Abstract
Assessing clinical pain and metrics related to function or quality of life predominantly relies on patient reported subjective measures. These outcome measures are generally not applicable to the preclinical setting where early signs pointing to analgesic value of a therapy are sought, thus introducing difficulties in animal to human translation in pain research. Evaluating brain function in patients and respective animal model(s) has the potential to characterize mechanisms associated with pain or pain-related phenotypes and thereby provide a means of laboratory to clinic translation. This review summarizes the progress made towards understanding of brain function in clinical and preclinical pain states elucidated using an imaging approach as well as the current level of validity of translational pain imaging. We hypothesize that neuroimaging can describe the central representation of pain or pain phenotypes and yields a basis for the development and selection of clinically relevant animal assays. This approach may increase the probability of finding meaningful new analgesics that can help satisfy the significant unmet medical needs of patients.
Keywords: Pain, translation, validity, animal model, brain imaging
Introduction
There are undoubtedly inherent differences and limitations to preclinical pain states in comparison to clinical pain, which can make laboratory to clinic translation difficult and confound the overall analgesic drug discovery process. For example, in clinical populations, chronic pain may be present for months to years and physically disabling, while preclinical models may harbor pain on the scale of hours to weeks with little to no observable impact on physical functioning (Figure 1). The poor translation between preclinical and clinical pain research may also be a consequence of how pain is distinctly assessed between preclinical and clinical pain states. In many preclinical pain experiments, transient, evoked somatosensory responses that are largely mediated by the peripheral and spinal segments of the nervous system are often studied, and these are not representative of or pertinent to many chronic pain conditions. In order to address this experimental disconnect, a number of strategies have evolved to evaluate drug effects on chronic pain such as conditioned place preference (Navratilova et al., 2012; Park et al., 2016), and probing normal rodent behaviors (Andrews et al., 2011; Muralidharan et al., 2016) including mobility or activity (Ishikawa et al., 2015; Miyagi et al., 2013). Using these novel approaches (Park et al., 2016; Xie et al., 2014), the efficacy of known analgesics has been back-translated to the preclinical setting, but to the best of our knowledge, identification of new analgesic drugs using these strategies has yet to come to fruition.
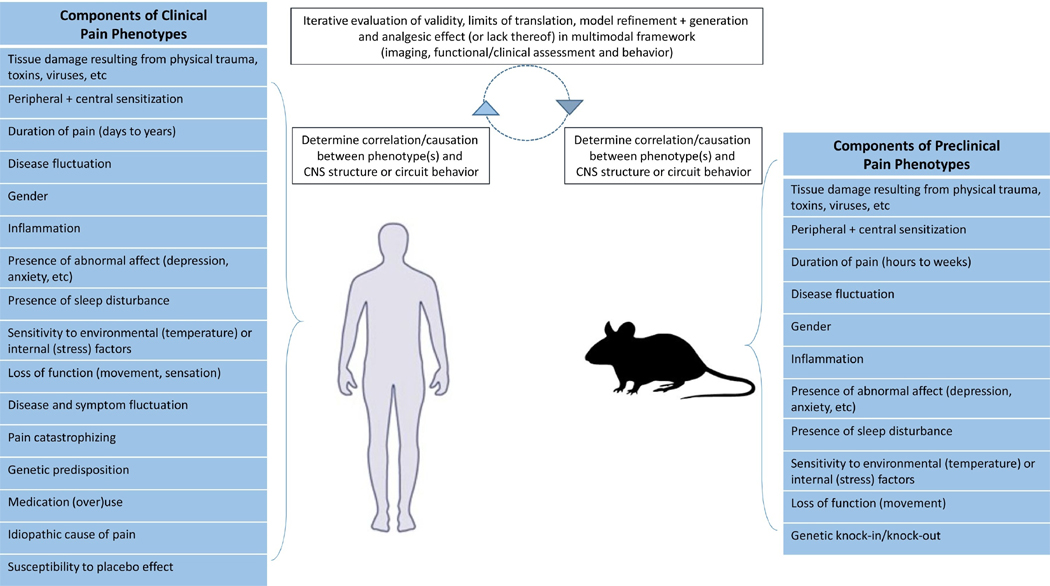
Figure 1. Convergence of phenotypes and CNS properties in clinical and pre-clinical settings.
A model of clinical and preclinical pain experimentation considers the use of pain-related phenotypes in conjunction CNS function to assess and improve the overall validity of preclinical pain investigations.
Functional brain imaging has emerged as a standalone tool with high potential to unravel the intricacies of central nervous system (CNS) pain neurobiology and neuropharmacology (Upadhyay et al., 2013; Zhao et al., 2014). With brain imaging techniques such as functional magnetic resonance imaging (fMRI), CNS structures and circuits hypothesized to mediate distinct aspects of pain processing (somatosensory, affective or motivational) and pain chronification have been characterized. Moreover, fMRI may be used in combination with other physiological and psychological methods to assess and monitor the evolution and resolution of acute and chronic pain states in a more holistic manner (Abaei et al., 2016; Xie et al., 2014). To date, multiple fMRI studies have also quantified central pharmacodynamic effects of current analgesics (Duff et al., 2015; Upadhyay et al., 2011a; Wager et al., 2013). The findings stemming from patient characterization and pharmacological fMRI studies suggest a framework by which central regions of interest are initially and reproducibly identified as key drivers of a clinical pain state, while subsequent investigations aim to determine if novel analgesic strategies normalize aberrant brain function in a robust and dose-dependent manner. Should parallel, functional modulation within the CNS be observed between a clinical pain cohort and the respective preclinical model(s) of pain, a fundamental component of a neuroimaging strategy would encompass preceding preclinical fMRI studies where early indications of a potential analgesic effect may be sought. We propose that fMRI studies have the potential to help identify the most relevant and valid animal assays (Table 1) for clinical pain conditions and provide experimental paradigms that could improve translation and validity of pain and analgesia research (Figure 1).
Table 1.
Definition of Validity in Pain Experiments
| Validity | Definition |
|---|---|
| Construct Validity | Degree to which an assay (paw withdrawal response or thermal pain detection threshold) appears to effectively measure a specific component of pain (thermal hyperalgesia) without the influence of other potentially related components of pain (skeletal or sensory deficits). |
| Face Validity | Degree to which an assay (paw withdrawal response or thermal pain detection threshold) appears to effectively measure a specific component of pain (thermal hyperalgesia). |
| Content Validity | Degree to which an assay (condition place preference or WOMAC) accounts for all components of pain (sensation, affect, function or motivation). |
| Predictive Validity | Degree to which a pain experiment* evaluating a therapy predicts an analgesic effect in a clinical pain population. |
The pain experiment is considered to encompass both the preclinical pain model that is utilized in conjunction with the assay implemented to measure pain. WOMAC - Western Ontario and McMaster Universities Osteoarthritis Index
This review demonstrates the rationale of utilizing brain imaging to improve lab to clinic translation in pain research. We aim to show the value of fMRI in describing clinical pain neurobiology, discuss the gaps and limitations in studies to date and consider where and how fMRI might project back into the preclinical space with the end goal of enhancing the discovery of novel analgesics. To meet this aim, an initial synopsis is given of known commonalities and differences of CNS properties across species as well as a description of functional responses measured during evoked stimulation in healthy animals and humans. In the following sections and for multiple clinical indications, we first overview the clinical phenotypes of a disease state, followed by the corresponding clinical pain imaging literature for that condition and where available, central properties of preclinical pain models elucidated with a neuroimaging approach. Clinical pain states reviewed herein were chosen based on the presence of a substantial body of neuroimaging work highlighting how CNS function and at times, structure are altered in those conditions. Importantly, pain indications where translational datasets were not available (i.e., fibromyalgia) were not excluded as such a gap reflects the need for preclinical to clinical translational neuroimaging studies in these specific domains. The body of work reviewed herein stems from PubMed searches where clinical pain conditions were combined with the following terms: ‘brain’, ‘CNS’, ‘pain’, ‘analgesia’, ‘fMRI’, ‘MRI’, ‘neuroimaging’, ‘resting-state’, ‘evoked-pain’, ‘volumetric’, ‘preclinical’, ‘mouse’ or ‘rat’. Moreover, our review only incorporated manuscripts published in English, while no bounds on the time of publication were implemented.
The Complexity of the Clinical Pain Condition
To pursue the goal of improving the translational process in chronic pain research, independent of whether neuroimaging is utilized or not, it is believed that current unknowns and hurdles present in the clinical space must be kept at the forefront. If there are substantial unknowns regarding the physiological, psychological or behavioral properties causing chronic, clinical pain, then the parameters that are important to recapitulate and therapeutically modulate in the preclinical setting can remain equivocal. With this in mind, we discuss factors that are associated with clinical phenotypic heterogeneity and ambiguity, which in turn, can pose challenges in translational pain research.
Pain Types and Pain Triggers
Two types of pain that are commonly present across patient populations include spontaneous pain (also referred to as clinical or ongoing pain) and evoked pain. Spontaneous pain is defined as pain occurring at rest and is difficult to objectively measure clinically or preclinically. Further complicating the overall clinical picture is that evoked pain may be disease-specific and is very often concomitant with spontaneous pain in the same patient. Depending on the disease population or patient, pain can be evoked by normally benign daily functions (walking or chewing), changes in environmental factors (temperature) or psychological factors (emotional stress and anxiety). These pain triggers are associated with intra-patient and inter-patient variability and impose distinct levels of impact on routine activities of daily function and quality of life. Given the multiple mechanisms underlying pain subtypes and the multiple factors that may contribute to a clinical pain condition, defining parallel processes in preclinical pain experiments is a challenging but important endeavor.
Phenotypic Heterogeneity
Although phenotypic descriptions are used clinically, these are at times far from uniform or diagnostic within a particular pain condition. Even in common chronic pain conditions such as knee osteoarthritis pain, the individual phenotype varies between patients in terms of the contributions of peripheral and central pathophysiology, psychological factors and the nature of disease evolution (Egsgaard et al., 2015; Neogi et al., 2016). Though pharmacological and non-pharmacological treatments have long been available for many pain conditions, what makes certain patients prone or resistant to therapy, or relatedly, prone or resistant to disease progression, is unknown. These uncertainties challenge our ability to produce clinically accurate pain assays within the preclinical setting.
Phenotype and Symptom Fluctuation
The intensity and duration of pain as well as the frequency of painful attacks can vary across time, gender and anatomy as well as between patients and distinct pain patient populations. In patients, chronic pain may last for many months to many years. During this time frame, pain, together with other associated symptoms such as stiffness, psychological distress or sensory hypersensitivity may fluctuate (Birklein et al., 1998a; Birklein et al., 1998b), thus adding complexity to the accurate characterization of pain patient populations. Comparatively, the pathophysiological changes underlying prolonged pain states in preclinical assays are often similar to the clinical counterpart but they progress and resolve over a time period of days to weeks.
Common CNS Circuits Across Species
Critical differences in size, structure and function are present between the rodent, non-human primate and human brain. While cortical differences such as those involving the prefrontal cortex are profound, subcortical structures have a more shared status across species in terms of phylogeny and function. Despite known functional or structural disparities, a preservation of CNS networks across species has in fact been observed using functional brain imaging (Gozzi and Schwarz, 2016). Notably, the default-mode network (DMN) and other intrinsic resting-state networks have been detected in rodents, non-human primates and humans (Becerra et al., 2011; Belcher et al., 2013; Jonckers et al., 2011; Lu et al., 2012; Mantini et al., 2011; Schroeder et al., 2016; Stafford et al., 2014; Upadhyay et al., 2011b). Further cross-species parallels viewed with pharmacological MRI (phMRI) stem from the consistent pharmacodynamic responses in the CNS reported for opioids (Becerra et al., 2013), NMDA-receptor antagonists (Becker et al., 2016) and other pharmacological compounds with analgesic profiles (Borsook and Becerra, 2011). (In-depth overview of phMRI methodology and its application in neuroscience research has been reviewed elsewhere (Jenkins, 2012; Jonckers et al., 2015). We consider these interspecies commonalities as fundamental grounds for using CNS circuits as key translational units in pain research.
Brain Activation During Experimental Pain in Healthy Populations
CNS Response to Acute, Evoked Pain in Healthy Human Volunteers
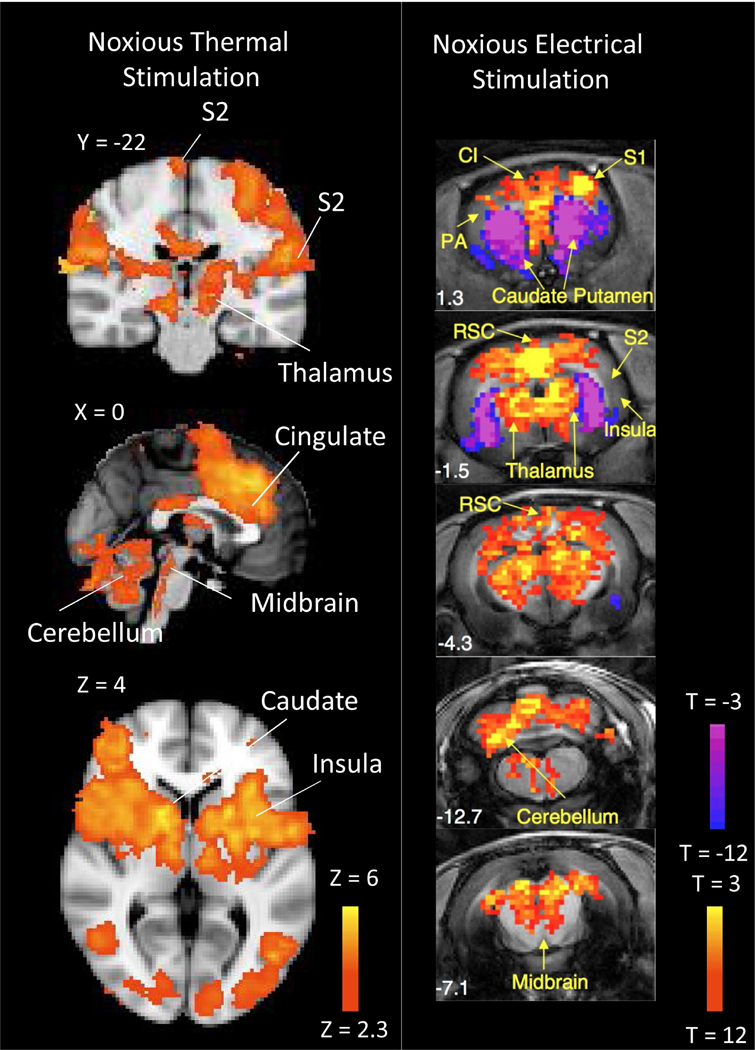
Experimental or evoked pain (noxious heat or cold, capsaicin and incision) activates nociceptors in an otherwise intact nervous system where no maladaptive plasticity or altered immune function exists. When nociceptors are activated, there is a normal physiological response consisting of signal propagation through the peripheral nervous system (A-delta fibers, C-fibers and ganglia) to the CNS (spinal cord, brainstem, subcortical and cortical regions). In neuroimaging studies, the central response to noxious heat stimuli is often measured; however, a range of mechanical, electrical, chemical and other types of stimuli have been explored in block and event related study paradigms. In general, administration of noxious pain stimulation induces a robust, widespread and rapidly reversible activation and deactivation pattern in the brainstem (periaqueductal gray (PAG), raphe nucleus and rostral ventromedial medulla (RVM)); subcortical structures (thalamus, basal ganglia, amygdala, hippocampus and hypothalamus); cortex (frontal, somatosensory, supplementary motor, cingulate and insular cortices); and cerebellum (lobule VI, Crus I, and VIIb) (Moulton et al., 2011b; Peyron et al., 2000; Tseng et al., 2015; Upadhyay et al., 2010; Wager et al., 2013) (Figure 2). The existence of a specific neuroimaging-based “pain matrix” in the CNS was hypothesized (Loggia et al., 2012; Tracey and Johns, 2010). The concept of a pain matrix has recently been challenged (Geha and Waxman, 2016) given the extensive overlap of brain activation by phasic noxious, non-noxious sensory, auditory and visual stimuli (Mouraux et al., 2011), which is also reflective of the multifaceted nature of pain processing (Kucyi and Davis, 2016). Relatedly, previous reports demonstrate activation of a salience detection network in response to phasic pain stimuli (i.e. attention towards the phasic stimulus in an otherwise stimulus free environment) (Legrain et al., 2011). In order to decrease a non-pain specific, saliency-type effect, tonic pain stimulation in conjunction with 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET) can be employed. Such an approach has revealed that the dorsal insula is specifically activated during tonic pain stimulation, while mid-insular cortex activation correlates with pain unpleasantness ratings (Schreckenberger et al., 2005).
