Abstract
In humans a chromosomal hemideletion of the 16p11.2 region results in variable neurodevelopmental deficits including developmental delay, intellectual disability and features of autism spectrum disorder (ASD). Serotonin is implicated in ASD but its role remains enigmatic. In this study we sought to determine if and how abnormalities in serotonin neurotransmission could contribute to the behavioral phenotype of the 16p11.2 deletion syndrome in a mouse model (Del mouse). Since ASD is frequently associated with altered response to acute stress and stress may exacerbate repetitive behavior in ASD, we studied the Del mouse behavior in the context of an acute stress using the forced swim test, a paradigm well characterized with respect to serotonin. Del mice perseverated with active coping (swimming) in the forced swim test and failed to adopt passive coping strategies with time as did their wild-type (WT) littermates. Analysis of monoamine content by HPLC provided evidence for altered endogenous serotonin neurotransmission in Del mice while there was no effect of genotype on any other monoamine. Moreover we found that Del mice were highly sensitive to the 5-HT2A antagonists M100907, which at a dose of 0.1 mg/kg normalized their level of active coping and restored the gradual shift to passive coping in the forced swim test. Supporting evidence for altered endogenous serotonin signaling was provided by observations of additional ligand effects including altered forebrain Fos expression. Taken together, these observations indicate notable changes in endogenous serotonin signaling in 16p11.2 deletion mice and support the therapeutic utility of 5-HT2A receptor antagonists.
Keywords: dorsal raphe, median raphe, hippocampus, monoamine, autism, serotonin, swim
Graphical abstract
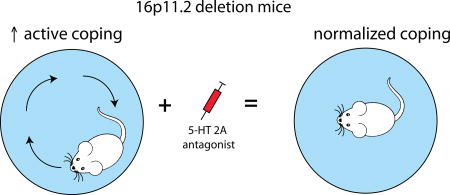
In humans a chromosomal hemideletion of the 16p11.2 region results in variable neurodevelopmental deficits including developmental delay, intellectual disability and features of autism spectrum disorder (ASD). Mice modeling this human chromosomal hemideletion of the 16p11.2 region show robust perseverative active coping in response to acute stress (swim). This phenotype is completely normalized by a 5-HT2A-receptor antagonist. These results indicate a strong potential for the therapeutic utility of 5-HT2A antagonists and also illustrate the utility of studying autism-relevant mouse models in the context of stress.
Introduction
In humans the 16p11.2 deletion syndrome is caused by a loss of a chromosomal segment that encodes about 27 genes, depending on the exact breakpoints. The phenotype of individuals with the deletion can be quite variable. Intellectual disability as well as problems with motor coordination, speech and language skills are common with varying severity (Hanson et al., 2015; Hippolyte et al., 2016). Human deletion carriers often exhibit some level of restricted and repetitive behavior patterns and children are often diagnosed with attention deficit or disruptive behavior disorders (Hanson et al., 2015). Depending on the combination and severity of behavioral features, a subset of 16p11.2 microdeletion carriers meet the diagnostic criteria for autism (about 20%) (Zufferey et al., 2012; Hanson et al., 2015; Steinman et al., 2016). Indeed copy number variants (CNVs; both deletions and duplications) of the 16p11.2 region is one of the most common chromosomal aberrations associated with autism accounting for approximately 1% of all patients diagnosed with ASD (Weiss et al., 2008; Hanson et al., 2015).
Serotonin (5-hydroxytriptamine; 5-HT) has been implicated in the behavioral impairments of ASD, however a consistent connection between brain serotonin abnormalities and ASD has yet to be established. Mouse models suggest that perturbing serotonin neurotransmission may be sufficient to generate deficits in social behavior as well as hyperactivity and repetitive behavior (Brunner et al., 1993; Veenstra-VanderWeele et al., 2012; Muller et al., 2016). Likewise, there is a rare human variant of the serotonin transporter (SERT) gene SLC6A4 is thought to be associated with the rigid-compulsive behaviors and hyperactivity of ASD (Sutcliffe et al., 2005; Veenstra-VanderWeele et al., 2012). In addition serotonin abnormalities have been discovered in other mouse models relevant to ASD (reviewed by (Muller et al., 2016)). In particular the inbred BTBR T+TF/J mouse model, commonly used as a model for idiopathic autism due to their severe decrease in social interaction and increased resistance to change, has altered response to 5-HT ligands, 5-HT forebrain tissue content, 5-HT axon density, and increased dorsal raphe 5HT neuron number and activity (Gould et al., 2011; Guo and Commons, 2017). These observations raise the possibility that an altered 5-HT system could contribute to a common neural basis for ASD.
Mouse models of the human 16p11.2 deletion syndrome have been generated with haploinsufficiency of the cognate chromosomal segment (Horev et al., 2011; Portmann et al., 2014; Arbogast et al., 2016). In the present study, we sought to characterize several aspects of serotonin neurotransmission in the 16p11.2 deletion mouse model to evaluate the potential contribution to the phenotype. These studies employed the forced swim test, where the rodent is faced with an acute and inescapable stress and has a choice to respond either actively (swimming or climbing) or passively (floating). Rodents typically flexibly switch between these coping strategies in the swim test, with active or escape-directed strategies predominating early while these are gradually supplanted by passive coping. The forced swim test is a behavioral paradigm that is well characterized with respect to serotonin neurotransmission and is often used in the context of depression research, likely because the neural circuits that coordinate the response to acute stress are highly relevant to depression (reviewed by: (Commons et al., 2017)). However, maladaptive response to acute stress is associated with other conditions including ASD (Taylor and Corbett, 2014) (Commons et al., 2017). Importantly altered behavioral response to acute stress and difficulty in adapting to change (behavioral inflexibility) is common in ASD (Spratt et al., 2012). Likewise repetitive behavior or stereotypy, a core feature of ASD, is often exacerbated by stress (Garcia-Villamisar and Rojahn, 2015). Indeed, several mouse models relevant to ASD exhibit altered behavior in the forced swim test and either do not mount the normal level of active coping or alternatively perseverate with an active strategy and fail to transition to passive strategies (Commons et al., 2017). Taken together these observations raise the possibility that the forced swim test may probe an endophenotype for ASD.
