Abstract
Background
Environmental enrichment (EE) confers benefits after traumatic brain injury (TBI) when provided daily for 6-hrs or greater, but not 2-hr or 4-hr, which more accurately reflects the daily amount of clinical rehabilitation. The lack of benefit with sub-therapeutic EE suggests that augmentation with galantamine (GAL), which enhances cognition after TBI, may be indicated to confer benefits.
Objective
To test the hypothesis that 2-hr and 4-hr of EE paired with GAL will provide benefits comparable to 24-hr EE alone. Moreover, all EE groups will perform better than the standard (STD)-housed GAL group.
Methods
Anesthetized rats received a TBI or sham injury and then were randomized to receive intraperitoneal injections of GAL (2 mg/kg) or saline vehicle (VEH; 1 mL/kg) beginning 24-hr after surgery and once daily while receiving EE for 2-hr, 4-hr, or 24-hr. Motor and cognitive assessments were conducted on post-operative days 1–5 and 14–19, respectively.
Results
Motor function was significantly improved in the TBI+24-hr EE group vs. the TBI+STD+VEH and TBI+STD+GAL groups [p<0.05]. Cognitive performance was enhanced in all EE groups, as well as in the TBI+STD+GAL vs. TBI+STD+VEH [p<0.05]. Moreover, the 2-hr EE and 4-hr EE groups receiving GAL did not differ from the 24-hr EE group [p>0.05] and performed better than GAL alone [p<0.05].
Conclusions
The findings support the hypothesis and have clinical relevance as often only brief rehabilitation may be available in the clinic and thus augmenting with a pharmacotherapy like GAL may lead to outcomes that are significantly better than either therapy alone.
Keywords: behavioral outcome, controlled cortical impact, environmental enrichment, functional recovery, galantamine, learning and memory, Morris water maze, traumatic brain injury
Introduction
An estimated 10 million people worldwide incur a traumatic brain injury (TBI) each year.1 While mild TBI may not consistently produce salient motor and cognitive disability, moderate-to-severe brain trauma results in substantial and persistent neurological impairments.2–4 The costs associated with acute medical care, long-term rehabilitation, and the loss of productivity resulting from the inability to regain pre-injury employment significantly taxes the health care system, which is estimated in the billions of dollars each year.5–7 To combat this problem, numerous preclinical studies evaluating a plethora of potential therapies have been conducted in the pursuit of elucidating effective strategies to promote cognitive and/or motor recovery after TBI.8–10
While many pharmacological agents have been found to confer significant improvement in the laboratory 8–11, few have translated to the clinic 12–14 suggesting that single therapeutic approaches may not be sufficient to promote recovery from an injury that is multifaceted.8,15 Hence, the inclusion of additional therapeutic manipulations, such as exercise 16–18, behavioral combinations 19,20, and environmental enrichment (EE), which can mimic clinical rehabilitation are warranted.
EE is an experimental paradigm designed to mimic the novelty and complexity of rehabilitation used with humans in a rat model where the environment and the range of activities are more limited. The exploratory (i.e., exposure to multiple objects of differing shape and size that can be manipulated), physical (i.e., vast living environment with ample room for walking, running, and climbing), and social (group-housing) elements provided by EE are considered enriching.11,21 This unique milieu is markedly different from the standard (STD) housing paradigm where paired subjects live in traditional-sized laboratory cages and receive only basic sustenance (i.e., food and water). Numerous studies have demonstrated that EE, provided continuously after experimental TBI, confers motor, cognitive, and histological benefits relative to STD-housed controls.21–27 It has also been shown that even relatively brief periods of EE after TBI produce effects that are comparable to the continuous EE paradigm in both male 28 and female 29 rats. However, when EE is reduced further and limited to just 2-hr or 4-hr per day the paradigm is rendered insufficient to promote benefits.28–29 Given that rehabilitation session times reported in the clinical literature range from 2–8 hours per day 30–32, and perhaps are more likely to fall within the lower range due to costs and resources, it is necessary to design preclinical studies such that sub-therapeutic EE can promote benefits. Demonstrating that significant benefits can be achieved with an EE paradigm that parallels the clinic in terms of time allotments would further validate EE as a preclinical model of neurorehabilitation.
