly all of the other PBPs in these two groups contain a C-terminal domain annotated as DUF1958 that is structurally distinct from the PBP5_C domain found in EcPBP5 and many other hydrolases (Figure S1). We hypothesized that the presence of this unusual domain might correlate with transpeptidase activity.
Figure 2.
Sequence similarity network of the Peptidase S11 Pfam family. “Edges” (lines) were drawn between PBP nodes with ≥40% sequence identity in the catalytic domain. PBPs having a DUF1958 domain are colored red; PBPs having a PBP5_C domain are colored blue. Group I contains staphylococcal PBPs, including SaPBP4. Group II contains primarily enterococcal and streptococcal PBPs.
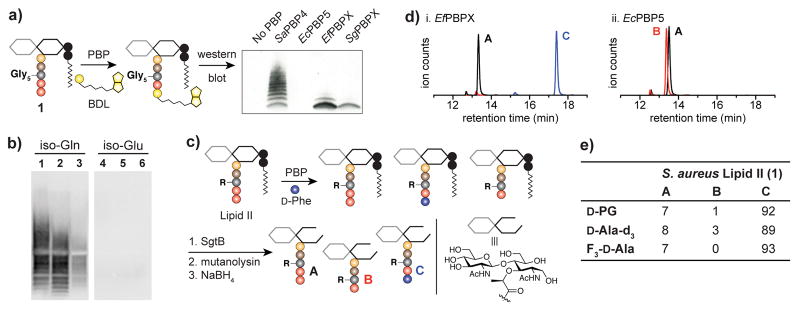
To test this hypothesis, we cloned, expressed, and purified PBPs with a DUF1958 C-terminal domain from two organisms, Enterococcus faecalis and Streptococcus gordonii (Figure S3). As neither protein has been named, we refer to them here as EfPBPX and SgPBPX, respectively. We first examined whether these PBPs would catalyze exchange of the unnatural amino acid biotin-D-lysine (BDL) into S. aureus Lipid II (1). Following incubation of the PBPs with 1 and BDL, we evaluated BDL incorporation by western blot (Figure 3a). The known transpeptidase SaPBP4 and the carboxy-peptidase EcPBP5 were used as positive and negative controls. SaPBP4, EfPBPX, and SgPBPX all catalyzed exchange of BDL into 1, but EcPBP5 did not (Figure 3a). The ladder of bands observed in the SaPBP4 reaction is due to extensive crosslinking of 1.7 We attribute the small amount of cross-linking observed with EfPBPX to the sterically unbiased glycine nucleophile of 1, which can occasionally intercept activated stem peptides. These results supported our hypothesis that DUF1958-containing PBPs have transpeptidase activity.
Figure 3.
EfPBPX and SgPBPX are transpeptidases. (a) Western blot (streptavidin-HRP) showing that PBPs containing DUF1958 catalyze D-amino acid exchange of BDL into 1. (b) Biotinylation of polymers made from 1–6 using EfPBPX. (c) Schematic of the LC-MS assay used to assess exchange of D-Phe into Lipid II. See Methods. (d) Extracted ion chromatograms of the products of labeling reactions with 1. (e) Exchange of D-amino acid labels into 1 using EfPBPX. Lipid II was labeled according to the scheme in panel (c). Values indicate the relative amount of each product. PG = propargyl glycine.
We next tested the Lipid II substrate scope of the PBP transpeptidases. S. aureus Lipid II (1) contains a pentaglycine branch on the stem peptide, while E. faecalis and S. gordonii contain L-Ala-L-Ala (2, Figure 1a).2, 10 We previously developed methods to synthesize (4) or isolate (1, 5, 6) four of the Lipid II variants shown in Figure 1a.3, 5d, 11 To expand the number of Lipid II structural variants, we isolated 2 from E. faecalis and 3 from a Streptococcus pneumoniae mutant (ΔmurMN) following similar methods.3 The structures of 2 and 3 were confirmed by LC-MS/MS (Figures S5, S6). To compare the stem peptide preferences of EfPBPX, SgPBPX, and SaPBP4, we made uncrosslinked peptidoglycan oligomers from the six Lipid II variants by incubating them with SgtBY181D (SgtB*), a peptidoglycan polymerase that makes short peptidoglycan chains.12 BDL and PBPs were then added and the reaction products analyzed by western blot. SaPBP4 labeled all the substrates, although four-fold higher concentrations of 4–6 were required to produce enough labeled material to detect (Figure S7). In contrast, EfPBPX and SgPBPX labeled polymers generated from 1–3 but not those from 4–6, even under forcing conditions (Figure 3b, S7). These results show that all three PBPs strongly prefer Lipid II containing iso-Gln, which is found in their native substrates, rather than iso-Glu, found in canonical Gram-negative Lipid II and B. subtilis Lipid II. Studies with additional Lipid II substrates were consistent with this preference. Curiously, SgPBPX strongly labeled only Lipid II variants bearing multiple glycines in the branch peptide (Figure S10), making it less versatile than the other PBPs as a possible labeling tool.