Figure 2. Common CNS Circuitry Implicated in Processing Evoked Pain Stimuli in Healthy Human Subjects and Naïve Rats.
Noxious thermal stimulation (7/10 pain rating) applied to the dorsum of the foot in healthy human subjects or noxious electrical stimulation administered to the paw of naïve rats commonly elicit BOLD (human fMRI data) or cerebral blood volume (rat fMRI data) changes in multiple CNS structures from the brainstem to cortex. CI – Cingulate cortex, S1 – Primary Somatosensory Cortex, S2 – Secondary Somatosensory Cortex, RSC – Retrospenial Cortex. Data was adapted and reproduced with permission from (Zhao et al., 2012) and (Upadhyay et al., 2015).
CNS Response to Acute, Evoked Pain in Healthy Animals
Pain fMRI paradigms that have utilized noxious thermal, electrical or mechanical stimuli in healthy volunteers, have been adapted for studying acute pain processing in rats (Abaei et al., 2016; Chang et al., 2016; Suzuki et al., 2015; Zhao et al., 2012), and to a lesser extent, non-human primates (Asad et al., 2016). When comparing evoked BOLD responses or changes in regional cerebral blood flow (rCBF) to pain stimuli between humans and other species, common activation can be observed in primary and secondary somatosensory cortex, cingulate cortex, frontal cortex, thalamus, cerebellum and brainstem (Figure 2). One obvious difference is the evoked deactivation observed in rodents within the caudate-putamen - a region normally possessing strong activation in healthy human subjects. Although the exact cause of this disparity between humans and other species in unknown, it is hypothesized that administration of anesthesia during fMRI acquisition may lead to differential activation patterns (Jonckers et al., 2014; Schlegel et al., 2015; Seah et al., 2014).
Translational Assessment (Table 2)
Table 2.
Validity of fMRI-Based Assessment of Acute Pain Responses in Healthy Volunteers and Naïve Rodents
| Construct Validity | Preclinical and clinical pain fMRI reliably and accurately elucidates the CNS circuitry mediating acute pain processing. Factors related to anesthetic use in preclinical setting may limit the level of construct validity. |
| Face Validity | Independent of mode of sensory stimulation (thermal or mechanical), CNS response to the stimulation can be reliably and accurately obtained. |
| Content Validity | Preclinical and clinical pain fMRI studies reflect involvement of multiple systems involved in processing sensory and affective components of acute pain. |
| Predictive Validity | A highly parallel response in the CNS to acute pain stimuli between the rodent and human CNS exists. However, given known functional and morphological differences between the two species, predictive validity of preclinical pain fMRI may be CNS region dependent. |
Application of noxious stimuli results in a highly parallel CNS response in both healthy human subjects and preclinical species. Differences in activation and deactivation patterns elicited by stimulation may result from experimental procedures (i.e., use of anesthetic) or region-specific differences between species. Whether evoked pain fMRI is performed pre-clinically or clinically, commonality in the CNS in response to acute pain stimulation may exist with other sensory types, for example, unpleasant visual or olfactory stimuli.
Brain Activation in Clinical Pain Populations
Migraine
Episodic Migraine Phenotype
Episodic (0–14 headaches/month) and chronic (>15 headaches/month for at least 3 months) migraine are complex conditions with evolving understanding of its pathophysiology, yet the clinical presentations of migraine are well-defined (Supplemental Table 1). Migraine symptoms vary across patients, but often involve aura, headache, sensory hypersensitivity, nausea, vomiting, deficits in cognition, memory or attention, increase urination, change in affect and fatigue (Bigal et al., 2008; Hirsch, 2005). Triggers of a migraine, while are also patient-dependent and multi-factorial, can include hormonal fluctuations, specific olfactory, visual or somatosensory stimulation, changes in environmental factors such as temperature or barometric pressure and excessive physical or psychological distress.
A migraine attack can consist of up to 4 phases: prodromal (pre-ictal), aura (peri-ictal to inter-ictal), headache (ictal) and post-dromal (post-ictal). The prodromal phase may occur hours to days before the migraine attack encompassing symptoms such as cognitive or language dysfunction, fatigue, pain and changes in mood. Migraineurs that do experience an aura phase, may perceive body distortion, allodynia, photophobia, hallucinations or vertigo. If visual auras are existent, the patients’ visual fields are commonly compromised by scotoma, wavy or blurry vision and flashes of light. Headaches are commonly the most debilitating phase of migraine, where the intensity of pain can range from mild to severe. The duration of a migraine headache can be between 4–72 hours with a uni- or bilateral, pulsating or throbbing-like pain that intensifies during physical efforts. In addition to headache, the migraine phase may involve allodynia, photophobia, phonophobia, osmophobia, autonomic dysfunction (dizziness, nausea and vomiting), diminished affect and cognitive or memory deficits. Lastly, the post-dromal phase of migraine can last from hours to days with a sense of fatigue, sleepiness, cognitive and memory deficits as well as diminish or heighted affect.
Treatment of migraine is primarily accomplished via management of symptoms, mainly migraine headache. Multiple classes of small molecule, pharmacological compounds have historically been prescribed to migraine patients (triptans, nonsteroidal anti-inflammatory drugs (NSAIDs), tricyclics, beta blockers, anti-convulsants, Ca2+ blockers and anti-emetics). However, recent efficacious results from early stage clinical trials involving monoclonal antibodies against calcitonin gene-related peptide (CGRP) or its receptor suggest a novel treatment strategy for migraine appears within reach (Schuster and Rapoport, 2016).
Imaging Migraine
The peripheral elements of migraine and the alleviation of migraine attacks via peripheral pharmacological action (dura, vasculature, trigeminal ganglion and retinal pathways) have been demonstrated (Hostetler et al., 2013; Noseda et al., 2016). Nonetheless, a multitude of neuroimaging studies have revealed functional and structural CNS alterations in migraine patients, which like other pain states, may be causal or correlational to the disease state (Chiapparini et al., 2010; Chong et al., 2016b; Demarquay and Mauguiere, 2016). From functional imaging studies involving assessment of central responses to sensory stimulation (thermal, visual or olfactory) applied across anatomical locations (hand, face and foot), a further comprehension of sensory processing, nociception, affective processing and aura or cortical spreading depression (CSD) have further contributed to understanding the neurobiology of migraine and its associated phenotypes.
Altered trigeminal system nociceptive processing of thermal stimuli during the inter-ictal period of migraine may be driven by temporal pole and entorhinal cortex dysfunction (Moulton et al., 2011a). Interestingly, if in the same patients, evoked BOLD responses are measured within a 4-hour period of a migraine attack, the magnitude of response is comparatively increased in the temporal pole, entorhinal cortex as well as the parahippocampal cortex. While these functional results reported by Moulton and colleagues remain intriguing, other investigators employing a differing pain fMRI paradigm, yet still probing trigeminal nociception in migraineurs, note more cingulate and frontal cortex involvement (Russo et al., 2016).
Many CNS structures and networks showing altered evoked or task-based fMRI responses in migraine patients also demonstrate functional connectivity and morphological changes (Chong et al., 2016b; Maleki and Gollub, 2016). For example, along with temporal lobe hyperactivity evoked by noxious stimulation, increases in both temporal lobe functional connectivity (Maleki et al., 2012a; Moulton et al., 2011a) and cortical thickness (Maleki et al., 2012a; Schwedt et al., 2015) have been associated with migraine. The insula also shows disparate evoked responses to somatosensory stimuli (Lee et al., 2016) in conjunction with altered functional connectivity (Hadjikhani et al., 2013; Maleki et al., 2012b; Niddam et al., 2016; Tso et al., 2015) and sex-specific morphometric properties (Maleki et al., 2015; Maleki et al., 2012b). The observations encompassing the insula are particularly remarkable as its properties objectively discriminate between migraine patients and healthy controls (Borsook et al., 2016; Chong et al., 2016a). Yet it remains unknown if functional and morphological changes of the anterior and posterior insula are specific to migraine pathophysiology considering other pain indications possessing abnormalities within this structure (i.e., fibromyalgia). Thus, an important component of future work could be further emphasis on how predictive insula-related findings are towards clinical symptoms or endpoints of migraine as well as their reproducibility, specificity and sensitivity. This is especially important to consider as consistent changes in, for example, cortical thickness or volume, have not been reported in sub-populations of migraineurs (Hougaard et al., 2016). Additionally, how use or over use of medication (e.g., triptans) can impact central morphology (Lai et al., 2016; Riederer et al., 2012), while also being a contributing factor to migraine chronification (Bigal and Lipton, 2011) would be pivotal for proper interpretation of imaging and phenotypic data.
Although challenging, the longitudinal investigation of patients going through complete migraine cycles would be fruitful as the fluctuating nature of migraine pathophysiology is likely better characterized. In a very recent case study, a migraine patient was followed up with fMRI every day for 30 days, where three complete, untreated migraine attacks were covered. These authors found that hypothalamic activity in response to trigeminal stimulation increases towards the next migraine attack. Furthermore, the hypothalamus was coupled with the spinal trigeminal nuclei and the dorsal rostral pons (so-called “migraine generator”) during the pre-ictal and the pain phase (Schulte and May, 2016). Such a design was highly unique and if replicated, the findings could significantly enhance our understanding of migraine.
Relative to pain processing in migraine patients, vision, olfaction, attention and cognition have been investigated with fMRI to a more limited degree. Compared to healthy controls and migraineurs without aura, migraine patients with visual aura have demonstrated increased primary visual cortex BOLD activity along with a positive correlation between fMRI signals and other clinical metrics (i.e., visual discomfort score) (Cucchiara et al., 2015). Moreover, altered excitation-inhibition coupling may underlie primary visual cortex hyperactivity as well as CSD (Bridge et al., 2015; Hadjikhani et al., 2001; Lauritzen et al., 2011). While normal olfactory responses may be present during the inter-ictal phase, heightened BOLD responses can be detected within the amygdala, insula and pons when olfaction is probed in close proximity to a migraine attack (i.e., 6 hours) – suggesting phase specific, interactions between the olfactory and trigeminal-nociceptive pathways (Stankewitz and May, 2011; Stankewitz et al., 2013). Although not ubiquitously observed (Pearson et al., 2006; Tessitore et al., 2015), deficits involving attention, cognition and memory have also been reported in migraine patients (Gil-Gouveia et al., 2016; Mickleborough et al., 2011). Migraineurs that do in fact develop reductions in cognitive capacities may harbor dysfunction within the so-called task positive and task negative networks (Mickleborough et al., 2016),(Mathur et al., 2015).
Imaging Preclinical Migraine Models
Migraine is a difficult disease to model, and it is unclear if the behaviors observed in animal models are driven by parallel pathological mechanisms occurring in the clinical settings. Current animal models of migraine include, but are not limited to: (i) a genetic mouse strain based on the gain-of-function mutation of Cav2.1 channels observed in familial hemiplegic migraine type 1 patients (van den Maagdenberg et al., 2004; van den Maagdenberg et al., 2010); (ii) models which recapitulate CSD (Becerra et al., 2016; Eikermann-Haerter et al., 2011; Green et al., 2014; Houben et al., 2016); and (iii) models where the cranial dura is manipulated with inflammatory soups or other noxious stimuli (Levy et al., 2008; Lundblad et al., 2015; Yan et al., 2012). From the preclinical fMRI work performed thus far using migraine models, common pathological components or phenotypes of migraine can be detected between rodents and migraine patients (Becerra et al., 2016). For example, seven days of triptan administration, but not saline exposure produced latent sensitization (observed at 14 days) as evidenced by sensitivity to bright light environmental stress and modulation within multiple CNS networks (i.e., default mode, autonomic, basal ganglia, salience, and sensorimotor networks). In the same triptan treated animals, Becerra et al. also demonstrated induction of a low threshold CSD involving cortical and sub-cortical structures. Given the common presence of CSD in migraine and preclinical, migraine-like conditions (Costa et al., 2013), CSD may very well serve as a translational imaging biomarker that further elucidates our understanding of migraine neurobiology as well as identification of new and effective treatment strategies for this indication.