One viable and clinically relevant approach is to augment EE with pharmacotherapies particularly because in the clinical rehabilitation setting many TBI patients have cognitive impairments that impede their ability to comprehend and function effectively and therefore necessitate pharmacological intervention. Because GAL has recently been shown to facilitate cognitive function after experimental TBI 33 and has also been reported in clinical studies to enhance vigilance and attention leading to better general function 34, significantly improve episodic memory 35, and enhance cognition for up to 12 months in Alzheimer’s disease 36, it is a logical choice as a treatment that could be combined with sub-therapeutic EE to determine whether synergism would lead to better outcomes relative to GAL treatment alone.
Hence, the specific aim of this study was to test the hypothesis that sub-therapeutic EE (i.e., 2-hr and 4-hr) combined with GAL will confer benefits beyond that of GAL alone and will be comparable to continuous EE. If the hypothesis is supported, the findings will refine, enhance, and further support the EE paradigm as a viable preclinical model of neurorehabilitation, particularly because it would mimic clinical rehabilitation, which is often limited in duration and in many instances augmented by pharmacotherapies.
Materials and methods
Subjects and pre-surgical procedures
Sixty-three adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1 °C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with food and water available ad libitum. After 1 week of acclimatization the rats underwent beam-walk training and then were randomly assigned to TBI and sham groups receiving STD housing, 2-hr, 4-hr, or continuous (24-hr) EE plus VEH or GAL treatment. Hence, the final group composition consisted of TBI + STD + VEH (1 mL/kg; n=9), TBI + STD + GAL (2 mg/kg; n=9), TBI + EE + VEH (1 mL/kg; n=9), TBI + 2-hr EE + GAL (2 mg/kg; n=9), TBI + 4-hr EE + GAL (2 mg/kg; n=9), and respective sham controls (n=18).
Surgery and acute neurological evaluation
Surgical anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane (IsoFlo®, Abbott Laboratories, North Chicago, IL), respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. After endotracheal intubation the rats were secured in a stereotaxic frame and ventilated mechanically during surgery. Utilizing aseptic techniques, a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6-mm in diameter) was made in the right hemisphere (encompassing bregma and lambda and between the sagittal suture and the coronal ridge) with a Michele trephine. The resulting bone flap was removed and the craniectomy was enlarged further with rongeurs. Controlled cortical impact (CCI) injury was produced in rats weighing 300–325 gm as previously described.37–44 Briefly, the impacting shaft was extended and the impact tip was centered and lowered over the craniectomy until it contacted the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/s). Immediately after the CCI, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome before being placed in a temporary cage until the effects of anesthesia waned (as indicated by spontaneous movement). Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately following the termination of anesthesia by gently squeezing the rats’ paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by quantifying the time required to turn from the supine to prone position on three consecutive trials. These neurological indices are used to determine the level of injury severity.42–44
Housing conditions: standard and environmental manipulation
After the effects of anesthesia waned (as evidenced by spontaneous movement in the holding cage), the rats were returned to the colony where those designated for continuous enrichment (i.e., 24-hr group) were immediately placed in an EE, which consists of a stainless steel-wire cage (91×76×50 cm) with three levels and ladders to ambulate from one level to another and contains various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water.42,45 To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was approximately every 3 days. Ten to 12 rats, which included both TBI and sham controls, as well as VEH and GAL-treated were housed in the EE at any given time. Rats in the STD conditions were placed in standard steel-wire mesh cages (37×25×18 cm, 2 rats per cage) with only food and water. The rats receiving the abbreviated enrichment sessions were removed from the STD cages and placed in the EE cages for 2-hr or 4-hr and then returned to the STD conditions. These rehabilitative manipulations occurred each day for 19 days.