Methods
Subjects
B6129S-Del (7Slx1b-spet1)4Aam/J RRID: IMSR_JAX: 013128 (‘Del’) mice were generated by Alea A. Mills at Cold Spring Harbor Laboratory (Horev et al., 2011) and purchased from Jackson Labs where they were bred with B6129F1/J for 7–8 generations. At Boston Children’s Hospital, male Del mice, heterozygous for the deletion allele, were bred with normal female B6129F1/J mice RRID: IMSR_JAX: 101043 such that maternal behavior would not be impacted by the genetic deficiency. Both male and female progeny born at Boston Children’s Hospital (8–10 generations back-crossed to B6129F1/J) were used for analysis at 28–35 postnatal days of age. Mice were housed in small mouse opti-cages with at least 2 but no more than 5 occupants, with free access to food and water. Mice weighed approximately 15 g when tested. Since autism is a disorder that manifests in childhood, we reasoned that this younger age is particularly relevant to understanding the neural mechanisms of the disorder. Postnatal day 28 is thought to represent an early adolescent state in rodent development (Laviola et al., 2003) and major postnatal developmental changes of the serotonin system have largely been completed by this age (Lidov and Molliver, 1982; Liu and Wong-Riley, 2010). While there are likely further refinements of the system into adulthood, age-dependent changes were not the focus of this study. Care and use of animals in these studies was approved by the Institutional Care and Use Committee at Boston Children’s Hospital under protocol approval number 15-03-2904R.
Drugs
M100907 (MDL-100907, Volinanserin, (R)-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol) (Sigma-Aldrich, St. Louis, USA) was dissolved in 0.9% normal saline and administered intraperitoneally in a single, acute dose of 0.003, 0.01, or 0.1 mg/kg 15 minutes before behavioral testing.
DOI ((±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride) (Sigma-Aldrich, St. Louis, USA) was dissolved in normal saline and administered intraperitoneally in a single, acute dose of 3.0 mg/kg.
RS-102221 (4-dione hydrochloride hydrate) (Sigma-Aldrich, St. Louis, USA) was dissolved in 0.9% normal saline and administered intraperitoneally in a single, acute dose of 2.0 mg/kg.
Locomotor Activity and Forced Swim Test
The locomotor activity of both Del and WT mice was measured automatically in an 18 in. × 9.5 in. × 5.5 in. Open Field Photobeam Activity System (San Diego Instruments). In this system, sequential beam brakes on the periphery of the field or the center of the field are quantified as ambulations, peripheral or central, respectively. Repetitive beam breaks are quantified as fine movement. Mice were injected intraperitoneally with either drug or 0.9%, saline and returned to their home cage for 15–20 minutes before being singly placed into the center of the open field chamber. Accumulated beam breaks of ambulatory, fine, and rearing movements were recorded in 5-minute intervals for a total of 10 minutes.
Following locomotor testing mice were tested in the forced swim. For this mice were placed into a cylindrical glass tank (46 cm high × 20 cm diameter) filled with water (25 ± 1 °C) to a depth of about 30 cm, where they were video recorded for 15 minutes. Swimming (active) and immobility (passive) behavior were scored using a 5 second time interval sampling method. Swimming behavior was defined as movements throughout the tank (usually horizontal), while immobility was scored as minimal movements made by the mice in order to stay above the water. After the 15-minute swim, the mice were removed from the tank, dried off with a towel, and returned to their home cage. Scoring of behavior was done by an observer blind to the treatment group.
Head Twitch (HTR) and Ear Scratch (ESR) Response
A separate group of Del and WT mice were injected intraperitoneally with either DOI or saline and returned to their home cage for 10 minutes. The mice were then singly placed into an empty, Plexiglas cage, where their behavior was video recorded for 10 minutes. The two movements that were counted were head twitch responses (rapid rotational side-to-side head movement) and ear scratch responses (a rapid scratching movement of the ear, head, or neck are by either hind limb).
HPLC Analysis
Mice that either were unperturbed or experienced the forced swim (without drug treatment) were sampled for HPLC. For this, mice were anesthetized using isoflurane 2-hours after swim. To measure the content of monoamines and their metabolites, whole brains were rapidly extracted and snap-frozen in 2-methyl butane cooled with dry ice (n = 10 per genotype per swim condition). The dorsal striatum (both medial and lateral), dorsal hippocampus (primarily the dentate gyrus and CA1 subfields) and dorsal raphe were punched out of serial 300-micron slices cut on a cryostat with a 2 mm diameter punch and analyzed by the Neurochemistry Core Facility at Vanderbilt University. These forebrain areas were selected because they represent termination fields with distinct areas of origin within the raphe nuclei, they were implicated as areas where serotonin neurotransmission may be compromised in BTBR T+TF/J mice and they could be sampled reproducibly from animal to animal (Gould et al., 2011; Amodeo et al., 2012; Commons, 2016). For the HPLC, frozen brain tissue samples were homogenized by using a tissue dismembrator (Misonix XL-2000; Qsonica) in a solution containing 100 mM TCA, 10 mM NaC2H3O2, 100 µM EDTA, 5 ng/mL isoproterenol (an internal standard), and 7.5% (vol/vol) methanol (pH 3.8). Using a Waters 2707 autosampler 10µL of supernatant from each sample was injected onto a Phenomenex Kintex (2.6 µm, 100 A) C18 HPLC column (100 ×4.6 mm). Biogenic amines were eluted with a mobile phase [100 mM TCA, 10mM NaC2H3O2, 100µM EDTA, and 7.5% methanol (pH 3.8)] delivered at 0.6 mL/min by using a Waters 515 HPLC pump. Analytes were detected by using an Antec Decade II (oxidation: 0.4) electrochemical detector operated at 33°C. Empower software was used to manage HPLC instrument control and data acquisition. Data were analyzed using ANOVA with factors for region, neurochemical and strain.
Immunohistochemistry/Fos Analysis
For analysis of Fos expression, mice were given an i.p. injection of either saline, 0.1 mg/kg M100907, or 3 mg/kg DOI and returned to their home cage. After 2 hour and 10 minute delay to allow maximal expression of Fos protein, mice were then anesthetized using pentobarbital and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. After equilibration in 30% sucrose, the brains were frozen and sectioned. Immunofluorescence processing was performed on floating sections. Fos was detected by incubating sections in primary antisera raised in rabbit (EMD Chemicals, Gibbstown, NJ, #PC38 RRID: AB_2106755) diluted 1:10,000 in 0.1 M phosphate buffered saline with 0.3% Triton X-100, 0.1% bovine serum albumin and 0.01% sodium azide at 4° C for 72 hours, followed by incubation in CY3 anti-rabbit (diluted 1:500) raised in donkey at room temperature for 90 minutes. Sections were rinsed in 0.05 M phosphate buffer, mounted, dried, and cover-slipped with a glycerol-based mounting medium. For analysis of serotonin neurons, tissue sections were processed for immunolabeling of tryptophan hydroxylase (TPH, Millipore, AB1541 raised in sheep RRID: AB_90754) as a marker for serotonin neurons using similar methods. To estimate the number of serotonin neurons, a nuclear stain (DAPI) was included in the cover slipping medium (Molecular Probes).