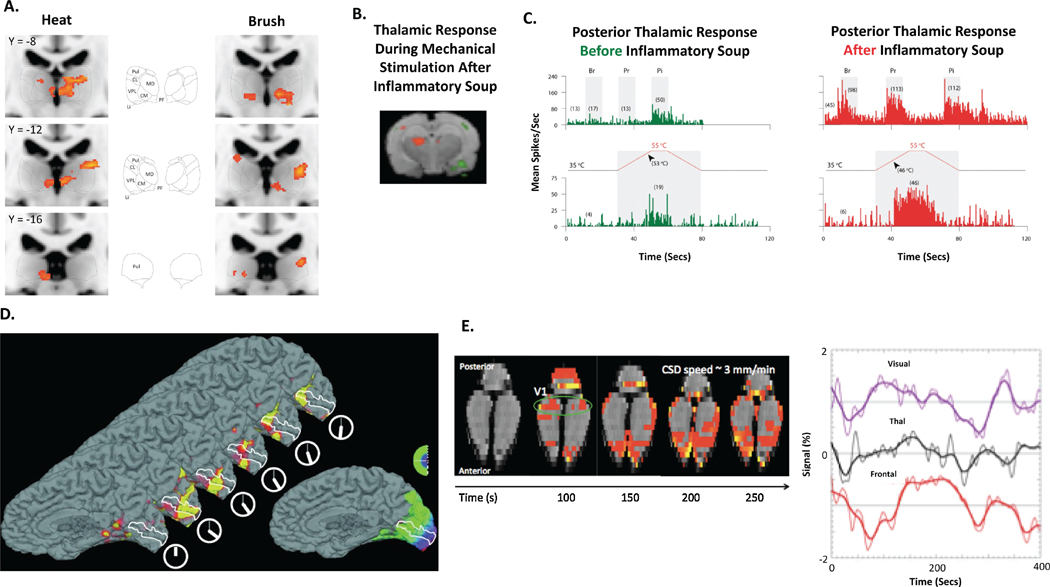
While translational functional imaging studies in the migraine literature are infrequent, clinical neuroimaging data could be utilized to better interpret electrophysiological, post-mortem and behavioral studies probing hyperalgesia and allodynia in rodent migraine models(Burstein et al., 2010). This strategy would in turn allow a determination of overlapping and compromised CNS systems between the clinical and preclinical migraine setting. As shown by Burstein et al., in rats whose dura was chemically stimulated, hypersensitivity to brush or heat stimulation induced enhanced electrophysiological activity within sensory neurons of the posterior thalamus, while similar somatosensory stimuli yielded thalamic hyperactive BOLD responses in migraineurs (Figure 3). Further validation of the rodent model of migraine utilized by Burstein and colleagues is perhaps achieved by measuring functional connectivity within CNS structures and networks implicated in the clinical migraine and a determination of dysfunctional visual processing as has been performed in other settings (Bailey et al., 2013).
Figure 3. Common CNS Functional Responses During Evoked, Sensory Stimulation and CSD in Clinical and Preclinical Migraine States.
In 8 migraine patients with extracephalic allodynia, increased thalamic BOLD responses to heat and brush stimuli were observed during migraine attack in comparison to migraine and allodynia free states in the same patients (A.) Thalamic activation to mechanical stimuli following dural administration of an Inflammatory soup (B.). Heightened electrophysiological responses of sensitized thalamic-trigeminovascular neurons to extracephalic skin stimuli following application of inflammatory soup to the respective dural afferents (C.). CSD can be measured and quantified in both migraine patients (D.) and preclinical migraine models (E.). Br – Brush, CL – Centrolateral thalamic nucleus, CM – Centromedian thalamic nucleus, CSD – Cortical spreading depression, LI – Limitans nucleus, MD – Mediodorsal thalamic group, PC – Posterior commissure, PF – Parafascicular thalamic nucleus, Pi – Pinch, Pr – Pressure, Pul – Pulvinar, V1 – Primary visual cortex, VPL – Ventral posterior lateral thalamic nucleus. Data was adapted and reproduced with permission from (Hadjikhani et al., 2001), (Burstein et al., 2010), (Becerra et al., 2016) and (Becerra et al., 2017).
Translational Assessment (Table 3)
Table 3.
Validity of fMRI Based Assessment of Migraine
| Construct Validity | In migraine models such as those involving a perturbation of the dura with an inflammatory soup, changes in basal physiology (CBF) may impact the global fMRI signal and thus, potentially impacting accuracy of fMRI readouts specific to migraine. |
| Face Validity | Downstream maladaptive CNS processes emanating from pathology within the dura, triptan over-exposure or sensory stimulation or triggering (e.g., bright lights or mechanical stimulation of trigeminal system) can be measured with fMRI, in particular if patients are followed-up before, during and after attacks. |
| Content Validity | Using a combination of evoked and resting-state fMRI, Distinct pathological features of migraine can be measured and quantified with fMRI including, but not limited to hyper-responsiveness to sensory stimuli or CSD. |
| Predictive Validity | Various components of the migraine phenotype such as triggering factors or dural perturbation can be modeled pre-clinically, while the subsequent CNS response with and without treatment can be measured with fMRI. However, a single preclinical migraine model, in which a therapy can be evaluated, which encompasses multiple, key clinical phenotypes is currently not available. |
Characterizing CNS function and structure in migraine patients can be considered as one of the more mature components of the pain imaging literature. However, a synonymous level of effort in preclinical migraine models is missing. From the preclinical migraine imaging literature performed thus far, key neurobiological components recapitulated from the clinical setting included detection of CSD and posterior thalamic hyperactivity. Similar to other pain indications (i.e., fibromyalgia), it is unknown how the rodent or human CNS is perturbed during normally benign environmental changes, or conversely, what role a maladapted CNS plays in amplifying the effect of external environmental or internal psychological factors to produce a pain state.
Neuropathic Pain (NP)
Neuropathic Pain Phenotypes
Injury or dysfunction of peripheral sensory nerve fibers can lead to chronic, neuropathic pain states characterized by positive (i.e., burning or shooting pain), but also negative (i.e., loss of sensation) symptoms. Distinct phenotypic expression of neuropathic pain, for example, burning versus shooting pain, may stem from differences in the nerve fiber type(s) that are pathologically affected, and from mechanisms behind nociceptive activity generation (Maier et al., 2010; Rowbotham and Fields, 1996). There is also evidence that somatosensory phenotypes can discriminate between mild neuropathic pain and moderate to severe neuropathic pain populations (Themistocleous et al., 2016). A wide range of medical conditions associated with nerve damage or dysfunction (diabetes, human immunodeficiency virus (HIV), herpes zoster, limb amputation, alcoholism and chemotherapy-induced peripheral neuropathy (CIPN)) as well as a number of core pathological events (hypoxia, aberrant ionic cellular influx, heighted inflammatory stimulants or nerve compression) modulate functional and structural properties of peripheral nerves (Supplemental Table 2). As a result of this diversity in conjunction with ill-defined pathological mechanisms, neuropathic pain can at times be a difficult to treat pain state. Adding to the complexity of neuropathic pain, sub-populations of pain patients classically described as possessing nociceptive pain (e.g., osteoarthritis (Hochman et al., 2011; Moreton et al., 2015) or rheumatoid arthritis (Koop et al., 2015)) or with a strong central pain component (e.g., fibromyalgia (Gauffin et al., 2013; Uceyler et al., 2013)) may also present with neuropathy and neuropathic-like pain features (Clauw, 2015). Pharmacological therapies used to treat neuropathic pain patients, include a very wide range of NSAIDs, opioids, anti-depressants, anti-convulsants and local or topical analgesics. Unfortunately, objective factors that link to either susceptibility or resistance to anti-neuropathic pain medication treatment remain largely unknown.
Imaging Clinical Neuropathic Pain Conditions
Presence of nerve injury or nerve dysfunction over a long period are each capable of producing maladaptive changes within the CNS. A potential consequence of such an adaptation is the central generation of spontaneous pain or amplification of normally, non-nociceptive peripheral signals to produce evoked pain. By probing central function in conditions such as trigeminal neuralgia, it has been possible to begin understanding common and distinct central mechanisms associated with spontaneous or evoked, disease-specific neuropathic pain (Borsook et al., 2007).
Patients with trigeminal neuralgia or multiple sclerosis often present with neuropathic pain in the V1-V3 distributions of the trigeminal system. The cause(s) of trigeminal neuropathic pain can be manifestations of trauma, inflammation, demyelination or vascular abnormalities, yet a central etiology may also be a driving factor of the pain experienced. On one hand, regional functional connectivity changes involving the amygdala are perhaps reflective of a robust affective component of trigeminal neuralgia (Wang et al., 2015). Such findings are in line with those observed in multiple sclerosis patients that develop chronic pain, as this population harbors functional and structural plasticity within motivational and affective systems (i.e., dorsal and ventral striatum as well as frontal-parietal and temporal cortices) (Seixas et al., 2016). On the other, increases in functional connectivity along the pre- and postcentral cortex may relate to patient reported pain severity (Wang et al., 2015). This latter functional finding in trigeminal pain is in accord with earlier work incorporating structural MRI, where pre- and postcentral cortex thinning overlapped with regions activated or deactivated during what was perceived as painful brushing (DaSilva et al., 2008) and whose abnormal function was mitigated by lamotrigine (Scrivani et al., 2010). Beyond the somatosensory cortices, structural MRI performed in trigeminal neuralgia patients revealed gray matter reduction in the fusiform and cuneus gyrus - brain areas associated with phasic pain processing and regions engaged in multisensory or cognitive processing (Obermann et al., 2013; Parise et al., 2014). Furthermore, successful surgical treatment of trigeminal pain reversed white matter changes in the trigeminal root entry zone and cortical thinning in the anterior insula, suggesting that these morphological changes, although potentially maladaptive, are reversible (DeSouza et al., 2015).
In spinal cord injury (SCI) patients, evoked fMRI data collected within the spinal cord and brainstem showed activation was either diminished or absent entirely - reflecting either pain, loss of sensory function or a combination of the two (Stroman et al., 2016). The reduced spinal cord and brainstem activation may also relate to an impairment of the descending pain control system as evidenced by functional connectivity changes between the hypothalamus and brainstem. Another potential source of persistent neuropathic pain experienced by SCI patients could originate at the primary somatosensory cortex level (Wrigley et al., 2009). The CNS atrophy reported in somatosensory cortex, anterior cingulate and insula as well as increases in motor cortex thickness certainly support the assertion of cortical drivers of neuropathic pain in SCI (Jutzeler et al., 2016). However, gray matter atrophy is also detected in the thalamus and spinal cord, and the correlations between cortical functional reorganization and pain intensity were not ubiquitously reported across neuroimaging studies performed in SCI patients (Jutzeler et al., 2015).
Conditions such as diabetic peripheral neuropathy (Scherens et al., 2009), CIPN (Geber et al., 2013) or idiopathic small fiber neuropathy (Forkert et al., 1989) can be painful or painless, with CNS factors differentiating between the two states. For example, compared to control subjects as well as painless diabetic patients, individuals with painful diabetic neuropathy possessed greater evoked responses to heat stimuli throughout the limbic system, striatum and somatosensory cortex (Tseng et al., 2013), where the magnitude of BOLD activity correlated with the duration of neuropathic pain. The involvement of the CNS in painful as well as painless diabetic neuropathy is further supported by structural changes observed in the spinal cord (Selvarajah et al., 2006) and supraspinal structures (somatosensory, supramarginal and cingulate cortices) (Selvarajah et al., 2014). In multiple myeloma patients with CIPN, heat stimulation applied to the foot or thigh induced decreased (relative to healthy volunteers) frontal cortex and increased precuneus activity (Boland et al., 2014). Interestingly, Nudelman and colleagues also reported similar cortical involvement in breast cancer patients with CIPN (Nudelman et al., 2016). In their work, increased blood perfusion in frontal and cingulate cortices was measured, with a correlation between rCBF with both gray matter density and CIPN symptom severity. What remains largely unknown is if various chemotherapy agents access and modulate the CNS by toxic effects directly in the brain or if a long-term presence of CIPN is the key driving factor of the observed central phenotypes. Similar to CIPN, HIV patients often present with neuropathy and neuropathic pain (Ellis et al., 2010; Wiebe et al., 2011), but here, nerve injury can be due to the infection itself or result from treatment-induced toxicity (Kranick and Nath, 2012). In HIV, changes in CNS structure (Chiang et al., 2007; Jahanshad et al., 2012; Keltner et al., 2014; Thompson et al., 2005; Thompson and Jahanshad, 2015), for instance, those occurring in posterior cingulate cortex, may contribute to the spectrum of symptoms reported by HIV patients and also objectively distinguish painful vs. painless HIV neuropathy (Keltner et al., 2016).
Generally speaking, neuropathic pain has been investigated with neuroimaging across many patient populations, yet common limitations of these clinical studies are their cross-sectional nature, small cohort sizes or paucity when considering a single neuropathic pain indication. Further emphasis on CNS imaging markers informing on spontaneous pain, rather than experimentally evoked pain is likewise much needed for improved characterization of neuropathic pain phenotypes. Nonetheless, the results stemming from this recent body of work involving these neuropathic pain patients importantly lend themselves towards initial comprehension of condition-specific central changes as well as better hypotheses generation for future work.
Imaging Preclinical Models of Neuropathic Pain
Common neuropathic pain models often involve unilateral transection (spared nerve injury (SNI)), loose ligation (chronic constriction injury (CCI)) or tight ligation (spinal nerve ligation (SNL)) of peripheral nerves (e.g., sciatic, tibial or sural nerves). Administration of exogenous substances such as chemotherapy agents (vincristine, paclitaxel or oxaliplatin), proteins (HIV-1 protein GP120) or hyperglycemia-inducing agents (streptozotocin) are often utilized to recapitulate mechanisms causing nerve injury unique to a specific disease with neuropathic pain. Reviews of preclinical models of neuropathic pain have been provided elsewhere (Bennett et al., 2003; Colleoni and Sacerdote, 2010; Mogil, 2009).
Preclinical imaging studies assessing neuropathic pain have primarily involved the CCI, SNL or SNI models, where biological processes occurring at the periphery or within the CNS have been monitored. Functional brain imaging in the SNL model revealed increased resting-state functional connectivity across the hippocampal formation, limbic system, somatosensory cortex and basal ganglia (Hooker et al., 2014); a finding in accord with other SNL-based investigations utilizing magnesium-enhanced MRI (Jeong et al., 2016). Interestingly, the function of these CNS systems in the presence of a neuropathic state can be pharmacologically modulated by acute gabapentin administration (Governo et al., 2008; Hooker et al., 2014; Takemura et al., 2011). Similar to fMRI studies performed in healthy volunteers and pain patient populations, CNS activity in the preclinical setting has been evoked using capsaicin, mechanical, electrical or thermal stimulation. In a recent longitudinal study by Hubbard et al, cold stimulation via application of acetone was performed during fMRI data acquisition in SNI animals (Hubbard et al., 2015). At the 20-week time point, primarily decreased fMRI responses were observed in subcortical regions (i.e., thalamus, hippocampus and periaqueductal gray) and increased activity was observed in cortical structures (i.e., limbic and somatosensory cortex). Yet these findings, particularly the decreased evoked fMRI responses in subcortical regions, are in contrast to functional observations in other pain models (Abaei et al., 2016). It can be considered that in preclinical neuropathic or neuropathic-like pain models investigated with fMRI, the observed mix of activation and deactivation patterns stem from a combination of factors ranging from stimulation paradigms to anesthetic protocol to time point(s) of model evaluation.