Drug administration
GAL (Sigma-Aldrich, St. Louis, MO) was prepared daily by dissolving in sterile saline, which also served as the VEH. GAL (2 mg/kg) or a comparable volume of VEH (1 mL/kg) was administered intraperitoneally beginning 24 hr after cortical impact or sham injury and once daily for 19 days. On the days of behavioral testing the injections were administered 1 hr prior to testing. The dose of GAL was selected based on published data from our laboratory showing a beneficial effect.33 The route of administration is standard protocol in our laboratory.38–41
Motor performance (beam-balance and beam-walk)
Established beam-balance and beam-walk tasks were utilized to assess motor function. Beam-balancing consists of placing the rat on an elevated (90 cm) narrow wooden beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 s. Beam-walking, originally devised by Feeney et al. (1982),46 and used extensively in our laboratory,38–44 consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and white noise by traversing an elevated narrow wooden beam (2.5 cm × 100 cm) and entering a darkened goal box situated at the opposite end. The aversive stimuli (light and noise) were terminated when the rat entered the goal box, thus serving as reinforcement (reward) for completing the task. Performance on the beam-walk consisted of recording the time to traverse the beam. Rats were tested on these motor tasks 1-hr prior to surgery to establish baseline performance and then again on post-operative days 1–5. Three trials (60 s allotted time) were provided per day on each task and the average daily scores for each subject were used in the statistical analyses.
Cognitive function: acquisition of spatial learning
Spatial learning was assessed in a Morris water maze task 47 that has been shown to be sensitive to cognitive function/dysfunction after TBI.38–44 Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) positioned 26 cm from the maze wall in the southwest quadrant. Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). On day 19 the platform was raised 2 cm above the water line and marked with white tape, which made it visible to the rats and served as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the escape platform within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: probe trial (memory retention)
One day after the final acquisition training session (day 19), all rats were given a probe trial to measure retention. Briefly, the platform was removed from the pool and the rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., target quadrant) and allowed to freely explore the pool for 30 s. Typically, rats that have learned the specific location of the escape platform exhibit a spatial bias and spend significantly more time in the target quadrant. The time spent in target quadrant was recorded using a spontaneous motor activity recording and tracking system. Swim speed was also recorded during this testing period. The percent time spent in the target quadrant was used in the statistical analysis.
All experimental procedures were preapproved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. All efforts were made to limit the number of rats used as well as to minimize their discomfort.
Data analysis
Statistical analyses were performed on data collected by observers blinded to drug treatment conditions using Statview 5.0.1 software. The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). Acute neurological assessments, swim speed, and visible platform performance were analyzed by one-factor ANOVAs. When the overall ANOVAs revealed a significant effect, the Newman-Keuls post-hoc test was utilized to determine specific group differences. The data are presented as the mean ± standard error of the mean (S.E.M.) and are considered significant when corresponding p values are ≤ 0.05.
Results
There were no significant differences in any assessment among the sham control groups, regardless of housing condition or treatment, and thus the data were pooled into a single SHAM group. Additionally, there were no variables that precluded accurate assessments and thus the statistical analyses are based on all sixty-three rats.
Acute neurological evaluation
No significant differences were observed among the TBI groups for return of hind limb reflex ability after a brief paw pinch (range for right: 166.9 ± 18.8 sec to 194.1 ± 9.2 sec; left range = 174.0 ± 18.6 sec to 200.5 ± 9.6 sec) or righting reflex latency (range = 333.3 ± 36.1 sec to 397.5 ± 36.0 sec) following the termination of anesthesia [p’s > 0.05]. The lack of neurological differences among the TBI groups indicates that all rats received similar levels of anesthesia and injury severity.
Motor performance: beam-balance
No baseline differences were observed among the groups as all rats balanced on the beam for the allotted 60 sec (Fig. 1). Post-TBI, the repeated-measures ANOVA revealed significant Group [F5,57 = 2.759, p = 0.027] and Day [F5,285 = 23.564, p < 0.0001] differences, as well as a significant Group x Day interaction [F25,285 = 2.412, p = 0.0003]. The post-hoc analysis revealed that the SHAM controls, which were able to balance for the full 60 sec were significantly better than the TBI+STD+VEH, TBI+STD+GAL, and TBI+4-hr EE+GAL groups [p’s < 0.05], but not the TBI+EE+VEH and TBI+2-hr EE+GAL groups [p’s > 0.05]. Over the course of the 5 days of testing, all TBI groups improved, however, the TBI+EE+VEH group reached baseline performance quicker than the TBI+STD+VEH and TBI+STD+GAL groups [p’s < 0.05], and did not differ from either the TBI+2-hr EE+GAL or TBI+4-hr EE+GAL groups [p’s > 0.05].