For image analysis, tissue was sampled in every third section through the region of interest. Wide-field images were obtained using an Olympus IX-81 fluorescence microscope with a 10× objective, a Hamamatsu Orca ER camera and Slidebook software (3i). Fos expression was quantified with ImageJ software using “threshold” and “analyze particles” functions. The threshold was set manually to include all nuclei that appeared filled with immunolabeling with minimal of noise. (n = 8–10 per genotype/drug). To quantify the number of serotonin neurons images containing DAPI labeling for nuclei and TPH labeling for serotonin neurons were analyzed. Images were split into separate color channels. For each channel, the background was subtracted and a threshold was applied to define labeled areas. A selection area generated by the area immunolabeled for TPH was applied to the cognate image of DAPI-defined nuclei. The number of DAPI-defined nuclei within the TPH selection area were counted using Analyze-Measure command. The mean number of DAPI cells per section per region was determined for each genotype and used for statistical comparisons.
Statistics
Behavior, HPLC and Fos analyses were done blind to genotype. If data points qualified as outliers as defined as differing from the mean by greater then two standard deviations, they were eliminated from the data set. This accounted for no more then 5% of data points, which are not included in the stated numbers of mice per group listed in the figure legends for each experiment. Siblings within litters were assigned to the same treatment groups such that any variables related to rearing were equivalent for both genotypes. Power analysis was not used, rather sample size was calculated based on experience with the assays from previous experiments. Since individuals were typically genotyped after completion of the experiments, allocation to treatment groups was non-systematically random. Data was analyzed using the appropriate one, two or three-factor ANOVA and follow up independent-samples t-tests between groups with Tukey HSD to control for multiple comparisons. When measures were repeated at different time points, a repeated measures ANOVA was used. All of the initial analysis included sex as an independent factor. The only endpoint where there was a significant effect of sex was body weight (described more fully in results) therefore in all other cases both sexes were subsequently pooled for analysis.
Results
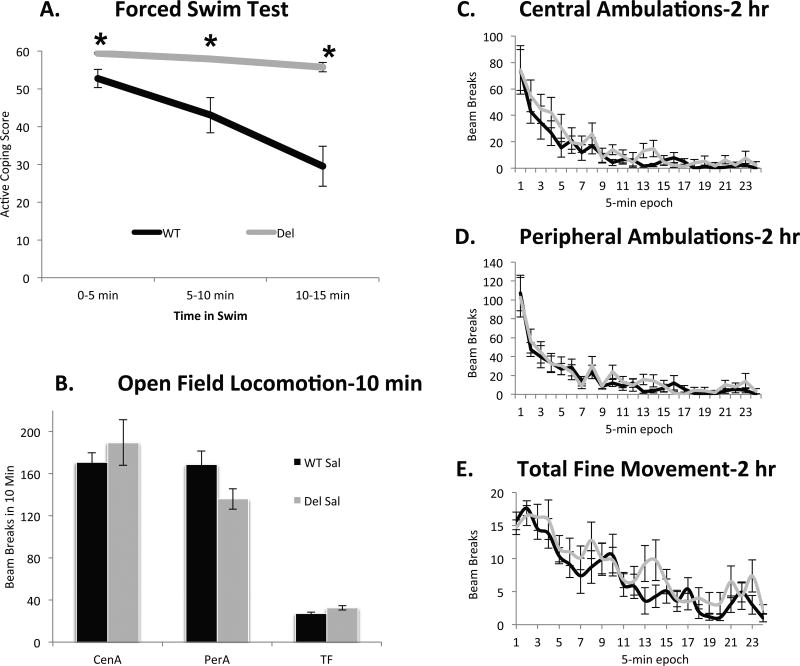
Since stress might exacerbate some symptoms of autism and serotonin is known to modify the behavioral response to stress, we evaluated coping behavior in Del and WT mice a 15-minute acute forced-swim stress, which we hypothesized could give insight into a new aspect of the behavioral phenotype of Del mice. Del mice showed a dramatic phenotype in which they were notably more active than WT mice at the beginning of the forced swim and persisted with an active coping strategy throughout the duration of the test (genotype × time, F(2,62) = 14.0, p < .001; Figure 1). As changes in locomotor behavior can non-selectively influence activity in the forced swim test and Del mice under certain testing conditions may be hyperlocomotive, particularly on an inbred genetic background (Horev et al., 2011; Brunner et al., 2015; Arbogast et al., 2016) we characterized locomotor behavior in a 10-minute open field test. However there was no effect of genotype on activity detected of our mice on an outbred background (Figure 1, p = .658). We followed locomotor behavior in a separate cohort of mice for 2 hours analyzed in 5-minute bins. Analyzed with a two factor ANOVA with repeated measures, we were unable to find statistically significant differences between genotypes (p = .393 for central ambulation, p =.363 for peripheral ambulation and p =.662 for fine movement) while both genotypes showed a gradual reduction in activity with time (Figure 1), consistent with prior observations that the locomotor phenotype can be subtle depending on how it is measured (Angelakos et al., 2016).
Figure 1.
A. Del mice begin the forced swim test with more active coping and perseverate with that strategy throughout the test while their WT sibling controls progressively adopt more passive coping strategies. At the 1st time-point, p = .003, at 2nd p <.002 and at 3rd p < .001. Group size, WT, n = 19 mice (10 male, 9 female); del, n = 16 mice (8 male, 8 female). B. In a 10 min open field test, there is no significant effect of genotype on central ambulations (CenA) perhipheral ambulations (PerA) or total fine movement (TF). Group size, WT, n = 19 mice (10 male, 9 female); del, n = 16 mice (8 male, 8 female). C. D. and E. In a separate cohort of mice examined for 2 hours, differences between genotypes in locomotor behavior were not statistically detectable and both genotypes showed a similar gradual reduction in locomotion. C. For central ambulations p = 0.393, D. peripheral ambulations p = .363, and E. total fine movement p = .662). Group size, WT, n = 10 mice (5 male, 5 female); del, n = 8 mice (4 male, 4 female).
It has been previously reported that Del mice weigh less than their WT littermates. Consistent with previous observations we found Del mice on average weighed slightly less than their WT siblings (for males, F(1,222) = 69.6, p < .001 ; Del, 14.5g +/− 2.9 s.d.; WT, 17.9g +/− 3.5 s.d.), with females weighing less than males in both genotypes (F(1, 222) = 31.4, p < .001; Del MD = −2.1g +/− .4; WT MD = −2.4g +/− 1.8). Weight was the only behavioral or biological endpoint examined where there was a detectable effect of sex. In both sexes there was considerable overlap in the distribution of individual body weights between genotypes as the means were within approximately one standard deviation of each other, thus weight was not a proxy for genotype. To determine if there was a possibility that body weight could be related to alterations in swimming behavior we further examined the data for correlations between weight and coping strategy within each genotype, and these tests were not significant for either genotype (for Del r = −0.016 p = 0.946; WT, r = −0.214 p = 0.462). Alternatively analyzed as an ANCOVA (genotype × sex on total swim count with weight as a covariate) there was no significant effect of weight (p = 0.461) while the main effect of genotype was still apparent (p = 0.001). Finally we matched the groups for weight by eliminating from the analysis the smallest 40% Del mice and the largest 40% of WT mice, yielding groups with almost identical mean weights (16.98 g +/− 2.92 s.d. for Del n = 12; 16.80 g +/− 3.92 for WT n = 9). These subsets of mice exhibited the identical swim scores as the complete groups (p = 0.80 Del and p = 0.96 WT). Further the comparative difference in cumulative immobility scores showed the same magnitude of genotype effect: cumulative immobility scores of weight-matched groups were 7.7 for Dels vs. 55.7 for WTs; as compared to the overall group scores of 7.0 and 54.8 (Del vs. WT). Thus even Del and WT mice that weigh the same have dramatically different behavior in the swim test. These results indicate that the robust increase in active coping strategy exhibited by Del mice in the forced swim test is neither easily attributable to generalized locomotor activation nor does it have any relationship to low weight.