Compared to CNS imaging efforts in neuropathic pain models, less emphasis has been placed on imaging peripheral pathology. Despite this limitation, recent results stemming from imaging muscle and nerve in preclinical, neuropathic conditions could be utilized to further hypothesize how peripheral abnormalities track with central function and structure as well as other markers (i.e., pain-like behaviors or soluble biomarkers). For example, using a combination of T1 or T2-weighted structural MRI along with 18F-FDG PET/CT, Lasko et al. and Biswal and colleagues independently demonstrated robust unilateral edema and enhanced glucose metabolism proximal to the nerve injury site, in distal nerve and in distal muscle within the SNI and CCI models, respectively (Behera et al., 2011; Lasko et al., 2013). It should be kept in mind that unilateral nerve injury can have widespread modulatory effects on the peripheral nervous system as suggested by bilateral increases of calcium flux in sciatic nerves (Behera et al., 2013). Importantly, utilization of imaging techniques such as 11C-PK11195 PET/CT, where macrophage or microglial activity is captured in preclinical neuropathic pain models may further detail when, where and how immune system responses are active following peripheral nerve injury (Imamoto et al., 2013).
Translational Assessment (Table 4)
Table 4.
Validity of fMRI Based Assessment of Neuropathic Pain States
| Construct Validity | In preclinical neuropathic pain models (SNI, SNL or CCI), fMRI readouts may be influenced by surgical procedures, particularly at early evaluation time points, in addition to factors stemming directly from nerve injury. In clinical and preclinical neuropathic pain states, the presence of loss-of-function may interact with the fMRI data. |
| Face Validity | An abnormal CNS response or set of responses to evoked pain can be obtained. Patient fMRI data has more variation than in healthy controls. |
| Content Validity | Using a combination of structural MRI as well as evoked and resting-state fMRI, distinct CNS circuits for sensation, affect, function or motivation can be simultaneously probed during a neuropathic condition. |
| Predictive Validity | Some neuropathic pain models (CCI) may recapitulate certain neuropathic conditions (sciatica) more than others (phantom limb pain). Human imaging is often correlative and cannot prove causality. It is therefore still unclear whether it measures causality for or side effects of chronic pain. |
Clinical neuropathic pain populations and neuropathic pain models show functional as well as structural plasticity within the somatosensory, limbic and reward systems in conjunction with the peripheral nervous system. What remains lacking in either the clinical or preclinical setting is a more concrete determination of how key features of neuropathy such as a gain- or loss-of-function longitudinally predict or are longitudinally predicted by CNS properties. A better determination of CNS-based measures susceptible to treatment effect is also needed.
Osteoarthritis (OA)
OA Pain Phenotype
Osteoarthritis, be it in the knee, hip or other joints, embodies multiple structural joint pathologies that range from degradation of subchondral bone and cartilage to presence of joint effusion or synovitis (Supplemental Table 3). It is well-known that the degree of structural degradation does not necessarily correlate with the levels of resting or evoked pain as well as functional impairment as measured with clinical tools such as the Western Ontario and McMaster Universities Osteoarthritis Index, Knee Injury and Osteoarthritis Outcome Scores or Patient Global Impression of Change. Thus, there is a strong need to consider multiple OA phenotypes outside of joint pathology contributing to the pain experienced by OA patients. These phenotypes can comprise of psychological distress, nervous system sensitization and pain catastrophizing levels (Egsgaard et al., 2015).
NSAIDs (topical or oral), opioids, acetaminophen and serotonin and norepinephrine reuptake inhibitors (SNRIs), which impact functionality of peripheral nerves, dorsal root ganglia and the CNS, are often prescribed to suppress OA pain, though inadequate treatment response to these pharmacological strategies is commonplace. Analgesia can also be achieved in OA using intra-articular injections of hyaluronates or corticosteroids. As demonstrated by O’Neill and colleagues, steroid treatment reduced synovial tissue volumes in knee OA patients and concomitantly decreased pain levels, while pain increased in conjunction with a ‘rebound’ effect on synovial tissue volume (O'Neill et al., 2016). Such observations are in accord with other findings where infrapatellar synovitis and joint effusion have shown a linkage with the evolution of knee OA and are suggested as having predictive capabilities with symptomatic progression (e.g., pain) (Bastick et al., 2015). The relationship between joint tissue pathology and reported pain levels indicate the key role played by the afferent sensory system in propagating noxious sensory signals in OA. Nonetheless, it would be beneficial to considered that factors associated with primary afferent drive may work in unison with mechanisms such as neurogenic inflammation (Ji et al., 2014) and central sensitization to produce an OA pain state.
Imaging Clinical Osteoarthritis Pain
In functional imaging experiments involving OA patients, arterial-spin labeling (ASL), BOLD fMRI and 18F-FDG-PET have been implemented during the resting-state (Baliki et al., 2014; Howard et al., 2012; Kulkarni et al., 2007), task performance (Sofat et al., 2013; Stafford et al., 2014) and evoked stimulation (Brown et al., 2014; Gimenez et al., 2014; Gwilym et al., 2009; Hiramatsu et al., 2014; Kulkarni et al., 2007). In a longitudinal study paradigm, Howard et al., demonstrated that individuals with OA of carpometacarpal joints possessed increased resting-state perfusion within brainstem (periaqueductal gray), subcortical (thalamus) and cortical (insula) structures, with a subset of regions showing a correlation with ongoing pain levels (Howard et al., 2012). Such functional alterations at a basal state are perhaps indicative of the CNS response or adaptation to the chronic presence of OA pain, but differentiating between the two is difficult. Given that joint movement can also induce pain, flexion of osteoarthritic, carpometacarpal joints during BOLD-fMRI can yield enhanced brain activity in pain processing regions such as the amygdala, thalamus, cingulate, insula and somatosensory cortex (Sanders et al., 2015; Sofat et al., 2013), and importantly, this activity is suppressed by NSAIDs. Movement of an OA joint during fMRI data collection offers a unique opportunity to evaluate central responses to OA specific pain. For example, based on regions of brain activation and deactivation, it may not only be possible to determine the somatosensory response to OA joint movement, but also, if and when regions implicated in fear and anxiety are modulated by the task, and as a result, potentially amplifying the overall pain experience (Timmermans et al., 2014). Similar to the work by Saunders et al., Gimenez and colleagues reported a suppression of BOLD responses induced by naproxen treatment in supramarginal gyrus, cingulate, insula, basal ganglia and amygdala, but here, pressure stimuli was applied to a hypersensitive region along medial articular interline of the OA knee (Gimenez et al., 2014). By evoking pain during fMRI with pressure, mechanical or thermal stimuli, particularly at the non-index OA joint, a determination of an overall hypersensitive, somatosensory state may be possible; yielding a presence of peripheral and central sensitization and providing a more comprehensive characterization of the pain phenotype.
From structural MRI work, gray matter atrophy appears to coincide with multiple affective or sensory processing structures demonstrating atypical function in OA. Using voxel-based morphometry, a decrease of gray matter in the anterior cingulate cortex, right insular cortex, operculum, dorsolateral prefrontal cortex (DLPFC), amygdala, thalamus and brainstem were described in OA of the hip (Gwilym et al., 2010; Rodriguez-Raecke et al., 2009). The majority of these changes were reversed after 6 weeks and 4 months following total hip replacement and if pain was successfully abolished (Rodriguez-Raecke et al., 2009). This longitudinal study nicely supports the specificity of focal grey matter atrophy for OA pain, but its reversibility challenges the concept of structural changes as a potential brain mechanism causal for or specific to chronic pain in OA (Rodriguez-Raecke et al., 2013).
Imaging Preclinical Models of Osteoarthritis Pain
Rodent models of OA that have been utilized to monitor disease progression, disease modifying OA drugs or analgesics include, but are not limited to the medial meniscus tear (MMT), anterior cruciate ligament transection (ACLT), destabilization of the medial meniscus (DMM) and monosodium iodoacetate (MIA) models. Similar to the clinical scenario, the focus in the preclinical OA imaging literature has centered around MRI, CT, PET or single-photon emission computed tomography (SPECT) based evaluation of bone turn over (Piscaer et al., 2013), cartilage degradation (Xie et al., 2014) and immune activity (Piscaer et al., 2011). This body of work has shown multiple parallelisms in peripheral joint pathology between preclinical and clinical OA settings. For example, just as in knee OA patients, cartilage degradation, subchondral bone lesions and synovitis have each been recapitulated in the MMT model (Bove et al., 2006; Upadhyay et al., 2013). These peripheral imaging studies can be used to build and test hypotheses related to the interplay between peripheral pathology in OA and maladaptive CNS processes that may together drive the evolution of OA pain.
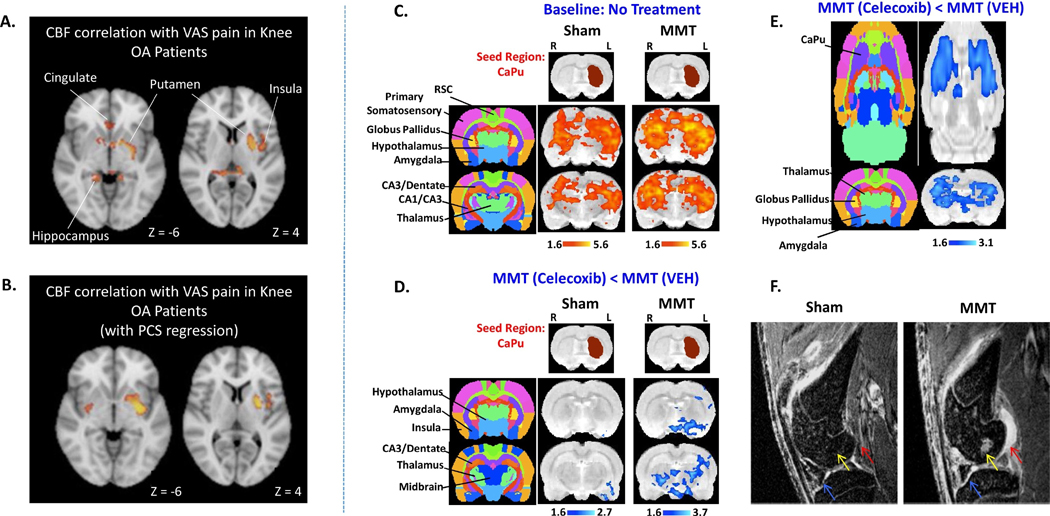
Upon investigating central plasticity with fMRI, MMT compared to sham operated animals showed increases in functional connectivity (Figure 4); an effect mitigated by prophylactic treatment with a peripherally restricted, broad-spectrum, matrix metalloproteinase (MMP) inhibitor. Interestingly, acute administration of celecoxib in MMT animals also resulted in decreased BOLD signals relative to both baseline and sham conditions with particularly strong pharmacodynamics measured within the caudate-putamen (Figure 4). More recently, Abaei et al. investigated nociceptive processing within the MIA model of OA. Relative to baseline, increases in functional connectivity or BOLD activity were reported especially in subcortical structures under conditions of intra-articular injection of capsaicin plus MIA (Abaei et al., 2016). Similar observations were also made (with the addition of cortical BOLD activation) during von Frey hair stimulation of the hindpaw.
Figure 4. Changes in Putaminal Function Commonly Observed in Knee OA Patients with Pain and the MMT Model of Knee OA.
In 26 knee OA patients with pain, the magnitude of cerebral blood flow (CBF) in the putamen correlated with patient reported levels of pain with (A.) and without regression of pain catastrophizing scores (PCS) (B.) Compared with the sham condition, increased functional connectivity in the MMT model was observed when the left caudate-putamen (contralateral to MMT knee) was used as the seed region (C.). Following intravenous celecoxib administration and compared to the saline condition, decreases in caudate-putamen functional connectivity was measured (D.), while BOLD fMRI signals within the bilateral caudate-putamen was present only in MMT animals (E.), but not in sham animals (data not shown). The MMT model possesses not only aberrant functional properties in the CNS, but also, the expected peripheral pathology in the knee joint (synovial lesions (red arrow), subchondral bone lesions (yellow arrow) and cartilage hyper-intensities (blue arrow)) as measured by contrast enhanced MRI (F). Data was adapted and reproduced with permission from (Upadhyay et al., 2013) and (Cottam et al., 2016).
Translational Assessment (Table 5)
Table 5.
Validity of fMRI Based Assessment of OA Pain States
| Construct Validity | In surgical OA models (DMM or MMT), fMRI as well as non-fMRI readouts may be influenced by surgical procedures particularly at early evaluation time points, in addition to factors stemming from peripheral joint pathology (e.g., synovitis and autoimmunity). |
| Face Validity | An abnormal CNS response or set of responses to evoked pain (experimental or disease-specific) specific to OA may be identified. Note that CNS responses to experimental pain may not importantly reflect evoked pain induced by joint movement or manipulation. |
| Content Validity | Using a combination of evoked and resting-state fMRI, distinct CNS circuits mediating sensation, affect, function or motivation can be simultaneously probed under an OA pain state. Sensory and affective responses specific to OA joint movement or manipulation may be possible. |
| Predictive Validity | While key peripheral (cartilage degradation, sensitization of primary afferent neurons) and central (central sensitization) pathology is present in preclinical and clinical OA conditions, it is unknown if temporal relationships between joint pathology vs. pain magnitude/CNS response is preserved in preclinical models. |
OA encompasses spontaneous and evoked pain in conjunction with functional deficits related to movement. Clinical and preclinical functional brain imaging studies involving OA patients and models implicate CNS structures such as the striatum and other subcortical regions; observations that may arise from persistent ongoing pain, movement-induced pain or changes in proprioception. What remains largely obscure is how and to what extent peripheral pathology (e.g., synovitis, joint effusion, subchondral bone lesions, inflammation and peripheral sensitization) present in OA longitudinally affect CNS function and vice versa.
Rheumatoid Arthritis (RA)
The RA Pain Phenotype
Rheumatoid arthritis is a chronic autoimmune condition consisting of inflammation induced joint degradation (Supplemental Table 4). Two major sub-populations of RA patients include those with (most common form and termed seropositive RA) or without antibodies to citrullinated protein antigens and rheumatoid factor. Factors that can help trigger or add susceptibility to development of RA include but are not limited to smoking (Saag et al., 1997) and the presence of genetic risk alleles (e.g., HLA class II alleles (Buckner and Nepom, 2002) and PTPN22 allele (Kallberg et al., 2007)). RA often involves pain, swelling and long-term deformity in multiple joints, yet patients commonly experience symptoms in hand joints (carpometacarpal, radius and ulna), which expectedly correlates with loss of mobility and strength (Horsten et al., 2010). In addition to pathology present within joints, skeletal (osteoporosis), dermatologic (rash), cardiovascular (vasculitis or atherosclerosis) and ophthalmologic (dry eyes or scleritis) disorders can often present concomitantly. Similar to other arthritic conditions such as osteoarthritis, a mismatch may exist between detectable peripheral pathology and magnitude of circulating inflammatory markers and reported pain levels (Taylor et al., 2010; Thompson and Carr, 1997). Interestingly, a number of disease-modifying anti-rheumatic drugs (DMARDs, adalimumab, infliximab, certolizumab, pegol and tofacitinib) and anti-inflammatory drugs effectively suppress joint inflammation and mitigate RA symptoms. Nevertheless, substantial levels of pain can remain in some patients. In accord with other chronic pain conditions, pain may be triggered or exasperated by environmental factors (i.e., cold temperatures), mental stress and physical activity levels.