Because PBP transpeptidases can catalyze stem peptide hydrolysis as a side reaction,13 we next assessed D-amino acid labeling vs. competing hydrolysis using an LC-MS assay (Figure 3c).11c In this assay, Lipid II is incubated with D-Phe and a PBP, resulting in three possible species: un-reacted starting material (A), hydrolyzed Lipid II (B), and Lipid II containing D-Phe (C). To quantify the relative abundance of each species, the material is polymerized and then enzymatically degraded into disaccharide units, which are subsequently reduced with NaBH4. Both EfPBPX and SgPBPX incorporated D-Phe into 1 with minimal competing hydrolysis (Figures 3d, S11). Hydrolysis could be suppressed by using a large excess of D-Phe to Lipid II (Figures S12, S13). In contrast, the carboxypeptidase EcPBP5 generated only hydrolysis product B (Figure 3d).
We next examined incorporation of a variety of potentially useful D-amino acids into Lipid II variants 1 and 2 using EfPBPX. The amino acids included D-propargyl glycine, which contains a click-able group, and D-Ala-d3 and trifluoro-D-Ala, which contain isotope/NMR-active tags. Good to excellent labeling yields were obtained for all the D-amino acids (Figure 3e). SgPBPX and SaPBP4 gave similarly good yields for labeling Lipid II variants 1 and 2, respectively (Tables S3, S4).
We have previously used radiolabeled synthetic Lipid II extensively to study cell wall biosynthetic enzymes. This material took months to synthesize.11a, b Using the conditions developed for D-Ala-d3, we labeled 1 with [14C]-D-Ala using EfPBPX and subjected the labeled material to a peptidoglycan polymerization assay using SgtB*. Radio-labeled products were readily detectable as a ladder of discrete bands on a PAGE autoradiograph (Figure S20). Thus, we have shortened the time required to obtain radiolabeled Lipid II for biochemical assays from several months to three days. Unrestricted access to labeled Lipid II variants should dramatically accelerate investigations of bacterial cell wall enzymes.
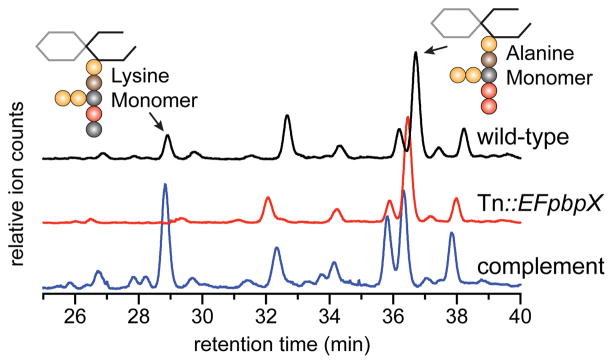
Our results show that SgPBPX and EfPBPX have transpeptidase activity that can be harnessed for various biochemical applications, but we have not yet addressed their cellular functions. Unlike SaPBP4, which crosslinks S. aureus peptidoglycan, we found that neither of these PBPs can crosslink polymer made from their native Lipid II substrate, 2 (Figure S21). Consistent with this result, we found little to no difference in the degree of crosslinking in peptidoglycan isolated from wild-type E. faecalis or S. gordonii compared with mutants lacking EfPBPX or SgPBPX (Figures S22, S24). However, for both organisms, we observed three peaks corresponding to modified muropeptides in digests of wild-type, but not mutant, cell walls (Figures 4, S22, S24). The modified muropeptides absent in the E. faecalis mutant were restored by complementation with a plasmid expressing EfPBPX. The masses of the modified muropeptides were consistent with exchange of Ala at the terminal position of the stem peptide for lysine (Figure S23). These results confirmed that EfPBPX and SgPBPX catalyze a transpeptidase reaction in cells.
Figure 4.
EfPBPX adds lysine to the cell wall. Cell walls isolated from wildtype, mutant (Tn::EFpbpX), or a complemented mutant strain were degraded and subjected to LC-MS analysis. The total ion chromatograms in Figure 4 were cropped to show only the region where muropeptide monomers elute. See Figure S22 for full chromatograms.
We considered two possible ways in which Lys could have been incorporated into the cell wall. First, D-Lys could be added by PBP transpeptidases via D-amino acid exchange. This would require a cellular enzyme capable of racemizing L-Lys. Second, Lys (either D or L) could be incorporated by attack of the primary ε-amine side chain on the activated peptide intermediate. Expecting to rule out reaction of the Lys side chain, which would be an unprecedented reaction for a PBP, we tested EfPBPX incorporation of D- and L-Lys into polymer generated from 2. We were surprised to find that EfPBPX incorporated both free amino acids (Figure S25). When we tested incorporation of Lys stereoisomers in which the side chain amines were Boc-protected, only D-Lys was incorporated (Figure S26). From these experiments, we conclude that EfPBPX can transfer L-Lys to peptidoglycan via the primary ε-amine. Surprisingly, L-Lys incorporation was comparable to D-amino acid exchange (62% conversion; negligible hydrolysis). In contrast, we observed 8% incorporation and 36% hydrolysis when SaPBP4 was incubated with 2 and excess L-Lys.