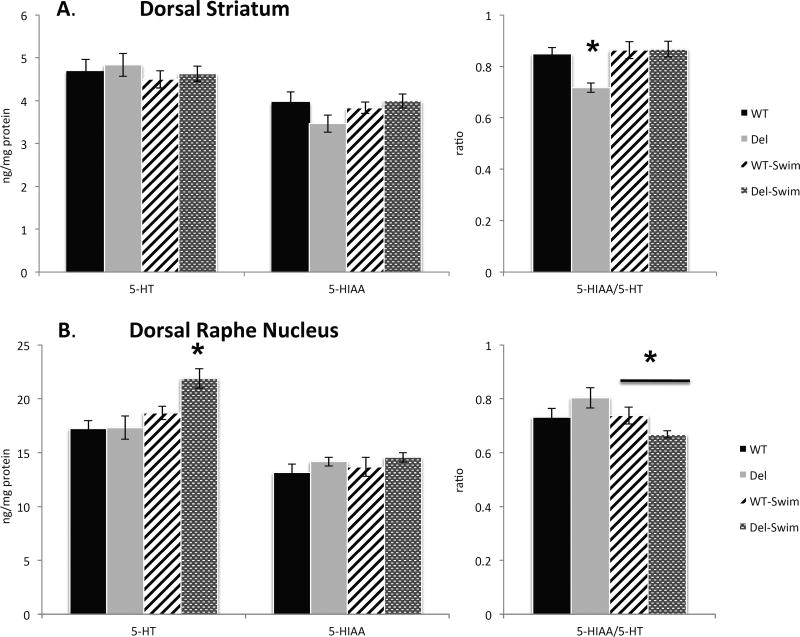
In order to investigate potential disruptions in monoamine neurotransmission that may play a role in altered swim behavior, we analyzed the tissue content by HPLC in samples of the dorsal striatum, dorsal raphe nucleus, and hippocampus taken from mice that were either unperturbed or had recently experienced the forced swim stress (Figure 2). We selected the dorsal striatum because alteration of this structure have been reported (Portmann et al., 2014), the dorsal raphe nucleus as the largest group of serotonin neurons, and the hippocampus because it is perturbed in the BTBR T+TF/J mice. While no effects of genotype or swim on 5-HT or 5-HIAA content in the dorsal striatum were revealed, there was a significant genotype by swim interaction on 5-HT turnover in the dorsal striatum (F(1,41) = 5.7, p = .02; Figure 2), with posthoc analyses suggesting that 5-HT turnover in Del mice was lower under non-stress conditions compared to WT mice (Tukey HSD < .05) and is normalized to the same level as WT mice after forced swim (Tukey HSD < .05). In contrast, no alterations in 5-HT turnover were exhibited in the dorsal striatum of WT mice (Figure 2).
Figure 2.
A. Dorsal striatal 5-HT, 5-HIAA and their ratio in Del and WT mice with or without a swim. There was a significant genotype × swim interaction on 5-HT turnover in the dorsal striatum. B. Same measures of the dorsal raphe nucleus. There is a significant effect of swim on 5-HT only in Del mice, and a significant genotype × swim interaction on 5-HT/5-HIAA ratio. (Asterisks indicate Tukey HSD < .05). Group size, n = 10–12 mice per genotype per condition, with at least 5 per sex in each group.
Analysis of dorsal raphe nucleus 5-HT tissue content (Figure 2) revealed a significant effect of swim (F(1,34) = 9.6, p = .004), with post hoc analyses revealing that despite similar 5-HT content between Del and WT mice in the non-stressed condition, 5-HT is significantly elevated following the forced swim exclusively in Del mice (Tukey HSD < .05) (Figure 2). Furthermore, there was a significant genotype by swim interaction on 5-HT turnover in the dorsal raphe (F(1,32) = 4.8, p = .036), with post hoc analyses revealing that 5-HT turnover in the dorsal raphe of Del mice is significantly decreased following forced swim (Tukey HSD < .05), with no similar effect of swim observed in WT mice (Figure 2). Neither swim nor genotype had an effect on 5-HIAA levels in the dorsal raphe. Finally, no differences between genotypes on 5-HT, 5-HIAA, or 5-HT turnover were observed in the hippocampus, although forced swim reduced both 5-HIAA (F(1,39) = 4.7, p = .035) and 5-HT turnover (F(1,37) = 4.0, p = .05) in both genotypes. The remaining HPLC data showed no significant effects of genotype on dopamine, any dopamine metabolite or norepinephrine. Collectively, these results suggest that of all the monoamines measured only serotonin could be implicated in the phenotype of the Del mice.
Given the alterations of serotonin metabolism combined with our previous findings of altered serotonin neuron cytoarchitecture in the BTBR T+TF/J model used to study autism (Guo and Commons, 2016), we examined the morphology of the dorsal raphe nucleus in Del vs. WT siblings. The cytoarchitecture of serotonin neurons in the dorsal raphe and quantitative estimates of the number of neurons present in each region revealed no obvious differences between genotypes (supplemental Figure 1).