Imaging Rheumatoid Arthritis (RA)
Presence of chronic or unresolved pain in RA may emanate in part from maladaptive plasticity and sensitization in the peripheral and central nervous systems. Early work measuring rCBF with PET suggested reduced CNS responses to heat stimulation within the prefrontal and anterior cingulate cortices (Jones and Derbyshire, 1997). Given the correlation between the evoked heat pain responses in the medial prefrontal cortex and depressive symptoms in addition to volumetric changes within the basal ganglia, a non-somatosensory and limbic component to pain perception in RA is likely and superimposed upon clinically obvious somatic components of RA (Schweinhardt et al., 2008; Wartolowska et al., 2012). Recently, Flodin et al. used seed region, resting-state fMRI to evaluate connectivity in RA patients (Flodin et al., 2016). Compared to healthy controls, increases in functional connectivity involving affective pain processing regions (mid-cingulate and mid-frontal cortices) and more sensorimotor circuitry (supplementary motor, motor and primary somatosensory cortices) were observed in the patient cohort. These and other functional alteration have been proposed to stem from adaptations in top-down processing between frontal and sensorimotor regions, but may also emanate from alterations in behavior or motor ability many RA patients experience. It is also noted that similar to many other fMRI studies involving pain patients, RA cohorts remained on treatment, primarily methothrexate, during fMRI acquisition, which likely impacts central function and potentially explains the lack of correlation between fMRI readouts and reported RA symptoms.
In studies by Hess et al. and Rech et al., evoked pain fMRI responses were observed during joint compression (Hess et al., 2011; Rech et al., 2013) in regions such as the anterior cingulate cortex, insula and prefrontal cortex. In the same investigations, a sustained suppression of BOLD activity could also be detected within 3 days (certolizumab pegol) or 24 hours (infliximab) of initiating anti-TNFα treatment and prior to improvements observed with conventional clinical assessments. Though the sample size in both studies by Schett and colleagues was small (N=5–8) and the study designs did not include a blinded, placebo controlled arm, single-subject treatment responses can be elucidated in conjunction with relationships between fMRI-based metrics, pain ratings and other clinical scores. The early findings in the CNS of RA patients receiving anti-TNFα therapies is of interest given patient reported improvements prior to robust detection of suppressed peripheral inflammation. The early therapeutic response to DMARDs may stem from drug effect on the CNS, but peripheral mechanism (e.g., modulation of the toll-like receptor signaling pathway and impact on afferent sensitization) must be considered as well (De Rycke et al., 2005; Kato et al., 2016).
Imaging Preclinical Models of RA
Preclinical imaging studies focusing on a functional assessment of the CNS in RA models have been sparse, yet from the limited amount of work performed thus far (Amirmohseni et al., 2016; Hess et al., 2011), parallels in brain function between clinical and preclinical RA states can be observed. In one study by Hess et al., transgenic mice overexpressing human TNFα showed higher BOLD signals to heat stimulation in brain regions such as the somatosensory cortex (Hess et al., 2011). This heightened CNS response was suppressed with anti-TNFα treatment, which is in accord with the clinical findings described above. In future preclinical neuroimaging investigations, it would be of interest to determine if joint compression, which may be more relevant to clinical RA, yields similar responses to thermal stimuli, or if the collagen-induced arthritis (CIA) model, a commonly utilized model in preclinical RA investigations, was assessed with fMRI. Moreover, results from studies implementing 18F-FDG PET/CT and 11C-PK11195 PET/CT in the Complete Freund's Adjuvant (CFA) model showed robust glucose metabolism and macrophage activity in inflamed joints (Chandrupatla et al., 2015), a finding similarly observed in the CIA model (Cha et al., 2012) and RA patients (Chaudhari et al., 2016; Suto et al., 2016). Such outcomes suggest that it may be feasible to simultaneous track peripheral inflammatory processes with central ones mediating pain processing in RA.
Translational Assessment (Table 6)
Table 6.
Validity of fMRI Based Assessment of RA
| Construct Validity | Spontaneous remission of RA joint pathology (limb swelling) especially in some preclinical RA models may occur. Thus, differentiation of fMRI response changes resulting from treatment or normal disease fluctuation may be difficult. |
| Face Validity | An abnormal response to experimental pain (heat, pressure or mechanical stimulation) may be specific to an RA pain state. CNS responses to experimental pain may not importantly reflect evoked pain induced by joint movement or manipulation (compression). |
| Content Validity | Using a combination of evoked and resting-state fMRI, distinct CNS circuits mediating sensation, affect, function or motivation can be simultaneously probed under an RA pain state. Sensory and affective responses specific to RA joint movement or manipulation may be possible. |
| Predictive Validity | While key peripheral (joint inflammation) and central (aberrant responses to sensory stimuli) pathologies are present in preclinical RA models and RA patients; it is unknown if clinically efficacious therapeutic strategies (anti-TNFα or anti-IL6) yield common fMRI signatures between preclinical and clinical settings. |
The use of fMRI in RA patients, be it at rest or during evoked pain condition, has not been a widespread effort. From the limited clinical studies performed, it is known that limbic and somatosensory circuits primarily show enhanced responses to noxious joint compression - a functional response modulated with treatment (i.e., anti-TNFα antibodies). In both the preclinical and clinical setting, it remains unknown if and how factors such as inflammation or impairments in joint mobility longitudinally track with brain function. Also, whether pharmacodynamic effects observed with fMRI in RA populations back-translate to preclinical RA models has yet to be concretely determined.
Clinical Conditions in Need of Translational Neuroimaging Datasets
For some clinical pain conditions, a substantial body of neuroimaging work in the clinical domain exists, yet the corresponding studies in the respective preclinical pain models are absent all together. Two examples of this circumstance are Complex Regional Pain Syndrome (CRPS) and Fibromyalgia. Interestingly, a central phenotype is thought to be key to the overall pathophysiology for both conditions. By exemplifying the CNS properties elucidated from clinical neuroimaging studies, it is hoped that impetus is provided to pursue preclinical pain imaging studies in models of CRPS or Fibromyalgia.
Complex Regional Pain Syndrome (CRPS)
Details on the CRPS Phenotype can be found in Supplementary Material and Supplemental Table 5.
Imaging CRPS
Resting-state fMRI has revealed a modulation within the DMN (Baliki et al., 2014; Bolwerk et al., 2013), with a particular reduction in connectivity between the medial prefrontal cortex (MPFC) to the posterior constituents of the DMN. Other alterations in functional connectivity were related to pain intensity ratings, particularly the increased connectivity of the MPFC to the insular cortex. Supporting these resting-state fMRI results and highlighting the role of the insula in CRPS, gray matter atrophy in the right insula, right ventromedial prefrontal cortex (VMPFC) and right nucleus accumbens have been reported (Geha et al., 2008). Relatedly, 18F-FDG-PET has shown increased glucose metabolism in many pain-related brain regions (Shiraishi et al., 2006), while magneto-encephalographic (MEG) studies at rest have demonstrated abnormal electrophysiology in the orbitofrontal–temporal cortices and somatosensory cortex, which respectively hint at deficits in affective pain perception and pain localization in CRPS patients (Walton et al., 2010). Although the results centered around the somatosensory cortex were challenged in favor of adaptive cortical changes of the healthy hand (Di Pietro et al., 2015), the contralateral, primary somatosensory cortex response during innocuous tactile stimulation of the affected CRPS hand was repeatedly reduced in fMRI (Pleger et al., 2006) as well as MEG (Juottonen et al., 2002; Maihofner et al., 2003) investigations. This impairment was correlated to tactile acuity and pain. The manifestation of an allodynic state can also be traced to central mechanisms. A study assessing allodynia in CRPS found that brush stimulation on the non-affected hand expectedly activated contralateral primary and secondary somatosensory cortices in addition to the bilateral insula. In contrast, allodynia (brushing the affected hand) led to much more activation within contralateral primary somatosensory, primary motor, parietal association, bilateral secondary somatosensory, insular, frontal, and cingulate cortices (Freund et al., 2010). Allodynic ratings were specifically correlated to activation in the primary somatosensory, bilateral secondary somatosensory, parietal, insular and cingulate cortices. Interestingly, successful treatment of CRPS induces a reversal of central reorganization in addition to aberrant contralateral primary somatosensory and anterior cingulate cortex activity induced by moving the painful hand and during nociceptive processing (Gustin et al., 2010; Maihofner et al., 2004). The observed normalizing-type effect in the CNS may signify the utility of predominantly cortical function as an imaging biomarker of disease activity or therapeutic effect specific to CRPS.
Investigating the motor function with fMRI in CRPS patients with dystonia has found that the imaginary movement of the affected hand also induced less activation ipsilaterally in the premotor, prefrontal cortex, frontal operculum, anterior insula and superior temporal cortices. Contralaterally, reduced activation was found in the inferior parietal and adjacent primary somatosensory cortex (Gieteling et al., 2008). There were no differences between patients and controls when they executed movements, nor when they imagined moving their unaffected hand. In contrast, fMRI of non-dystonic CRPS patients described increased activation in several areas of the central motor circuit during a motor task of the affected hand. Subsequent covariate analyses demonstrated that specific activation of the posterior parietal cortices (i.e. intraparietal sulcus), supplementary motor area and the primary motor cortex were related to the extent of active motor dysfunction (Maihofner et al., 2007).
An understanding of the evolution and devolution of CRPS pain-related brain changes can provide insights into brain plasticity as it relates to pain persistence or resilience. Pediatric CRPS provides a major opportunity to explore these issues given that the brain is (i.) more plastic and therefore adaptive; (ii.) most pediatric CRPS gets symptomatically better and (iii) the potential to evaluate reversal of functional and structural brain changes (Becerra et al., 2014; Erpelding et al., 2016). Moreover, changes specific to emotional circuitry in children with CRPS (Simons et al., 2015) may offer insights regarding affective disorders possessing onset in adulthood (e.g., depression, anxiety and recrudescence of pain).
Translational Assessment
Preclinical models of CRPS (Coderre et al., 2004; Guo et al., 2004; Siegel et al., 2007) have not been evaluated using a functional brain imaging approach. From a clinical perspective, modulation of somatosensory and motor cortex in addition to the insula appears as a consistent phenotype of CRPS; which is reflective of the presence of pain and prevalent motor deficits in this patient population. Therefore, testing hypotheses centered around sensory and motor systems in the preclinical setting could be fruitful in terms of assessing validity of CRPS models.
Fibromyalgia
Details on the Fibromyalgia Pain Phenotype can be found in Supplementary Material and Supplemental Table 6.
Imaging Fibromyalgia
A central etiology for fibromyalgia pain appears highly plausible considering the body of neuroimaging work showing modulations in neurotransmitter system function (i.e., dopamine and μ-opioid receptors) (Albrecht et al., 2016; Harris et al., 2009), and the several functional studies outlined below describing abnormal brain responses to pain and sensory stimuli. Initial work by Clauw and colleagues demonstrated that compared to controls, fibromyalgia patients subjected to a normally non-noxious pressure stimulation, possessed both heightened behavioral pain measures and BOLD activity in regions such as the insula, putamen and somatosensory cortex (Gracely et al., 2002). More recent investigations involving resting-state fMRI, evoked pain fMRI and MR spectroscopy have further implicated the role of the insula in fibromyalgia pain (Harris et al., 2008; Ichesco et al., 2016; Ichesco et al., 2014; Pujol et al., 2014). A common trend observed in these studies has been the presence of altered levels of functional connectivity between the insula and structures of the DMN and executive network. While atypical evoked functional responses and functional connectivity strengths involving other brain regions (e.g., medial prefrontal and secondary somatosensory cortices) are noted, the functional relationship amongst the insula and DMN can be considered particularly important for fibromyalgia given its correlation with clinical pain levels (Pujol et al., 2014). Based on this correlation, one would hypothesize that ‘effective’ analgesics used to treat fibromyalgia pain may directly or indirectly alter function of the insula and DMN. Such pharmacodynamic effects in the CNS have indeed been shown (Harris et al., 2013; Harte et al., 2016; Jensen et al., 2014; Kim et al., 2013; Lopez-Sola et al., 2010). For example, Harris et al. recently reported pregabalin-induced decreases in insular glutamate and glutamine levels and decreases in functional connectivity amongst the insula and constituents of the DMN. Jensen et al., demonstrated higher posterior cingulate cortex activity during evoked pressure stimulation in milnacipran responders compared to non-responders. Despite these central pharmacodynamic observations, the findings must be viewed in the context of clinical value of the analgesics tested. As with most analgesics, pain relief in fibromyalgia with drugs such as pregabalin yield moderate to substantial analgesia in only a minority of patients (Hauser et al., 2010; Straube et al., 2010). Moreover, other symptoms (fatigue, memory and mood) that markedly affect quality of life for fibromyalgia patients, but indirectly relate to pain levels experienced, may not be impacted by currently available analgesics. Therefore, therapies targeting other peripheral and central systems or mechanisms must be considered.
Translational Assessment
Preclinical models of fibromyalgia (Gibson et al., 2009; Sluka et al., 2001; Sluka and Rasmussen, 2010; Xiao et al., 2015) have not been evaluated using a functional brain imaging approach. A dominant feature stemming from the clinical fibromyalgia imaging literature is the involvement of the insula and DMN structures. While examining insular function and morphology in preclinical fibromyalgia models seems logical, interpretation of imaging data should be performed with caution considering inter-species differences involving this structure (Craig, 2009). Moreover, it remains unknown how normally innocuous stimuli (e.g., changes in temperature, low or high levels of activity) elicit a robust pain state in fibromyalgia patients. Understanding how these environmental triggers are correlative or causative to CNS function in a fibromyalgia pain state should be probed.
Conclusions
Functional brain imaging has been widely used across many clinical pain conditions, and to a lesser extent, in corresponding preclinical pain models. The impetus for further utilizing the behavior of CNS circuits as translational units in pain and analgesia research stems from the body of neuroimaging results summarized herein demonstrating that CNS responses can be: (i) parallel between clinical and preclinical settings; (ii) unique to distinct types of pain or pain conditions; (iii) correlated with patient reported pain magnitude and duration; (vi) have a dynamic range amendable to a therapeutic challenge and (v) combined with other modalities (i.e., peripheral imaging) in order to provide an integrated view of pain or pain chronification. In conclusion, a neuroimaging approach for analyzing pain and analgesia can further our understanding of various pathological conditions harboring pain in conjunction with evaluating novel therapeutic strategies.