In conclusion, we have identified a unique family of low molecular weight PBP transpeptidases that are useful for labeling peptidoglycan precursors and display unusual chemistry. Unlike SaPBP4, these enzymes do not crosslink peptidoglycan but, instead, appear to have an as-yet unknown roll in modifying the cell wall. We are currently working to understand the biological roles of these PBPs.
Supplementary Material
Acknowledgments
This research was supported by NIH grants R01GM76710, P01AI083214, U19AI109764, and F32GM123579 (to M.A.W.). A.T. is supported by the Funai Overseas Scholarship.
Footnotes
Notes
The authors declare no competing financial interest.
SUPPORTING INFORMATION: Methods, strains, plasmids, MS data, western blots. This information is available free of charge on the ACS Publications website.
References
- 1.Silhavy TJ, Kahne D, Walker S. Cold Spring Harb Perspect Biol. 2010;2:a000414–a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollmer W. FEMS Microbiol Rev. 2008;32:287–306. doi: 10.1111/j.1574-6976.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- 3.Qiao Y, Srisuknimit V, Rubino F, Schaefer K, Ruiz N, Walker S, Kahne D. Nat Chem Biol. 2017;13:793–798. doi: 10.1038/nchembio.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Cava F, de Pedro MA, Lam H, Davis BM, Waldor MK. EMBO J. 2011;30:3442–3453. doi: 10.1038/emboj.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kuru E, Tekkam S, Hall E, Brun YV, Van Nieuwenhze MS. Nat Protoc. 2015;10:33–52. doi: 10.1038/nprot.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fura JM, Kearns D, Pires MM. J Biol Chem. 2015;290:30540–30550. doi: 10.1074/jbc.M115.683342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Lupoli TJ, Tsukamoto H, Doud EH, Wang TSA, Walker S, Kahne D. J Am Chem Soc. 2011;133:10748–10751. doi: 10.1021/ja2040656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, VanNieuwenhze MS. Angew Chem Int Ed. 2012;51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR. ACS Chem Biol. 2013;8:500–505. doi: 10.1021/cb3004995. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lebar MD, May JM, Meeske AJ, Leiman SA, Lupoli TJ, Tsukamoto H, Losick R, Rudner DZ, Walker S, Kahne D. J Am Chem Soc. 2014;136:10874–10877. doi: 10.1021/ja505668f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Wyke AW, Ward JB, Hayes MV, Curtis NA. Eur J Biochem. 1981;119:389–393. doi: 10.1111/j.1432-1033.1981.tb05620.x. [DOI] [PubMed] [Google Scholar]; (b) Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]; (c) Qiao Y, Lebar MD, Schirner K, Schaefer K, Tsukamoto H, Kahne D, Walker S. J Am Chem Soc. 2014;136:14678–14681. doi: 10.1021/ja508147s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W, Schaefer K, Qiao Y, Srisuknimit V, Steinmetz H, Müller R, Kahne D, Walker S. J Am Chem Soc. 2016;138:100–103. doi: 10.1021/jacs.5b11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Atkinson HJ, Morris JH, Ferrin TE, Babbitt PC. PLoS ONE. 2009;4:e4345. doi: 10.1371/journal.pone.0004345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gerlt JA. Biochemistry. 2017;56:4293–4308. doi: 10.1021/acs.biochem.7b00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Shi Q, Meroueh SO, Vakulenko SB, Mobashery S. Biochemistry. 2007;46:10113–10121. doi: 10.1021/bi700777x. [DOI] [PubMed] [Google Scholar]
- 10.Bizzini A, Majcherczyk P, Beggah-Möller S, Soldo B, Entenza JM, Gaillard M, Moreillon P, Lazarevic V. Microbiology. 2007;153:490–498. doi: 10.1099/mic.0.29256-0. [DOI] [PubMed] [Google Scholar]
- 11.(a) Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]; (b) Lupoli TJ, Taniguchi T, Wang TS, Perlstein DL, Walker S, Kahne DE. J Am Chem Soc. 2009;131:18230–18231. doi: 10.1021/ja908916z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lebar MD, Lupoli TJ, Tsukamoto H, May JM, Walker S, Kahne D. J Am Chem Soc. 2013;135:4632–4635. doi: 10.1021/ja312510m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebets Y, Lupoli T, Qiao Y, Schirner K, Villet R, Hooper D, Kahne D, Walker S. ACS Chem Biol. 2014;9:459–467. doi: 10.1021/cb4006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srisuknimit V, Qiao Y, Schaefer K, Kahne D, Walker S. J Am Chem Soc. 2017;139:2791–2794. doi: 10.1021/jacs.7b04881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.