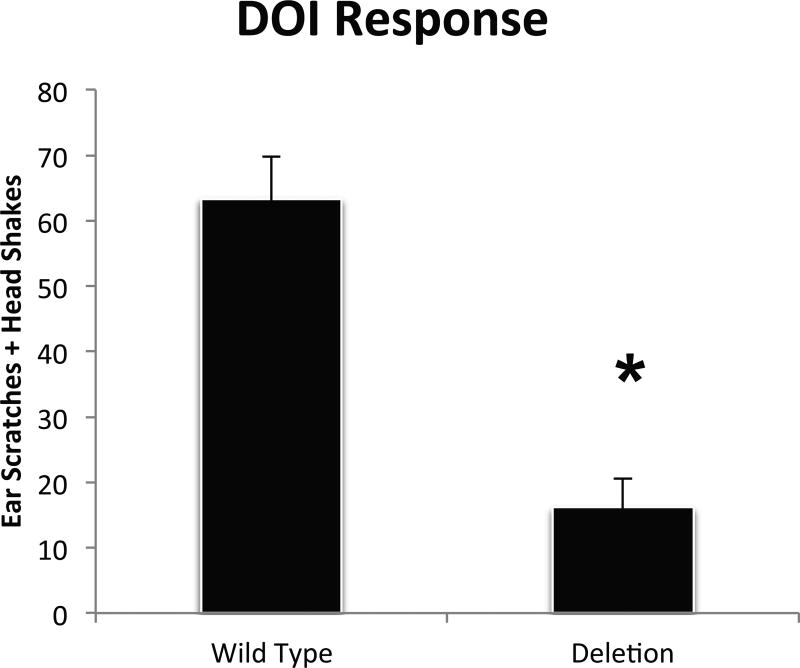
Risperidone is a drug often prescribed in ASD and is most well known as a D2 receptor antagonist. However risperidone also acts as an inverse agonist of serotonin 5-HT2A and 5-HT2C receptors. In a similar 16p11.2 deletion mouse model, risperidone reportedly has reduced effects in Del mice (Portmann et al., 2014). While this is thought to be attributable to changes in neurons that have D2 receptors, altered 5-HT2 receptor functionality has never been evaluated. Furthermore, we found no changes in levels of dopamine or any dopamine metabolite in Del mice leading us to investigate 5-HT2 receptor actions. Although there is evidence that 5-HT2 receptor ligands can modify the actions of antidepressants in the forced swim test, typically they do not have robust effects on swim behavior by themselves. However, a well-established bioassay for 5-HT2 receptor function in that the agonist DOI elicits well characterized and quantifiable head-twitch or ear scratch responses (Halberstadt and Geyer, 2017). Therefore we examined the functional state of 5-HT2 receptors in Del mice by measuring the effect of the DOI on these movements. This revealed that while DOI promoted a considerable response in WT mice, Del mice appeared relatively insensitive (Figure 3). This observation suggested that in addition to alterations in endogenous 5-HT metabolism, there are likely alterations in 5-HT2 receptor function in Del mice.
Figure 3.
Ear scratch and head shake events were counted for 10 minutes after 3 mg/kg i.p. DOI injection. P = 0.0002 (asterisk). Not shown are scores for mice receiving saline injections of both genotypes that had negligible quantifiable movements (averaging < 1 event for both groups). Group size, n = 13–28 per group.
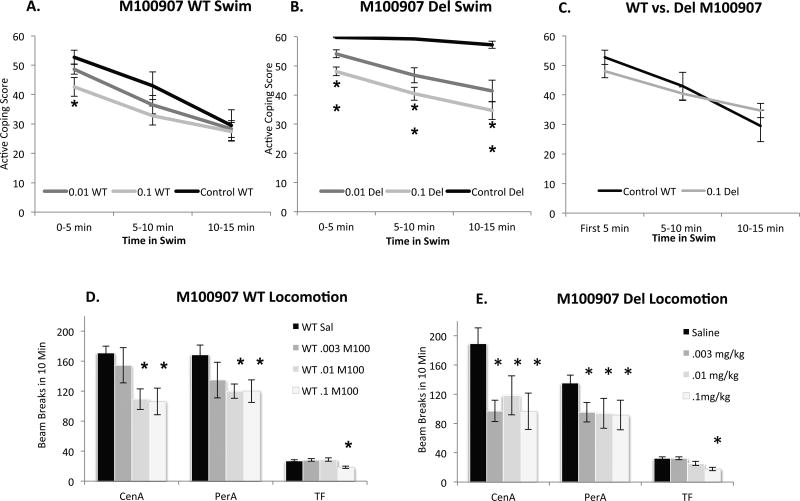
Given the attenuated effect of the 5-HT2 agonist in Del mice, next we assessed the effects of 5-HT2 antagonists on behavior in Del mice. Using both swim and locomotor activity as behavioral endpoints, first we tested the effect of the 5-HT2A antagonist M100907 (MDL-100,907, Volinanserin) (.003, .01, .1 mg/kg) in both Del and WT mice (Figure 4). This highest dose is 20-fold lower than doses that have a detectable effect on vigilance state measured 3 hours following treatment (Popa et al., 2005). With respect to the swim for M100907, mixed-ANOVA with genotype (Del; WT) and Drug (M100907, .003, .01, .1 mg/kg; sal) as between subjects factors and time (1st, 2nd, 3rd 5 minutes) as the within-subjects factor revealed a significant genotype × drug × time interaction (F(6,286) = 3.342, p = .003). Following administration of M100907, Del mice display a significant reduction in active coping behavior (swimming) across the entire forced swim test at all doses administered (Tukey HSD p < .05 at all doses/time points). In contrast, WT mice display a reduction in active coping behavior only at the highest dose of M100907 administered and only within the first 5-minutes of the forced swim test (Tukey HSD p < .05)(Figure 4). Collectively, these results suggest that the perseverative active coping behavior that Del mice display in the forced swim test is normalized by administration of a 0.1 mg/kg dose of the 5-HT2A antagonist M100907 (Figure 4C).
Figure 4. Effects of the 5-HT2A antagonist M-100907.
A. In the forced swim test in WT mice, 0.01 mg/kg M-100907 has no significant effect, while 0.1 mg/kg reduces active coping for the first 5 minutes (* indicates p < .05). B. More dramatically in Del mice M-100907 dose-dependently reduces active coping throughout the test, and partially restores the adaptive shift from active to passive strategy (Significant genotype × drug × time interaction) ** indicates p < .05 for both doses and timepoints compared to saline Del treated; N = 16–18 mice per genotype/dose with at least 8 of each sex. C. Posthoc comparison between saline treated-WT and Del mice receiving 0.1 mg/kg M-100907 reveal no significant differences (Tukey HSD p > .05 at all time points). D. In WT mice, .1 mg/kg and .01 mg/kg reduce central ambulations (CenA) and perhipheral ambulations (PerA) while only the higher dose reduced total fine movement (TF). The lowest dose, .003 mg/kg had no significant effect. E. In Del mice, all doses reduced central ambulations (CenA) and peripheral ambulations (PerA) while only the highest dose reduced total fine movement (TF). Group size, n = 16–18 mice per genotype/dose with at least 8 of each sex.
Our data already showed that Del mice display fairly normal locomotor activity in the open field. However to evaluate the possibility that 5-HT2A acting ligands may broadly impact locomotor activity in Del and WT mice we next assessed the effect of acute administration of M100907 (.003, .01, .1mg/kg) in the open field (Figure 4), measuring ambulatory movements (beam breaks) in both the open field center and periphery regions.