Fig. 1.
Mean (± S.E.M.) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or sham injury. There were no significant differences among the sham groups and thus the data were pooled. *p < 0.05 vs. TBI + STD + VEH and TBI + STD + GAL. #p < 0.05 vs. TBI + STD + VEH, TBI + STD + GAL, and TBI + 4-hr EE + GAL groups. No difference was revealed between the TBI + EE + VEH vs. the TBI + 2-hr EE + GAL or TBI + 4-hr EE + GAL groups [p’s > 0.05].
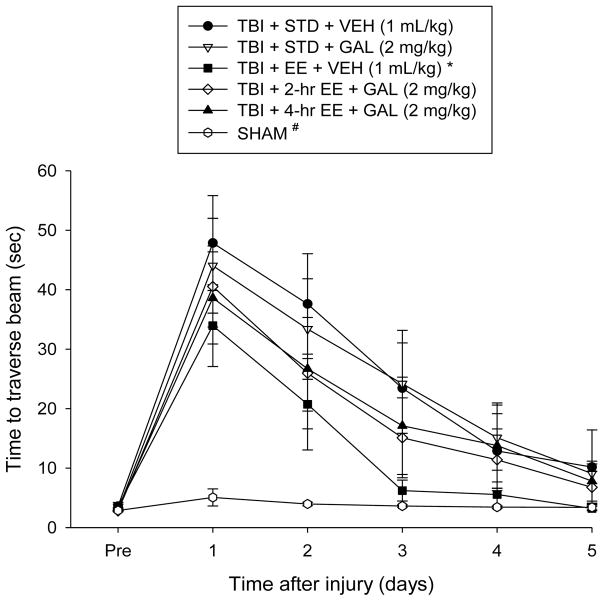
Motor performance: beam-walk
No significant differences were observed among any of the groups in the time to traverse the beam prior to surgery (Fig. 2). However, after surgery, the repeated-measures ANOVA revealed significant Group [F5,57 = 5.393, p = 0.0004] and Day [F5,285 = 65.276, p < 0.0001] differences, as well as a significant Group x Day interaction [F25,285 = 4.128, p < 0.0001]. The post-hoc analysis indicated that all TBI groups were significantly impaired compared to the SHAM controls [p’s < 0.05]. Like the beam-balance findings, the TBI+EE+VEH group recovered better than the TBI+STD+VEH and TBI+STD+GAL groups [p’s < 0.05], and did not differ from either the TBI+2-hr EE+GAL or TBI+4-hr EE+GAL groups [p’s > 0.05].
Fig. 2.
Mean (± S.E.M.) time (sec) to traverse an elevated narrow beam prior to, and after, TBI or sham injury. There were no significant differences among the sham groups and thus the data were pooled. *p < 0.05 vs. TBI + STD + VEH and TBI + STD + GAL. #p < 0.05 vs. all TBI groups, regardless of treatment. No difference was revealed between the TBI + EE + VEH vs. the TBI + 2-hr EE + GAL or TBI + 4-hr EE + GAL groups [p’s > 0.05].
Cognitive function: acquisition of spatial learning
Analysis of the spatial learning data revealed significant Group [F5,57 = 3.119, p = 0.0148] and Day [F4,228 = 37.157, p < 0.0001] differences. The post-hoc analysis revealed that the SHAM group was better than the TBI+STD+VEH and TBI+STD+GAL groups [p’s < 0.05], but did not differ from the TBI+EE+VEH, TBI+2-hr EE+GAL, and TBI+4-hr EE+GAL groups [p’s > 0.05]. The TBI+EE+VEH, TBI+2-hr EE+GAL, and TBI+4-hr EE+GAL groups were markedly better at learning the location of the escape platform relative to both the TBI+STD+VEH and TBI+STD+GAL groups [p’s < 0.05], but did not differ from one another [p > 0.05]. Additionally, TBI+STD+GAL group was better than the TBI+STD+VEH group [p < 0.05; Fig. 3]. No significant differences in swim speed (range = 28.5 ± 1.7 cm/sec to 31.4 ± 1.4 cm/sec) or time to reach the visible platform were observed among the TBI or SHAM groups [p’s > 0.05].