Supplementary Material
Highlights.
Most clinical measures informing on chronic pain are non-translatable to the preclinical setting
Parallels in CNS function are observed between preclinical & clinical pain states
Measuring CNS function may further the comprehension of pain and pain phenotypes
A translational neuroimaging assay in pain research may facilitate discovery of new analgesic
Acknowledgments
JU is currently an employee of Regeneron Pharmaceuticals. CG is supported by intramural funding from the University Medical Centre (UMM), Mainz and a grant from Eli Lilly. RH is currently an employee of Celgene Corporation. FB is supported by grants from the DFG Bi 579/8-1 and the Berufsgenossenschaft für das Gesundheitswesen, Mainz. DB is supported by US National Institutes of Health grant NINDS K24NS064050.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abaei M, Sagar DR, Stockley EG, Spicer CH, Prior M, Chapman V, Auer DP. Neural correlates of hyperalgesia in the monosodium iodoacetate model of osteoarthritis pain. Mol Pain. 2016;12 doi: 10.1177/1744806916642445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, MacKie PJ, Kareken DA, Hutchins GD, Chumin EJ, Christian BT, Yoder KK. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016;10:829–839. doi: 10.1007/s11682-015-9459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirmohseni S, Segelcke D, Reichl S, Wachsmuth L, Gorlich D, Faber C, Pogatzki-Zahn E. Characterization of incisional and inflammatory pain in rats using functional tools of MRI. Neuroimage. 2016;127:110–122. doi: 10.1016/j.neuroimage.2015.11.052. [DOI] [PubMed] [Google Scholar]
- Andrews N, Harper S, Issop Y, Rice AS. Novel, nonreflex tests detect analgesic action in rodents at clinically relevant concentrations. Ann N Y Acad Sci. 2011;1245:11–13. doi: 10.1111/j.1749-6632.2011.06342.x. [DOI] [PubMed] [Google Scholar]
- Asad AB, Seah S, Baumgartner R, Feng D, Jensen A, Manigbas E, Henry B, Houghton A, Evelhoch JL, Derbyshire SW, Chin CL. Distinct BOLD fMRI Responses of Capsaicin-Induced Thermal Sensation Reveal Pain-Related Brain Activation in Nonhuman Primates. PLoS One. 2016;11:e0156805. doi: 10.1371/journal.pone.0156805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Sanganahalli BG, Herman P, Blumenfeld H, Gjedde A, Hyder F. Analysis of time and space invariance of BOLD responses in the rat visual system. Cereb Cortex. 2013;23:210–222. doi: 10.1093/cercor/bhs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastick AN, Runhaar J, Belo JN, Bierma-Zeinstra SM. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17:152. doi: 10.1186/s13075-015-0670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Bishop J, Barmettler G, Kainz V, Burstein R, Borsook D. Brain network alterations in the inflammatory soup animal model of migraine. Brain Res. 2017 doi: 10.1016/j.brainres.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Bishop J, Barmettler G, Xie Y, Navratilova E, Porreca F, Borsook D. Triptans disrupt brain networks and promote stress-induced CSD-like responses in cortical and subcortical areas. J Neurophysiol. 2016;115:208–217. doi: 10.1152/jn.00632.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Pendse G, Chang PC, Bishop J, Borsook D. Robust reproducible resting state networks in the awake rodent brain. PLoS One. 2011;6:e25701. doi: 10.1371/journal.pone.0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Sava S, Simons LE, Drosos AM, Sethna N, Berde C, Lebel AA, Borsook D. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 2014;6:347–369. doi: 10.1016/j.nicl.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Upadhyay J, Chang PC, Bishop J, Anderson J, Baumgartner R, Schwarz AJ, Coimbra A, Wallin D, Nutile L, George E, Maier G, Sunkaraneni S, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Borsook D. Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J Pharmacol Exp Ther. 2013;345:41–51. doi: 10.1124/jpet.112.201145. [DOI] [PubMed] [Google Scholar]
- Becker R, Braun U, Schwarz AJ, Gass N, Schweiger JI, Weber-Fahr W, Schenker E, Spedding M, Clemm von Hohenberg C, Risterucci C, Zang Z, Grimm O, Tost H, Sartorius A, Meyer-Lindenberg A. Species-conserved reconfigurations of brain network topology induced by ketamine. Transl Psychiatry. 2016;6:e786. doi: 10.1038/tp.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera D, Behera S, Jacobs KE, Biswal S. Bilateral peripheral neural activity observed in vivo following unilateral nerve injury. Am J Nucl Med Mol Imaging. 2013;3:282–290. [PMC free article] [PubMed] [Google Scholar]
- Behera D, Jacobs KE, Behera S, Rosenberg J, Biswal S. (18)F-FDG PET/MRI can be used to identify injured peripheral nerves in a model of neuropathic pain. J Nucl Med. 2011;52:1308–1312. doi: 10.2967/jnumed.110.084731. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, Silva AC, Stein EA. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci. 2013;33:16796–16804. doi: 10.1523/JNEUROSCI.3146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Chung JM, Honore M, Seltzer Z. Models of neuropathic pain in the rat. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0914s22. Chapter 9, Unit 9 14. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB, Group A. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–1533. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Migraine chronification. Curr Neurol Neurosci Rep. 2011;11:139–148. doi: 10.1007/s11910-010-0175-6. [DOI] [PubMed] [Google Scholar]
- Birklein F, Riedl B, Claus D, Neundorfer B. Pattern of autonomic dysfunction in time course of complex regional pain syndrome. Clin Auton Res. 1998a;8:79–85. doi: 10.1007/BF02267817. [DOI] [PubMed] [Google Scholar]
- Birklein F, Riedl B, Neundorfer B, Handwerker HO. Sympathetic vasoconstrictor reflex pattern in patients with complex regional pain syndrome. Pain. 1998b;75:93–100. doi: 10.1016/S0304-3959(97)00209-1. [DOI] [PubMed] [Google Scholar]
- Boland EG, Selvarajah D, Hunter M, Ezaydi Y, Tesfaye S, Ahmedzai SH, Snowden JA, Wilkinson ID. Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PLoS One. 2014;9:e96474. doi: 10.1371/journal.pone.0096474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwerk A, Seifert F, Maihofner C. Altered resting-state functional connectivity in complex regional pain syndrome. J Pain. 2013;14:1107–1115. e1108. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L. CNS animal fMRI in pain and analgesia. Neurosci Biobehav Rev. 2011;35:1125–1143. doi: 10.1016/j.neubiorev.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Pendse G, Morris S, Cole SH, Aiello-Lammens M, Scrivani S, Becerra LR. Comparison of evoked vs. spontaneous tics in a patient with trigeminal neuralgia (tic doloureux) Mol Pain. 2007;3:34. doi: 10.1186/1744-8069-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Veggeberg R, Erpelding N, Borra R, Linnman C, Burstein R, Becerra L. The Insula: A "Hub of Activity" in Migraine. Neuroscientist. 2016;22:632–652. doi: 10.1177/1073858415601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, Hook KE, Juneau PL, Connor JR, Kilgore KS. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14:1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia. 2015;35:1025–1030. doi: 10.1177/0333102414566860. [DOI] [PubMed] [Google Scholar]
- Brown CA, El-Deredy W, Jones AK. When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci. 2014;39:663–672. doi: 10.1111/ejn.12420. [DOI] [PubMed] [Google Scholar]
- Buckner JH, Nepom GT. Genetics of rheumatoid arthritis: is there a scientific explanation for the human leukocyte antigen association? Curr Opin Rheumatol. 2002;14:254–259. doi: 10.1097/00002281-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JH, Lee SH, Lee SW, Park K, Moon DH, Kim K, Biswal S. Assessment of collagen-induced arthritis using cyanine 5.5 conjugated with hydrophobically modified glycol chitosan nanoparticles: correlation with 18F-fluorodeoxyglucose positron emission tomography data. Korean J Radiol. 2012;13:450–457. doi: 10.3348/kjr.2012.13.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrupatla DM, Weijers K, Gent YY, de Greeuw I, Lammertsma AA, Jansen G, van der Laken CJ, Molthoff CF. Sustained macrophage infiltration upon multiple intra-articular injections: an improved rat model of rheumatoid arthritis for PET guided therapy evaluation. Biomed Res Int. 2015;2015:509295. doi: 10.1155/2015/509295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Procissi D, Bao Q, Centeno MV, Baria A, Apkarian AV. Novel method for functional brain imaging in awake minimally restrained rats. J Neurophysiol. 2016;116:61–80. doi: 10.1152/jn.01078.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari AJ, Ferrero A, Godinez F, Yang K, Shelton DK, Hunter JC, Naguwa SM, Boone JM, Raychaudhuri SP, Badawi RD. High-resolution (18)F-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: findings of a prospective pilot study. Br J Radiol. 2016;89:20160138. doi: 10.1259/bjr.20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapparini L, Ferraro S, Grazzi L, Bussone G. Neuroimaging in chronic migraine. Neurol Sci. 2010;31(Suppl 1):S19–22. doi: 10.1007/s10072-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Chong CD, Gaw N, Fu Y, Li J, Wu T, Schwedt TJ. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia. 2016a doi: 10.1177/0333102416652091. [DOI] [PubMed] [Google Scholar]
- Chong CD, Schwedt TJ, Dodick DW. Migraine: What Imaging Reveals. Curr Neurol Neurosci Rep. 2016b;16:64. doi: 10.1007/s11910-016-0662-5. [DOI] [PubMed] [Google Scholar]
- Clauw DJ. What is the meaning of "small fiber neuropathy" in fibromyalgia? Pain. 2015;156:2115–2116. doi: 10.1097/j.pain.0000000000000311. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Colleoni M, Sacerdote P. Murine models of human neuropathic pain. Biochim Biophys Acta. 2010;1802:924–933. doi: 10.1016/j.bbadis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Costa C, Tozzi A, Rainero I, Cupini LM, Calabresi P, Ayata C, Sarchielli P. Cortical spreading depression as a target for anti-migraine agents. J Headache Pain. 2013;14:62. doi: 10.1186/1129-2377-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam WJ, Condon L, Alshuft H, Reckziegel D, Auer DP. Associations of limbicaffective brain activity and severity of ongoing chronic arthritis pain are explained by trait anxiety. Neuroimage Clin. 2016;12:269–276. doi: 10.1016/j.nicl.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. A rat is not a monkey is not a human: comment on Mogil (Nature Rev. Neurosci. 10, 283–294 (2009)) Nat Rev Neurosci. 2009;10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J. Measurement of visual sensitivity in migraine: Validation of two scales and correlation with visual cortex activation. Cephalalgia. 2015;35:585–592. doi: 10.1177/0333102414547782. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52:2146–2158. doi: 10.1002/art.21155. [DOI] [PubMed] [Google Scholar]
- Demarquay G, Mauguiere F. Central Nervous System Underpinnings of Sensory Hypersensitivity in Migraine: Insights from Neuroimaging and Electrophysiological Studies. Headache. 2016;56:1418–1438. doi: 10.1111/head.12651. [DOI] [PubMed] [Google Scholar]
- DeSouza DD, Davis KD, Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain. 2015;156:1112–1123. doi: 10.1097/j.pain.0000000000000156. [DOI] [PubMed] [Google Scholar]
- Di Pietro F, Stanton TR, Moseley GL, Lotze M, McAuley JH. Interhemispheric somatosensory differences in chronic pain reflect abnormality of the healthy side. Hum Brain Mapp. 2015;36:508–518. doi: 10.1002/hbm.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff EP, Vennart W, Wise RG, Howard MA, Harris RE, Lee M, Wartolowska K, Wanigasekera V, Wilson FJ, Whitlock M, Tracey I, Woolrich MW, Smith SM. Learning to identify CNS drug action and efficacy using multistudy fMRI data. Sci Transl Med. 2015;7:274ra216. doi: 10.1126/scitranslmed.3008438. [DOI] [PubMed] [Google Scholar]
- Egsgaard LL, Eskehave TN, Bay-Jensen AC, Hoeck HC, Arendt-Nielsen L. Identifying specific profiles in patients with different degrees of painful knee osteoarthritis based on serological biochemical and mechanistic pain biomarkers: a diagnostic approach based on cluster analysis. Pain. 2015;156:96–107. doi: 10.1016/j.pain.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Yuzawa I, Qin T, Wang Y, Baek K, Kim YR, Hoffmann U, Dilekoz E, Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice. J Neurosci. 2011;31:5755–5763. doi: 10.1523/JNEUROSCI.5346-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I, Group CS. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding N, Simons L, Lebel A, Serrano P, Pielech M, Prabhu S, Becerra L, Borsook D. Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct Funct. 2016;221:1095–1111. doi: 10.1007/s00429-014-0957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P. Intrinsic Brain Connectivity in Chronic Pain: A Resting-State fMRI Study in Patients with Rheumatoid Arthritis. Front Hum Neurosci. 2016;10:107. doi: 10.3389/fnhum.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkert PG, Vessey ML, Park SS, Gelboin HV, Cole SP. Cytochromes P-450 in murine lung. An immunohistochemical study with monoclonal antibodies. Drug Metab Dispos. 1989;17:551–555. [PubMed] [Google Scholar]
- Freund W, Wunderlich AP, Stuber G, Mayer F, Steffen P, Mentzel M, Weber F, Schmitz B. Different activation of opercular and posterior cingulate cortex (PCC) in patients with complex regional pain syndrome (CRPS I) compared with healthy controls during perception of electrically induced pain: a functional MRI study. Clin J Pain. 2010;26:339–347. doi: 10.1097/AJP.0b013e3181cb4055. [DOI] [PubMed] [Google Scholar]
- Gauffin J, Hankama T, Kautiainen H, Hannonen P, Haanpaa M. Neuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort study. BMC Neurol. 2013;13:21. doi: 10.1186/1471-2377-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber C, Breimhorst M, Burbach B, Egenolf C, Baier B, Fechir M, Koerber J, Treede RD, Vogt T, Birklein F. Pain in chemotherapy-induced neuropathy--more than neuropathic? Pain. 2013;154:2877–2887. doi: 10.1016/j.pain.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Geha P, Waxman SG. Pain Perception: Multiple Matrices or One? JAMA Neurol. 2016;73:628–630. doi: 10.1001/jamaneurol.2016.0757. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Taguchi T, Mizumura K, Graven-Nielsen T. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308. doi: 10.1007/s00221-008-1699-8. [DOI] [PubMed] [Google Scholar]
- Gieteling EW, van Rijn MA, de Jong BM, Hoogduin JM, Renken R, van Hilten JJ, Leenders KL. Cerebral activation during motor imagery in complex regional pain syndrome type 1 with dystonia. Pain. 2008;134:302–309. doi: 10.1016/j.pain.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Gil-Gouveia R, Oliveira AG, Martins IP. Subjective Cognitive Symptoms During a Migraine Attack: A Prospective Study of a Clinic-Based Sample. Pain Physician. 2016;19:E137–150. [PubMed] [Google Scholar]