For M100907, ANOVA with sex (male; female), genotype (Del; WT) and drug (M100907, .003, .01, .1mg/kg; sal) as between subjects factors revealed a significant effect of drug on center ambulatory movements (F(3,140) = 6.102, p = .001), and main effects of both drug (F(3, 140) = 2.655, P = .051) and genotype (F(1,140) = 7.533, p = .007) on periphery ambulatory movements. M100907 decreased center ambulatory movements in both Del and WT mice only at the highest doses administered (.01 and .1mg/kg), while a significant genotype by drug interaction was observed at the lowest dose (.003mg/kg) (F(1,84) = 4.410, p = .039), revealing that this lowest dose of M100907 decreased center ambulatory movements selectively in del mice (Tukey HSD < .05) (Figure 4). While M100907 decreased periphery ambulatory movements in both genotypes at all doses administered, Del mice displayed lower periphery ambulatory movements compared to WT mice across all doses of M100907 (Figure 4). Collectively, these results suggest that Del mice may be more sensitive to the locomotor effects of M100907, particularly on center ambulatory movement, compared to WT mice.
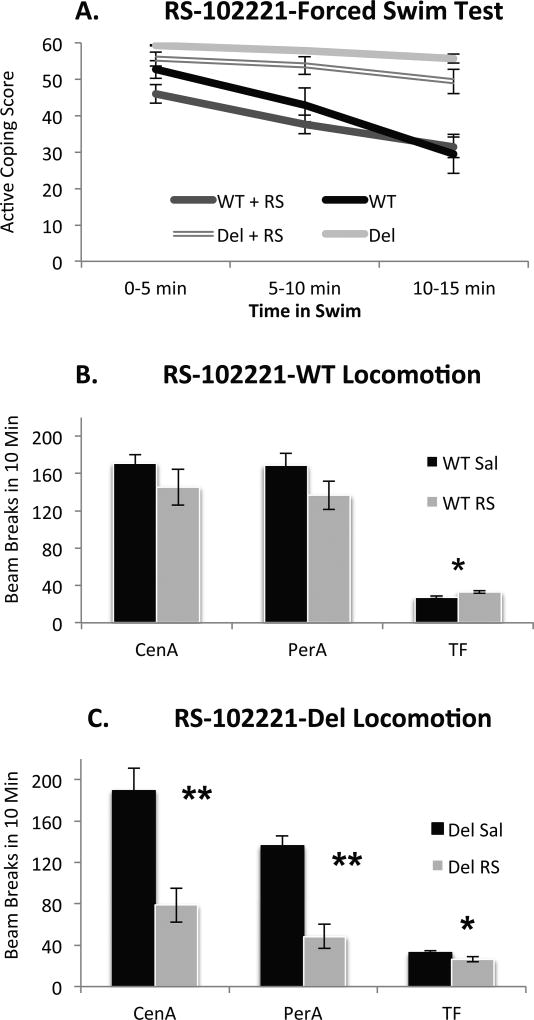
In addition to 5-HT2A receptors, both risperidone and DOI may act at 5-HT2C receptors. Therefore we tested the effects of the 5-HT2C antagonist RS102221 (2 mg/kg) on both behavior in the swim and locomotor behavior (Figure 5). In contrast to the 5-HT2A antagonist, the 5-HT2C antagonist RS10221 did not alter coping behavior in either WT or Del mice suggesting a unique contribution of 5-HT2A receptors in this particular behavioral phenotype. In the open field, an ANOVA with sex, genotype, and drug (RS102221; sal) as between subjects factors revealed a significant genotype by drug interaction on center ambulatory movement (F(1,59) = 5.083, p = .028), significant main effects of both genotype (F(1,59) = 20.307, p < .001) and drug (F(1,59) = 28.631, p < .001) on periphery ambulatory movement, and no interactions with sex. Posthoc analyses revealed that RS102221 decreased center ambulatory movements in Del mice (Tukey HSD < .05) but had no effect in WT mice (Figure 5). While RS102221 decreased peripheral ambulatory movements in both genotypes, this effect only reached statistical significance in Del mice (Tukey HSD < .05) (Figure 5). Collectively, these results indicate an altered locomotor response to RS10221 in Del mice but no detectable effect in forced swim behavior. While increasing the dose of RS102221 might reveal effects on swim, we elected not to pursue those experiments since increasing doses might also be less specific for 5-HT2C receptors and potentially engage confounding behavioral effects.
Figure 5. Effect of the 5-HT2C antagonist, RS-102221 at 2 mg/kg.
A. There is no significant effect of drug or drug by genotype interaction on behavior in the forced swim test. B. In WT mice, RS-10221 increased total fine (TF) movement (Tukey HSD p < 0.05) while both central and peripheral ambulations (CenA, PerA) were not detectably effected. C. In Del mice, all indices of movement were significantly reduced; Genotype*Drug Interaction at all measurements (p < .05) * = p < .05 ** = p < .01; Group size, n = 16–18 mice per genotype/drug condition with at least 8 of each sex
Fos
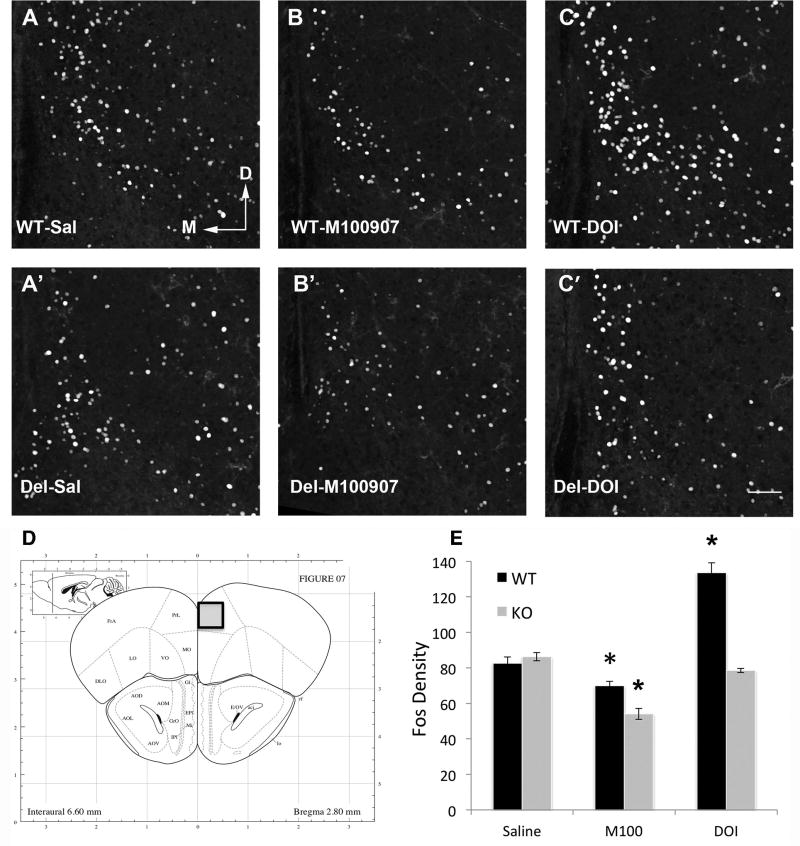
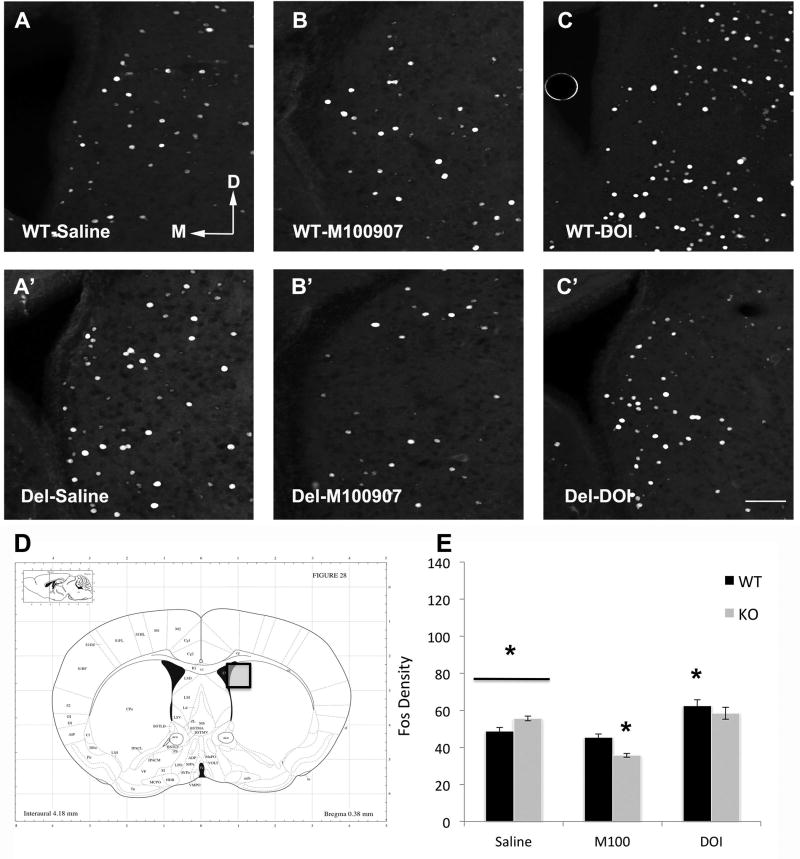
Previous studies have suggested altered striatal function in Del mice (Portmann et al., 2014). However 5-HT2A also receptors have prominent location in cortical interneurons, which have been suggested as a common location of defect in autism (Jacob, 2016). To assess whether Del mice display an altered neural response to 5-HT2A acting drugs in the prelimbic cortex (PLC) and/or dorsal striatum (DS) we measured Fos expression in these regions following administration of M100907 (.01mg/kg), DOI (3mg/kg) or saline (Figure 6 & Figure 7) in non-swim exposed mice. ANOVA revealed a significant genotype × drug interaction in both the PLC (F(2,37) = 39.239, p < .001) and DS (F(2,37) = 11.200, p < .001). Following saline administration, Del and WT mice had similar PLC Fos expression, while Del mice displayed lower DS Fos expression compared to WT mice (t(15) = 3.072, p = .008). When administered the 5HT2A antagonist M100907, Fos expression was significantly decreased in the PLC of both WT and Del mice, however, Del mice were notably more sensitive to this effect (genotype × drug, F(1,29) = 11.815, p = .002) (Figure 6). Similarly, M100907 decreased Fos expression in the DS of Del mice yet had no effect on the DS of WT mice (genotype × drug, F(1,29) = 30.228, p < .001). In sharp contrast, when administered the 5HT2A agonist DOI Fos expression was significantly enhanced only in WT mice and had no effect on Del mice in both the PLC (genotype × drug, F(1,23) = 74.404, p < .001) and the DS (genotype × drug, F(1,23) = 5.903, p = .023) (Figure 7). Collectively, these results suggest that Del mice are more sensitive to the effects of the 5-HT2A antagonist M100907 yet completely insensitive to the effects of the 5HT2A agonist DOI on PLC and DS Fos activity.