Fig. 3.
Mean (± S.E.M.) time (sec) to locate a hidden and visible platform in the water maze. There were no significant differences among the sham groups and thus the data were pooled. *p < 0.05 vs. TBI + STD + VEH. ^, #p < 0.05 vs. TBI + STD + VEH and TBI + STD + GAL. No difference was revealed between the TBI + EE + VEH vs. the TBI + 2-hr EE + GAL or TBI + 4-hr EE + GAL groups [p’s > 0.05]. No significant differences were revealed among the groups in the visible platform assessment.
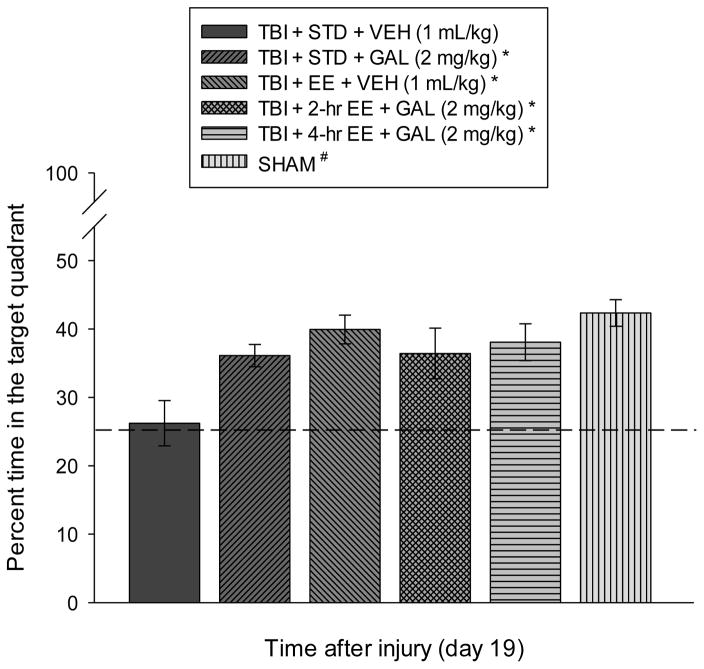
Cognitive function: probe trial
Analysis of the probe trial (i.e., memory retention) data revealed a significant Group effect [F5,57 = 4.717, p = 0.0011]. The post-hoc analysis revealed that the SHAM control, TBI+EE+VEH, TBI+2-hr EE+GAL, TBI+4-hr EE+GAL, and TBI+STD+GAL groups spent a greater percentage of the allotted time in the target quadrant (42.3 ± 1.9 %, 39.9 ± 2.1 %, 36.4 ± 3.7 %, and 38.1 ± 2.7 %, respectively) vs. the TBI + STD (26.2 ± 3.3 %) group [p’s < 0.05], but did not differ from one another [p’s > 0.05; Fig. 4].
Fig. 4.
Mean (± S.E.M.) percent time spent in the target quadrant (i.e., where escape platform was previously located) following a single probe trial 19 days after TBI or sham injury. *, #p < 0.05 vs. TBI + STD + VEH. No other comparisons were significant [p > 0.05]. The dotted line represents performance at the chance level (25%).
Discussion
The aim of the study was to test the hypothesis that EE abridged to the point where it becomes sub-therapeutic can still be transformed into an effective rehabilitative paradigm by combining it with a clinically relevant pharmacotherapy like GAL. In support of this hypothesis, the data showed that 2-hr or 4-hr of EE combined with GAL was as effective as continuous EE in promoting cognitive benefits after a CCI injury of moderate severity in adult male rats. The ameliorative effects were not due solely to GAL as the abbreviated EE groups combined with GAL performed better relative to the GAL-only group suggesting that the combination lead to a synergistic effect resulting in increased performance. This additive effect was observed even after minimal enrichment (i.e., 2-hr) as there were no differences between the GAL-treated 2-hr EE and 4-hr EE groups.