- Gimenez M, Pujol J, Ali Z, Lopez-Sola M, Contreras-Rodriguez O, Deus J, Ortiz H, Soriano-Mas C, Llorente-Onaindia J, Monfort J. Naproxen effects on brain response to painful pressure stimulation in patients with knee osteoarthritis: a double-blind, randomized, placebo-controlled, single-dose study. J Rheumatol. 2014;41:2240–2248. doi: 10.3899/jrheum.131367. [DOI] [PubMed] [Google Scholar]
- Governo RJ, Morris PG, Marsden CA, Chapman V. Gabapentin evoked changes in functional activity in nociceptive regions in the brain of the anaesthetized rat: an fMRI study. Br J Pharmacol. 2008;153:1558–1567. doi: 10.1038/bjp.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 2016;127:496–509. doi: 10.1016/j.neuroimage.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Green AL, Gu P, De Felice M, Dodick D, Ossipov MH, Porreca F. Increased susceptibility to cortical spreading depression in an animal model of medication-overuse headache. Cephalalgia. 2014;34:594–604. doi: 10.1177/0333102413515344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signalling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Schwarz A, Birbaumer N, Sines N, Schmidt AC, Veit R, Larbig W, Flor H, Lotze M. NMDA-receptor antagonist and morphine decrease CRPS-pain and cerebral pain representation. Pain. 2010;151:69–76. doi: 10.1016/j.pain.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, Tinelli E, Mainero C. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33:1264–1268. doi: 10.1177/0333102413490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on muopioid receptors (MORs) Neuroimage. 2009;47:1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain. 2016;157:1933–1945. doi: 10.1097/j.pain.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 2010;11:505–521. doi: 10.1016/j.jpain.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, Straub RH, Sporns O, Doerfler A, Brune K, Schett G. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108:3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu T, Nakanishi K, Yoshimura S, Yoshino A, Adachi N, Okamoto Y, Yamawaki S, Ochi M. The dorsolateral prefrontal network is involved in pain perception in knee osteoarthritis patients. Neurosci Lett. 2014;581:109–114. doi: 10.1016/j.neulet.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Hirsch AR. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache. 2005;45:763–764. doi: 10.1111/j.1526-4610.2005.05147_2.x. author reply 764. [DOI] [PubMed] [Google Scholar]
- Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–654. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Hooker BA, Tobon G, Baker SJ, Zhu C, Hesterman J, Schmidt K, Rajagovindan R, Chandran P, Joshi SK, Bannon AW, Hoppin J, Beaver J, Fox GB, Day M, Upadhyay J. Gabapentin-induced pharmacodynamic effects in the spinal nerve ligation model of neuropathic pain. Eur J Pain. 2014;18:223–237. doi: 10.1002/j.1532-2149.2013.00364.x. [DOI] [PubMed] [Google Scholar]
- Horsten NC, Ursum J, Roorda LD, van Schaardenburg D, Dekker J, Hoeksma AF. Prevalence of hand symptoms, impairments and activity limitations in rheumatoid arthritis in relation to disease duration. J Rehabil Med. 2010;42:916–921. doi: 10.2340/16501977-0619. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–486. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- Houben T, Loonen IC, Baca SM, Schenke M, Meijer JH, Ferrari MD, Terwindt GM, Voskuyl RA, Charles A, van den Maagdenberg AM, Tolner EA. Optogenetic induction of cortical spreading depression in anesthetized and freely behaving mice. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard A, Amin FM, Arngrim N, Vlachou M, Larsen VA, Larsson HB, Ashina M. Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin. 2016;11:322–327. doi: 10.1016/j.nicl.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Sanders D, Krause K, O'Muircheartaigh J, Fotopoulou A, Zelaya F, Thacker M, Massat N, Huggins JP, Vennart W, Choy E, Daniels M, Williams SC. Alterations in resting-state regional cerebral blood flow demonstrate ongoing pain in osteoarthritis: An arterial spin-labeled magnetic resonance imaging study. Arthritis Rheum. 2012;64:3936–3946. doi: 10.1002/art.37685. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Khan SA, Xu S, Cha M, Masri R, Seminowicz DA. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: a longitudinal MRI study. Neuroimage. 2015;107:333–344. doi: 10.1016/j.neuroimage.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Ichesco E, Puiu T, Hampson JP, Kairys AE, Clauw DJ, Harte SE, Peltier SJ, Harris RE, Schmidt-Wilcke T. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain. 2016;20:1079–1089. doi: 10.1002/ejp.832. [DOI] [PubMed] [Google Scholar]
- Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014;15:815–826. e811. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N, Momosaki S, Fujita M, Omachi S, Yamato H, Kimura M, Kanegawa N, Shinohara S, Abe K. [11C]PK11195 PET imaging of spinal glial activation after nerve injury in rats. Neuroimage. 2013;79:121–128. doi: 10.1016/j.neuroimage.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2015;23:925–932. doi: 10.1016/j.joca.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Valcour VG, Nir TM, Kohannim O, Busovaca E, Nicolas K, Thompson PM. Disrupted brain networks in the aging HIV+ population. Brain Connect. 2012;2:335–344. doi: 10.1089/brain.2012.0105-Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage. 2012;62:1072–1085. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Petzke F, Carville S, Choy E, Fransson P, Gracely RH, Vitton O, Marcus H, Williams SC, Ingvar M, Kosek E. Segregating the cerebral mechanisms of antidepressants and placebo in fibromyalgia. J Pain. 2014;15:1328–1337. doi: 10.1016/j.jpain.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Jeong KY, Kim HM, Kang JH. Investigation of the functional difference between the pathological itching and neuropathic pain-induced rat brain using manganese-enhanced MRI. Acta Radiol. 2016;57:861–868. doi: 10.1177/0284185115604514. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckers E, Delgado y Palacios R, Shah D, Guglielmetti C, Verhoye M, Van der Linden A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn Reson Med. 2014;72:1103–1112. doi: 10.1002/mrm.24990. [DOI] [PubMed] [Google Scholar]
- Jonckers E, Shah D, Hamaide J, Verhoye M, Van der Linden A. The power of using functional fMRI on small rodents to study brain pharmacology and disease. Front Pharmacol. 2015;6:231. doi: 10.3389/fphar.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckers E, Van Audekerke J, De Visscher G, Van der Linden A, Verhoye M. Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One. 2011;6:e18876. doi: 10.1371/journal.pone.0018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Derbyshire SW. Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:601–607. doi: 10.1136/ard.56.10.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juottonen K, Gockel M, Silen T, Hurri H, Hari R, Forss N. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Jutzeler CR, Freund P, Huber E, Curt A, Kramer JL. Neuropathic Pain and Functional Reorganization in the Primary Sensorimotor Cortex After Spinal Cord Injury. J Pain. 2015;16:1256–1267. doi: 10.1016/j.jpain.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Jutzeler CR, Huber E, Callaghan MF, Luechinger R, Curt A, Kramer JL, Freund P. Association of pain and CNS structural changes after spinal cord injury. Sci Rep. 2016;6:18534. doi: 10.1038/srep18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallberg H, Padyukov L, Plenge RM, Ronnelid J, Gregersen PK, van der Helm-van Mil AH, Toes RE, Huizinga TW, Klareskog L, Alfredsson L, Epidemiological Investigation of Rheumatoid Arthritis study, g Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007;80:867–875. doi: 10.1086/516736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Agalave NM, Svensson CI. Pattern recognition receptors in chronic pain: Mechanisms and therapeutic implications. Eur J Pharmacol. 2016;788:261–273. doi: 10.1016/j.ejphar.2016.06.039. [DOI] [PubMed] [Google Scholar]
- Keltner JR, Connolly CG, Vaida F, Jenkinson M, Fennema-Notestine C, Archibald S, Akkari C, Schlein A, Lee J, Wang D, Kim S, Li H, Rennels A, Miller DJ, Kesidis G, Franklin DR, Sanders C, Corkran S, Grant I, Brown GG, Atkinson JH, Ellis RJ, Group C. HIV Distal Neuropathic Pain Is Associated with Smaller Ventral Posterior Cingulate Cortex. Pain Med 2016 [Google Scholar]
- Keltner JR, Fennema-Notestine C, Vaida F, Wang D, Franklin DR, Dworkin RH, Sanders C, McCutchan JA, Archibald SL, Miller DJ, Kesidis G, Cushman C, Kim SM, Abramson I, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Corkran S, Cherner M, Duarte NA, Alexander T, Robinson-Papp J, Gelman BB, Simpson DM, Collier AC, Marra CM, Morgello S, Brown G, Grant I, Atkinson JH, Jernigan TL, Ellis RJ, Group C. HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol. 2014;20:209–218. doi: 10.1007/s13365-014-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Lee S, Mun CW. Evaluation of the effectiveness of pregabalin in alleviating pain associated with fibromyalgia: using functional magnetic resonance imaging study. PLoS One. 2013;8:e74099. doi: 10.1371/journal.pone.0074099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop SM, ten Klooster PM, Vonkeman HE, Steunebrink LM, van de Laar MA. Neuropathic-like pain features and cross-sectional associations in rheumatoid arthritis. Arthritis Res Ther. 2015;17:237. doi: 10.1186/s13075-015-0761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneap Minn) 2012;18:1319–1337. doi: 10.1212/01.CON.0000423849.24900.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The Neural Code for Pain: From Single-Cell Electrophysiology to the Dynamic Pain Connectome. Neuroscientist. 2016 doi: 10.1177/1073858416667716. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- Lai TH, Chou KH, Fuh JL, Lee PL, Kung YC, Lin CP, Wang SJ. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia. 2016;36:1324–1333. doi: 10.1177/0333102416630593. [DOI] [PubMed] [Google Scholar]
- Lasko L, Huang X, Voorbach MJ, Lewis LG, Stavropoulos J, Carriker J, Seifert TR, Baker SJ, Upadhyay J. Multimodal assessment of nervous and immune system responses following sciatic nerve injury. Pain. 2013;154:2782–2793. doi: 10.1016/j.pain.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lin RL, Garcia RG, Kim J, Kim H, Loggia ML, Mawla I, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V. Reduced insula habituation associated with amplification of trigeminal brainstem input in migraine. Cephalalgia. 2016 doi: 10.1177/0333102416665223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27:917–922. doi: 10.1111/j.1460-9568.2008.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Edwards RR, Kim J, Vangel MG, Wasan AD, Gollub RL, Harris RE, Park K, Napadow V. Disentangling linear and nonlinear brain responses to evoked deep tissue pain. Pain. 2012;153:2140–2151. doi: 10.1016/j.pain.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sola M, Pujol J, Hernandez-Ribas R, Harrison BJ, Contreras-Rodriguez O, Soriano-Mas C, Deus J, Ortiz H, Menchon JM, Vallejo J, Cardoner N. Effects of duloxetine treatment on brain response to painful stimulation in major depressive disorder. Neuropsychopharmacology. 2010;35:2305–2317. doi: 10.1038/npp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad C, Haanes KA, Grande G, Edvinsson L. Experimental inflammation following dural application of complete Freund's adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J Headache Pain. 2015;16:91. doi: 10.1186/s10194-015-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- Maleki N, Barmettler G, Moulton EA, Scrivani S, Veggeberg R, Spierings EL, Burstein R, Becerra L, Borsook D. Female migraineurs show lack of insular thinning with age. Pain. 2015;156:1232–1239. doi: 10.1097/j.pain.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012a;32:607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Gollub RL. What Have We Learned From Brain Functional Connectivity Studies in Migraine Headache? Headache. 2016;56:453–461. doi: 10.1111/head.12756. [DOI] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012b;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin. 2015;7:347–358. doi: 10.1016/j.nicl.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleborough MJ, Ekstrand C, Gould L, Lorentz EJ, Ellchuk T, Babyn P, Borowsky R. Attentional Network Differences Between Migraineurs and Non-migraine Controls: fMRI Evidence. Brain Topogr. 2016;29:419–428. doi: 10.1007/s10548-015-0459-x. [DOI] [PubMed] [Google Scholar]
- Mickleborough MJ, Hayward J, Chapman C, Chung J, Handy TC. Reflexive attentional orienting in migraineurs: The behavioral implications of hyperexcitable visual cortex. Cephalalgia. 2011;31:1642–1651. doi: 10.1177/0333102411425864. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Sakuma Y, Orita S, Oikawa Y, Aoki Y, Toyone T, Takahashi K, Inoue G, Ohtori S. Assessment of pain behavior in a rat model of intervertebral disc injury using the CatWalk gait analysis system. Spine (Phila Pa 1976) 2013;38:1459–1465. doi: 10.1097/BRS.0b013e318299536a. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Moreton BJ, Tew V, das Nair R, Wheeler M, Walsh DA, Lincoln NB. Pain phenotype in patients with knee osteoarthritis: classification and measurement properties of painDETECT and self-report Leeds assessment of neuropathic symptoms and signs scale in a cross-sectional study. Arthritis Care Res (Hoboken) 2015;67:519–528. doi: 10.1002/acr.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, Burstein R, Borsook D. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011a;21:435–448. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011b;31:3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the "pain matrix". Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Muralidharan A, Kuo A, Jacob M, Lourdesamy JS, Carvalho LM, Nicholson JR, Corradini L, Smith MT. Comparison of Burrowing and Stimuli-Evoked Pain Behaviors as End-Points in Rat Models of Inflammatory Pain and Peripheral Neuropathic Pain. Front Behav Neurosci. 2016;10:88. doi: 10.3389/fnbeh.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, Woolf C, Niu J, Bradley LA, Quinn E, Law LF. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016;68:654–661. doi: 10.1002/art.39488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam DM, Lai KL, Fuh JL, Chuang CY, Chen WT, Wang SJ. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2016;36:53–66. doi: 10.1177/0333102415583144. [DOI] [PubMed] [Google Scholar]
- Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139:1971–1986. doi: 10.1093/brain/aww119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O'Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ. Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. J Clin Oncol. 2016;34:677–683. doi: 10.1200/JCO.2015.62.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill TW, Parkes MJ, Maricar N, Marjanovic EJ, Hodgson R, Gait AD, Cootes TF, Hutchinson CE, Felson DT. Synovial tissue volume: a treatment target in knee osteoarthritis (OA) Ann Rheum Dis. 2016;75:84–90. doi: 10.1136/annrheumdis-2014-206927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Parise M, Kubo TT, Doring TM, Tukamoto G, Vincent M, Gasparetto EL. Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain. 2014;15:17. doi: 10.1186/1129-2377-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Sandor K, McQueen J, Woller SA, Svensson CI, Corr M, Yaksh TL. The effect of gabapentin and ketorolac on allodynia and conditioned place preference in antibodyinduced inflammation. Eur J Pain. 2016;20:917–925. doi: 10.1002/ejp.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AJ, Chronicle EP, Maylor EA, Bruce LA. Cognitive function is not impaired in people with a long history of migraine: a blinded study. Cephalalgia. 2006;26:74–80. doi: 10.1111/j.1468-2982.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Piscaer TM, Muller C, Mindt TL, Lubberts E, Verhaar JA, Krenning EP, Schibli R, De Jong M, Weinans H. Imaging of activated macrophages in experimental osteoarthritis using folate-targeted animal single-photon-emission computed tomography/computed tomography. Arthritis Rheum. 2011;63:1898–1907. doi: 10.1002/art.30363. [DOI] [PubMed] [Google Scholar]
- Piscaer TM, Sandker M, van der Jagt OP, Verhaar JA, de Jong M, Weinans H. Realtime assessment of bone metabolism in small animal models for osteoarthritis using multi pinhole-SPECT/CT. Osteoarthritis Cartilage. 2013;21:882–888. doi: 10.1016/j.joca.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Pleger B, Ragert P, Schwenkreis P, Forster AF, Wilimzig C, Dinse H, Nicolas V, Maier C, Tegenthoff M. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage. 2006;32:503–510. doi: 10.1016/j.neuroimage.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Pujol J, Macia D, Garcia-Fontanals A, Blanco-Hinojo L, Lopez-Sola M, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014;155:1492–1503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Rech J, Hess A, Finzel S, Kreitz S, Sergeeva M, Englbrecht M, Doerfler A, Saake M, Schett G. Association of brain functional magnetic resonance activity with response to tumor necrosis factor inhibition in rheumatoid arthritis. Arthritis Rheum. 2013;65:325–333. doi: 10.1002/art.37761. [DOI] [PubMed] [Google Scholar]
- Riederer F, Marti M, Luechinger R, Lanzenberger R, von Meyenburg J, Gantenbein AR, Pirrotta R, Gaul C, Kollias S, Sandor PS. Grey matter changes associated with medication-overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatry. 2012;13:517–525. doi: 10.3109/15622975.2012.665175. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS One. 2013;8:e54475. doi: 10.1371/journal.pone.0054475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Fields HL. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain. 1996;119(Pt 2):347–354. doi: 10.1093/brain/119.2.347. [DOI] [PubMed] [Google Scholar]
- Russo A, Esposito F, Conte F, Fratello M, Caiazzo G, Marcuccio L, Giordano A, Tedeschi G, Tessitore A. Functional interictal changes of pain processing in migraine with ictal cutaneous allodynia. Cephalalgia. 2016 doi: 10.1177/0333102416644969. [DOI] [PubMed] [Google Scholar]
- Saag KG, Cerhan JR, Kolluri S, Ohashi K, Hunninghake GW, Schwartz DA. Cigarette smoking and rheumatoid arthritis severity. Ann Rheum Dis. 1997;56:463–469. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Krause K, O'Muircheartaigh J, Thacker MA, Huggins JP, Vennart W, Massat NJ, Choy E, Williams SC, Howard MA. Pharmacologic modulation of hand pain in osteoarthritis: a double-blind placebo-controlled functional magnetic resonance imaging study using naproxen. Arthritis Rheumatol. 2015;67:741–751. doi: 10.1002/art.38987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherens A, Maier C, Haussleiter IS, Schwenkreis P, Vlckova-Moravcova E, Baron R, Sommer C. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain. 2009;13:711–718. doi: 10.1016/j.ejpain.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Schlegel F, Schroeter A, Rudin M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: Implications on analysis of mouse fMRI data. Neuroimage. 2015;116:40–49. doi: 10.1016/j.neuroimage.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- Schroeder MP, Weiss C, Procissi D, Disterhoft JF, Wang L. Intrinsic connectivity of neural networks in the awake rabbit. Neuroimage. 2016;129:260–267. doi: 10.1016/j.neuroimage.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–1993. doi: 10.1093/brain/aww097. [DOI] [PubMed] [Google Scholar]
- Schuster NM, Rapoport AM. New strategies for the treatment and prevention of primary headache disorders. Nat Rev Neurol. 2016;12:635–650. doi: 10.1038/nrneurol.2016.143. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015;10:e0116687. doi: 10.1371/journal.pone.0116687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. Neuroimage. 2008;40:759–766. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Scrivani S, Wallin D, Moulton EA, Cole S, Wasan AD, Lockerman L, Bajwa Z, Upadhyay J, Becerra L, Borsook D. A fMRI evaluation of lamotrigine for the treatment of trigeminal neuropathic pain: pilot study. Pain Med. 2010;11:920–941. doi: 10.1111/j.1526-4637.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- Seah S, Asad AB, Baumgartner R, Feng D, Williams DS, Manigbas E, Beaver JD, Reese T, Henry B, Evelhoch JL, Chin CL. Investigation of cross-species translatability of pharmacological MRI in awake nonhuman primate - a buprenorphine challenge study. PLoS One. 2014;9:e110432. doi: 10.1371/journal.pone.0110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas D, Palace J, Tracey I. Chronic pain disrupts the reward circuitry in multiple sclerosis. Eur J Neurosci. 2016;44:1928–1934. doi: 10.1111/ejn.13272. [DOI] [PubMed] [Google Scholar]
- Selvarajah D, Wilkinson ID, Emery CJ, Harris ND, Shaw PJ, Witte DR, Griffiths PD, Tesfaye S. Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care. 2006;29:2664–2669. doi: 10.2337/dc06-0650. [DOI] [PubMed] [Google Scholar]
- Selvarajah D, Wilkinson ID, Maxwell M, Davies J, Sankar A, Boland E, Gandhi R, Tracey I, Tesfaye S. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care. 2014;37:1681–1688. doi: 10.2337/dc13-2610. [DOI] [PubMed] [Google Scholar]
- Shiraishi S, Kobayashi H, Nihashi T, Kato K, Iwano S, Nishino M, Ishigaki T, Ikeda M, Kato T, Ito K, Kimura T. Cerebral glucose metabolism change in patients with complex regional pain syndrome: a PET study. Radiat Med. 2006;24:335–344. doi: 10.1007/s11604-006-0035-0. [DOI] [PubMed] [Google Scholar]
- Siegel SM, Lee JW, Oaklander AL. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth Analg. 2007;105:1820–1829. doi: 10.1213/01.ane.0000295234.21892.bc. table of contents. [DOI] [PubMed] [Google Scholar]
- Simons LE, Erpelding N, Hernandez JM, Serrano P, Zhang K, Lebel AA, Sethna NF, Berde CB, Prabhu SP, Becerra L, Borsook D. Fear and Reward Circuit Alterations in Pediatric CRPS. Front Hum Neurosci. 2015;9:703. doi: 10.3389/fnhum.2015.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofat N, Smee C, Hermansson M, Howard M, Baker EH, Howe FA, Barrick TR. Functional MRI demonstrates pain perception in hand osteoarthritis has features of central pain processing. J Biomed Graph Comput. 2013;3 doi: 10.5430/jbgc.v3n4p20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, Lahvis GP, Lattal KM, Mitchell SH, David SV, Fryer JD, Nigg JT, Fair DA. Large-scale topology and the default mode network in the mouse connectome. Proc Natl Acad Sci U S A. 2014;111:18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia. 2013;33:256–265. doi: 10.1177/0333102412470215. [DOI] [PubMed] [Google Scholar]
- Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford) 2010;49:706–715. doi: 10.1093/rheumatology/kep432. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Khan HS, Bosma RL, Cotoi AI, Leung R, Cadotte DW, Fehlings MG. Changes in Pain Processing in the Spinal Cord and Brainstem after Spinal Cord Injury Characterized by Functional Magnetic Resonance Imaging. J Neurotrauma. 2016;33:1450–1460. doi: 10.1089/neu.2015.4257. [DOI] [PubMed] [Google Scholar]
- Suto T, Okamura K, Yonemoto Y, Okura C, Tsushima Y, Takagishi K. Prediction of Large Joint Destruction in Patients With Rheumatoid Arthritis Using 18F-FDG PET/CT and Disease Activity Score. Medicine (Baltimore) 2016;95:e2841. doi: 10.1097/MD.0000000000002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Dongmin K, Nagase M, Saitoh Y, Someya T, Sekino M. An MRI-compatible and quantifiable mechanical stimulator for allodynia in a rat model of neuropathic pain. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4298–4301. doi: 10.1109/EMBC.2015.7319345. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Yamashita A, Horiuchi H, Furuya M, Yanase M, Niikura K, Imai S, Hatakeyama N, Kinoshita H, Tsukiyama Y, Senba E, Matoba M, Kuzumaki N, Yamazaki M, Suzuki T, Narita M. Effects of gabapentin on brain hyperactivity related to pain and sleep disturbance under a neuropathic pain-like state using fMRI and brain wave analysis. Synapse. 2011;65:668–676. doi: 10.1002/syn.20898. [DOI] [PubMed] [Google Scholar]
- Taylor P, Manger B, Alvaro-Gracia J, Johnstone R, Gomez-Reino J, Eberhardt E, Wolfe F, Schwartzman S, Furfaro N, Kavanaugh A. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res. 2010;38:1213–1224. doi: 10.1177/147323001003800402. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Russo A, Conte F, Giordano A, De Stefano M, Lavorgna L, Corbo D, Caiazzo G, Esposito F, Tedeschi G. Abnormal Connectivity Within Executive Resting-State Network in Migraine With Aura. Headache. 2015;55:794–805. doi: 10.1111/head.12587. [DOI] [PubMed] [Google Scholar]
- Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS, Bennett DL. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Jahanshad N. Novel Neuroimaging Methods to Understand How HIV Affects the Brain. Curr HIV/AIDS Rep. 2015;12:289–298. doi: 10.1007/s11904-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PW, Carr AJ. Pain in the rheumatic diseases. Ann Rheum Dis. 1997;56:395. doi: 10.1136/ard.56.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans EJ, van der Pas S, Schaap LA, Sanchez-Martinez M, Zambon S, Peter R, Pedersen NL, Dennison EM, Denkinger M, Castell MV, Siviero P, Herbolsheimer F, Edwards MH, Otero A, Deeg DJ. Self-perceived weather sensitivity and joint pain in older people with osteoarthritis in six European countries: results from the European Project on OSteoArthritis (EPOSA) BMC Musculoskelet Disord. 2014;15:66. doi: 10.1186/1471-2474-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Johns E. The pain matrix: reloaded or reborn as we image tonic pain using arterial spin labelling. Pain. 2010;148:359–360. doi: 10.1016/j.pain.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Tseng MT, Chiang MC, Chao CC, Tseng WY, Hsieh ST. fMRI evidence of degeneration-induced neuropathic pain in diabetes: enhanced limbic and striatal activations. Hum Brain Mapp. 2013;34:2733–2746. doi: 10.1002/hbm.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng MT, Kong Y, Chiang MC, Chao CC, Tseng WY, Hsieh ST. Brain imaging signatures of the relationship between epidermal nerve fibers and heat pain perception. Neuroimage. 2015;122:288–297. doi: 10.1016/j.neuroimage.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Tso AR, Trujillo A, Guo CC, Goadsby PJ, Seeley WW. The anterior insula shows heightened interictal intrinsic connectivity in migraine without aura. Neurology. 2015;84:1043–1050. doi: 10.1212/WNL.0000000000001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Anderson J, Schwarz AJ, Coimbra A, Baumgartner R, Pendse G, George E, Nutile L, Wallin D, Bishop J, Neni S, Maier G, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Becerra L, Borsook D. Imaging drugs with and without clinical analgesic efficacy. Neuropsychopharmacology. 2011a;36:2659–2673. doi: 10.1038/npp.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Chandran P, Miller L, Lee Y, Marek GJ, Sakoglu U, Chin CL, Luo F, Fox GB, Day M. Default-mode-like network activation in awake rodents. PLoS One. 2011b;6:e27839. doi: 10.1371/journal.pone.0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Rajagovindan R, Hart M, Chandran P, Hooker BA, Cassar S, Mikusa JP, Tovcimak A, Wald MJ, Joshi SK, Bannon A, Medema JK, Beaver J, Honore P, Kamath RV, Fox GB, Day M. Pharmacological modulation of brain activity in a preclinical model of osteoarthritis. Neuroimage. 2013;64:341–355. doi: 10.1016/j.neuroimage.2012.08.084. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Lemme J, Anderson J, Bleakman D, Large T, Evelhoch JL, Hargreaves R, Borsook D, Becerra L. Test-retest reliability of evoked heat stimulation BOLD fMRI. J Neurosci Methods. 2015;253:38–46. doi: 10.1016/j.jneumeth.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Pendse G, Anderson J, Schwarz AJ, Baumgartner R, Coimbra A, Bishop J, Knudsen J, George E, Grachev I, Iyengar S, Bleakman D, Hargreaves R, Borsook D, Becerra L. Improved characterization of BOLD responses for evoked sensory stimuli. Neuroimage. 2010;49:2275–2286. doi: 10.1016/j.neuroimage.2009.10.053. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, Barrett CF, Gherardini L, van de Ven RC, Todorov B, Broos LA, Tottene A, Gao Z, Fodor M, De Zeeuw CI, Frants RR, Plesnila N, Plomp JJ, Pietrobon D, Ferrari MD. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Dubois M, Llinas RR. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010;150:41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Guan Q, Wan L, Yi Y, Liu CF. Altered regional homogeneity of spontaneous brain activity in idiopathic trigeminal neuralgia. Neuropsychiatr Dis Treat. 2015;11:2659–2666. doi: 10.2147/NDT.S94877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64:371–379. doi: 10.1002/art.33326. [DOI] [PubMed] [Google Scholar]
- Wiebe LA, Phillips TJ, Li JM, Allen JA, Shetty K. Pain in HIV: an evolving epidemic. J Pain. 2011;12:619–624. doi: 10.1016/j.jpain.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Lei J, Ye G, Xu H, You HJ. Role of thalamic nuclei in the modulation of Fos expression within the cerebral cortex during hypertonic saline-induced muscle nociception. Neuroscience. 2015;304:36–46. doi: 10.1016/j.neuroscience.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, Borsook D, Porreca F. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain. 2014;155:1659–1666. doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Welsh D, Williams M, Coimbra A, Urban MO, Hargreaves R, Evelhoch J, Williams DS. fMRI of pain processing in the brain: a within-animal comparative study of BOLD vs. CBV and noxious electrical vs. noxious mechanical stimulation in rat. Neuroimage. 2012;59:1168–1179. doi: 10.1016/j.neuroimage.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Zhao F, Williams M, Bowlby M, Houghton A, Hargreaves R, Evelhoch J, Williams DS. Qualification of fMRI as a biomarker for pain in anesthetized rats by comparison with behavioral response in conscious rats. Neuroimage. 2014;84:724–732. doi: 10.1016/j.neuroimage.2013.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.