Figure 6.
Fos in the prelimbic cortex in Del mice shows increased sensitivity to the 5-HT2A antagonist M100907 and decreased sensitivity to the agonist DOI. A, A’ Fos in saline treated WT and Del mice is comparable. B, B’ M100907 produces a significant reduction in Fos in both genotypes, to a greater degree in Del mice Tukey HSD p = 0.008 for WT; p = 8.2×10−7 Del. C, C’ DOI increases Fos in WT mice but not Del mice p = 1.8×10−6. N = 6–9 per group * = p < 0.05. D Boxed area shows region sampled. E. Quantification * = p < 0.05, Group size, n = 5–9 mice in each group, with at least 2 of each sex.
Figure 7.
Images of Fos in dorsal striatum from mice injected with saline, 0.01 mg/kg M-100907 (5-HT2A antagonist), or 3 mg/kg DOI (5-HT2 agonist). A. WT mice have less Fos activation then Del mice p = 0.008 (A’) with saline treatment. B. While there is no significant effect of M100907 in WT mice, Fos in Del mice is reduced (Tukey HSD p = 6.8×10−9)(B’). C. DOI increases Fos in WT mice but has no effect in Del mice (C’). D. Boxed area indicates area photographed. E. Quantification of Fos density. Arrows M, D indicate medial and dorsal directions. All images same scale bar in C’ = 100 um. * = p < 0.05, Group size, n = 5–9 mice in each group, with at least 2 of each sex.
Discussion
This report is the first to provide evidence for altered endogenous serotonin signaling in the 16p11.2 deletion model mice. In particular, Del mice are very sensitive to a 5-HT2A antagonist, which has the striking capacity to normalize their behavior when challenged with a forced swim test despite modest effects of the same doses on WT siblings in the test. Evidence from experiments examining agonist effects, forebrain Fos expression and serotonin metabolism provide confirming lines of evidence for changes in endogenous serotonin signaling.
Del mice exhibit a pronounced phenotype in the forced swim test, where they are hyperactive and perseverate with swimming throughout a 15-minute epoch. In contrast, their WT littermates began the test with an active coping strategy but with time used an increasingly passive strategy. Increasing passivity with time of exposure is a normal response to the inescapability of the swim and it has been argued that this shift is adaptive and serves to conserve energy (reviewed by: (de Kloet and Molendijk, 2016; Commons et al., 2017)). 5-HT is well characterized in the forced swim test because pro- and antidepressant manipulations change the balance between active and passive coping strategies (Porsolt et al., 1978; Cryan et al., 2005). However not all manipulations that change coping strategy in the swim are relevant to depression and this test cannot be interpreted in isolation with respect to affective state (Commons et al., 2017). Careful consideration of the behavioral phenotype of the Del mouse model does not provide support for an interpretation of the forced swim in the context of depression. Specifically, there is no corroborating evidence of an ‘anti-depressed’ mood from either the animal model or human literature. In fact, the observations that Del mice are less responsive to reward might suggest the opposite conclusion (Grissom et al., 2016). Likewise adult human carriers of the 16p11.2 deficiency are not immune from mood disorder diagnosis (Hanson et al., 2015). Instead, Del mice tend to be hyperactive in novel environments and exhibit little habituation to novelty (Horev et al., 2011; Portmann et al., 2014) characteristics that seem more directly relevant to their persistence with active coping. Thus, 16p11.2 Del mice share the propensity for excessive active coping with several other mouse models relevant to autism including Fragile-X Mental Retardation 1 (FMR1) knockout (Uutela et al., 2014), Timothy Syndrome Type 2 (TS2-neo) mice (Ehlinger et al., 2016) and BTBR T+tf/J (Guo and Commons, 2016).