The augmented benefits with the combination of GAL and abbreviated EE therapy is contrary to previous reports where various pharmacotherapies provided in conjunction with continuous EE in adult male rats did not produce benefits beyond those of the individual therapies.33,40–42,44 The lack of additional benefit when combining effective drug therapies with the typical EE paradigm is theorized to be due to the robustness of continuous EE affording little room for additional recovery. To this end, the results from the current study indicate that the rehabilitation-relevant abbreviated EE paradigm may provide an experimental model with which to test combination treatments that include limited or sub-therapeutic EE and various potentially efficacious pharmacotherapies. Moreover, the paradigm may have clinical relevance because rehabilitation is not provided continuously in the clinic, but rather may range from 2–8 hr per day.30–32 Indeed, a recent study required patients to engage for a minimum of 3 hr of physical therapy, occupational therapy, or speech-language pathology each day for 5 days per week.48
GAL is a dual-functioning drug that increases acetylcholine (ACh) levels and stimulates nicotinic ACh receptors (nAChRs) on neurons and glia 49 in brain regions such as the frontal cortex and hippocampus 50 that are subject to injury after clinical or experimental TBI.51,52 Stimulation of microglial nAChRs may be involved in GAL’s inhibitory effects on inflammation and oxidative stress 53,54 as the nicotinic antagonist mecamylamine blocks the GAL-mediated reduction of inflammatory cytokines and subsequent cognitive recovery after brain injury.55 GAL increases synaptic plasticity, as evidenced by enhanced long-term potentiation.56 Moreover, pharmacological enhancement of α7nAChR activation has been shown to produce potent neuroprotective and anti-inflammatory effects by reducing blood brain barrier permeability, TUNEL-positive apoptotic cells, and reactive gliosis after TBI.57 Additionally, GAL increases firing activity of dopaminergic cells in the ventral tegmental area and dopamine output from the prefrontal cortex.58 These GAL-induced influences on dopamine-mediated function are intriguing as pharmacotherapies with D2 receptor agonist properties have been shown in preclinical and clinical studies to enhance neurobehavior after TBI.38,39,43,44,59
Potential mechanisms of EE are plentiful 11,21 and consist of, but are not limited to, protection of the cholinergic system by reducing TBI-induced ChAT-positive cell loss 40, a reduction of CA1 and CA3 cell loss after TBI 11,21, and induction of hippocampal and cortical plasticity, which includes long-term potentiation.60 EE also produces higher levels of nerve growth factor mRNA in the hippocampus, increases brain derived neurotrophic factor gene upregulation and progenitor cell survival, enhances dendritic branching, and reduces markers of oxidative stress and inflammation (for comprehensive review see Bondi and colleagues).11,21
Aside from the potential mechanisms attributed to GAL or EE alone or in combination that may have led to the robust cognitive benefits observed, 11,21 GAL could also be increasing the arousal of the TBI rats during their time in EE, thus making shorter periods of EE (i.e., rehabilitation) as effective as longer periods through increased interaction with the multiple and various cage accoutrements (i.e., novel stimuli) thus providing additional cognitive therapy. As shown in previous studies 28,29 there appears to be a threshold amount of EE necessary to confer benefit, with 6-hr being sufficient, but 2-hr and 4-hr being ineffective. Thus, increased arousal during the time spent in EE may be successful in lowering this threshold. Another possibility is that the increased dopaminergic or cholinergic activity induced with GAL may be promoting neural remodeling, and subsequently enhancing the effect of EE outside of any increase in arousal.
In conclusion, abbreviated EE and GAL produce benefits that are comparable to the robust, albeit not clinically relevant, continuous EE approach. While we acknowledge that EE does not simulate the skill-based training elements of rehabilitation in humans, EE does provide a unique opportunity to examine the effects of novelty and complexity on functional recovery over time.
Acknowledgments
This work was supported, in part, by NIH grants HD069620, HD069620-S1, NS060005, NS084967 (AEK), NS094950, NS099683 (COB), the University of Pittsburgh Physicians/UPMC Academic Foundation, and the UMPC Rehabilitation Institute (COB).
Footnotes
There are no conflicts of interest to report
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. Neurorehabilitation. 2007;22:341–355. [PubMed] [Google Scholar]
- 2.Sosin DM, Sniezek JE, Waxweiler RJ. Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA. 1995;273:1778–1780. [PubMed] [Google Scholar]
- 3.Binder L. Persistent symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol. 1986;8:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- 4.Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- 5.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of longterm disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 6.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 7.Max W, Mackenzie EJ, Rice DP. Head injuries: costs and consequences. J Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- 8.Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokiko ON, Hamm RJ. A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 2007;21:259–274. doi: 10.1080/02699050701209964. [DOI] [PubMed] [Google Scholar]
- 10.Wheaton P, Mathias JL, Vink R. Impact of early pharmacotherapy treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J Clin Psychopharmacol. 2009;29:468–477. doi: 10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- 11.Bondi CO, Semple BD, Noble-Haeusslein LJ, Osier ND, Carlson SW, Dixon CE, Giza CC, Kline AE. Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neurosci Biobehav Rev. 2015;58:123–146. doi: 10.1016/j.neubiorev.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doppenberg EMR, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp K, Mutlak H, Smith WR, Shohami E, Stahel PF. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon DK. Unique challenges in clinical trials in traumatic brain injury. Crit Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 15.Margulies S, Hicks R. Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Kreber LA, Griesbach GS. The interplay between neuropathology and activity based rehabilitation after traumatic brain injury. Brain Res. 2016;1640:152–63. doi: 10.1016/j.brainres.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Adkins DL, Ferguson L, Lance S, Pevtsov A, McDonough K, Stamschror J, Jones TA, Kozlowski DA. Combining multiple types of motor rehabilitation enhances skilled forelimb use following experimental traumatic brain injury in rats. Neurorehabil Neural Repair. 2015;29:989–1000. doi: 10.1177/1545968315576577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combs HL, Jones TA, Kozlowski DA, Adkins DL. Combinatorial motor training results in functional reorganization of remaining motor cortex after controlled cortical impact in rats. J Neurotrauma. 2016;33:741–747. doi: 10.1089/neu.2015.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondi CO, Klitsch KC, Leary JB, Kline AE. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J Neurotrauma. 2014;31:873–888. doi: 10.1089/neu.2014.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briones TL, Woods J, Rogozinska M. Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol Commun. 2013;1:57. doi: 10.1186/2051-5960-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- 25.Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–637. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman AN, Malena RR, Westergom BP, Luthra P, Cheng JP, Aslam HA, Zafonte RD. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- 28.de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radabaugh HL, Carlson LJ, O’Neil DA, LaPorte MJ, Monaco CM, Cheng JP, de la Tremblaye PB, Lajud N, Bondi CO, Kline AE. Abbreviated environmental enrichment confers neurobehavioral, cognitive, and histological benefits in brain-injured female rats. Exp Neurol. 2016;286:61–68. doi: 10.1016/j.expneurol.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackerby WF. Intensity of rehabilitation and length of stay. Brain Inj. 1990;4:167–173. doi: 10.3109/02699059009026162. [DOI] [PubMed] [Google Scholar]
- 31.Shiel A, Burn JP, Henry D, Clark J, Wilson BA, Burnett ME, McLellan DL. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin Rehabil. 2001;15:501–514. doi: 10.1191/026921501680425225. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XL, Poon WS, Chan CC, Chan SS. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj. 2007;21:681–690. doi: 10.1080/02699050701468941. [DOI] [PubMed] [Google Scholar]
- 33.de la Tremblaye PB, Bondi CO, Lajud N, Cheng JP, Radabaugh HL, Kline AE. Galantamine and environmental enrichment enhance cognitive recovery after experimental traumatic brain injury but do not confer additional benefits when combined. J Neurotrauma. 2017;34:1610–1622. doi: 10.1089/neu.2016.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenovuo O. Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury – clinical experience in 111 patients. Prog Neuro-Psychopharmacol & Biol Psychiatry. 2005;29:61–67. doi: 10.1016/j.pnpbp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.McAllister TW, Zafonte R, Jain S, Flashman LA, George MS, Grant GA, Stein MB. Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology. 2016;41:1191–1198. doi: 10.1038/npp.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blesa R, Davidson M, Kurz A, Reichman W, van Baelen B, Schwalen S. Galantamine provides sustained benefits in patients with ‘advanced moderate’ Alzheimer’s disease for at least 12 months. Dement Geriatr Cogn Disord. 2003;15:79–87. doi: 10.1159/000067974. [DOI] [PubMed] [Google Scholar]
- 37.Bondi CO, Cheng JP, Tennant HM, Monaco CM, Kline AE. Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J Neurotrauma. 2014;31:926–937. doi: 10.1089/neu.2013.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- 39.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 40.Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- 44.Leary JB, Bondi CO, LaPorte MJ, Carlson LJ, Radabaugh HL, Cheng JP, Kline AE. The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J Neurotrauma. 2017;34:444–450. doi: 10.1089/neu.2016.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 47.Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.McLafferty FS, Barmparas G, Ortega A, Roberts P, Ko A, Harada M, Nuno M, Black KL, Ley EJ. Predictors of improved functional outcome following inpatient rehabilitation for patients with traumatic brain injury. NeuroRehabilitation. 2016;39:423–430. doi: 10.3233/NRE-161373. [DOI] [PubMed] [Google Scholar]
- 49.Stavrakov G, Philipova I, Zheleva-Dimitrova D, Valkova I, Salamanova E, Konstantinov S, Doytchinova I. Docking-based design and synthesis of galantamine-camphane hybrids as inhibitors of acetylcholinesterase. Chem Biol Drug Des. 2017 doi: 10.1111/cbdd.12991. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Reid RT, Sabbagh MN. Effects of cholinesterase inhibitors on rat nicotinic receptor levels in vivo and in vitro. J Neural Transm Vienna. 2008;115:1437–1444. doi: 10.1007/s00702-008-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arciniegas D, Adler L, Topkoff J, Cawthra E, Filley CM, Reite M. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13:1–13. doi: 10.1080/026990599121827. [DOI] [PubMed] [Google Scholar]
- 52.Gorman LK, Fu K, Hovda DA, Murray M, Traystman RJ. Effects of traumatic brain injury on the cholinergic system in the rat. J Neurotrauma. 1996;13:457–463. doi: 10.1089/neu.1996.13.457. [DOI] [PubMed] [Google Scholar]
- 53.Egea J, Martin-de-Saavedra MD, Parada E, Romero A, Del Barrio L, Rosa AO, Garcia AG, Lopez MG. Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology. 2012;62:1082–1090. doi: 10.1016/j.neuropharm.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Ezoulin MJ, Ombetta JE, Dutertre-Catella H, Warnet JM, Massicot F. Antioxidative properties of galantamine on neuronal damage induced by hydrogen peroxide in SK-NSH cells. Neurotoxicology. 2008;29:270–277. doi: 10.1016/j.neuro.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Lorrio S, Sobrado M, Arias E, Roda JM, Garcia AG, Lopez MG. Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J Pharmacol Exp Ther. 2007;322:591–599. doi: 10.1124/jpet.107.122747. [DOI] [PubMed] [Google Scholar]
- 56.Moriguchi S, Shioda N, Han F, Yeh JZ, Narahashi T, Fukunaga K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus. 2009;19:844–854. doi: 10.1002/hipo.20572. [DOI] [PubMed] [Google Scholar]
- 57.Gatson JW, Simpkins JW, Uteshev VV. High therapeutic potential of positive allosteric modulation of alpha7 nAChRs in a rat model of traumatic brain injury: proof-of-concept. Brain Res Bull. 2015;112:35–41. doi: 10.1016/j.brainresbull.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32:43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- 59.Frenette AJ, Kanji S, Rees L, Williamson DR, Perreault MM, Turgeon AF, Bernard F, Fergusson DA. Efficacy and safety of dopamine agonists in traumatic brain injury: a systematic review of randomized controlled trials. J Neurotrauma. 2012;29:1–18. doi: 10.1089/neu.2011.1812. [DOI] [PubMed] [Google Scholar]
- 60.Hullinger R, O’Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126–134. doi: 10.1016/j.nlm.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]