5-HT2A receptors are not thought to be major contributors to behavior in the forced swim test in normal rodents (reviewed by: (Cryan et al., 2005)). Consistent with this observation, in WT mice M100907 had only had a very modest effect in reducing active swim coping in the first 5 minutes of the forced swim test, and only at the highest dose administered. However M100907 had a striking effect on Del mice, normalizing swim coping by both reducing the level of active coping on initial exposure to the swim and improving the gradual shift to a more passive strategy with time. In combination with altered behavioral effects of DOI, as well as changed patterns of Fos expression after either DOI or M100907, these findings indicate alterations in endogenous 5-HT2A receptor function in Del mice. These results also raise the possibility that differential effects of risperidone in Del mice may be related to altered 5-HT2A function. That is, a previous study using slightly different 16p11.2 deletion mice reported they were relatively insensitive to the locomotor inhibition produced by risperidone (Portmann et al., 2014). While risperidone is a well-known antagonist of dopamine receptors (preferentially D2-type) and alpha 2C receptors, it also has very high affinity for 5-HT2A receptors were it acts as an inverse agonist. The relationship of 5-HT2A receptors to ASD has also been noted in other mouse models. For example, in the BTBR T+TF/J mouse model of idiopathic autism M100907 likewise improves reversal learning (Amodeo et al., 2014). In both cases, the effective dose (0.1 mg/kg) was considerably lower than doses that have detectable effects on vigilance states (Popa et al., 2005). Taken together these results indicate that 5-HT2A receptors may constitute an important target for pharmacotherapy independent of D2 receptors.
There was no simple-direct relationship between locomotor behavior under stressful vs. non-stressful conditions. While Del mice are hyperlocomotive under some conditions (Horev et al., 2011; Brunner et al., 2015; Angelakos et al., 2016; Arbogast et al., 2016), this effect is not robust or pervasive enough to be detected in a 10–15 minute open field test as we and others using the same mutation and background strain have found (Angelakos et al., 2016). However, our results show Del mice were clearly hyperlocomotive in the swim. In addition, our experiments revealed that M100907 depressed locomotion at doses of 0.1 and 0.01 mg/kg in both Del and WT mice, but only had a subtle effect on swim behavior in WT mice at these doses. Thus there was a dissociation of the effects of M100907 on locomotion depending on the stress condition and genotype. We speculate that the stress condition accentuates both an underlying hyperlocomotion in Del mice as well as their tendency to poorly habituate to new situations, two aspects of their behavioral phenotype that have been described with other assays (Horev et al., 2011; Portmann et al., 2014). Thus these studies revealed a new aspect of their overall behavioral phenotype: that they respond differently to an acute stress, and stress may reveal or accentuate other behavioral deficits. This would be consistent with altered behavioral responses to stress observed in ASD, in that stress exacerbates repetitive behavior in ASD, and impaired coping response to stress is characteristic of ASD including a tendency for inflexibility. Likewise it is consistent with a deficit in 5-HT2A receptor function, which has a well characterized role in recognition of novelty (Aznar and Hervig Mel, 2016; Hervig et al., 2017). The present results also indicate the utility of behavioral tests examining stress coping strategy such as the forced swim test for ASD (Commons et al., 2017) and raise the possibility that the impact of stress on other behavioral phenotypes relevant to ASD such as social interaction may be an interesting avenue of exploration.
Del mice exhibit reduced behavioral sensitivity to the 5-HT2A agonist DOI yet increased sensitivity to the antagonist M100907. Similar shifts at the level of behavior are sometimes seen in G-protein coupled receptor systems following chronic agonist treatment. For example, following chronic mu opioid receptor agonist exposure there is typically a reduced behavioral effect of the agonist (tolerance) accompanied by marked sensitivity to the antagonist, shown by its’ ability to precipitate withdrawal. Thus, the observed alterations in 5-HT2A receptor function could be an adaptation to chronic increases in endogenous 5-HT signaling in the Del mice. Possibly supporting this, the only monoamines exhibiting genotype-dependent changes in both naïve and stressed mice were either serotonin, it’s metabolite or their ratio (serotonin turnover); although these changes were somewhat nuanced. In addition, while increasing in extracellular serotonin would increase active coping in the swim, 5-HT2A receptors would not be predicted to contribute to this effect (Quesseveur et al., 2013) pointing to additional or alternative underlying mechanisms. Another factor that could account for the alterations in 5-HT2A receptor signaling is altered phosphorylation state, which are implicated in mediating some of the long-term adaptations of G-protein couple receptor-systems to chronic activation. One of the genes within the 16p11.2 chromosomal region is MAPK3, which encodes ERK1 MAP kinase. Previous studies have shown ERK1/2 signaling has the capacity to regulate 5-HT2A-receptor activity (Franklin and Carrasco, 2013). Likewise another kinase, RSK2 has the ability to profoundly change agonist efficacy at 5-HT2A receptors (Strachan et al., 2009). These changes in agonist efficacy can be both profound and specific for individual ligands (Strachan et al., 2010) thus it’s certainly plausible that altered intracellular signaling cascades could contribute to the observed alterations in the effects of serotonergic ligands in Del mice.
In the present study, we also find evidence for altered neural activation following 5-HT2 ligand administration within both the striatum and pre-limbic cortex. Alterations in striatal function including evidence of increased excitability have previously been reported in Del mice (Portmann et al., 2014). Consistent with those observations we find a baseline hyperactivation of dorsal striatum Fos in Del mice. Intriguingly, this hyperactivation of Fos is rescued by the 5-HT2A antagonist in Del mice, while this drug has no effect on Fos in WT mice thus these effects on Fos parallel the behavioral effects of the drug. While it’s unknown if Fos-expressing neurons drive the behavioral difference, they seem to reflect the altered brain states associated with the difference in behavioral performance. At the level of the prelimbic cortex, Fos activation was similar between Del and WT mice at baseline. However both the increased sensitivity to the 5-HT2A antagonist and decreases sensitivity to the 5-HT2A agonist DOI was apparent. 5-HT2A receptors are expressed in both a subpopulation of pyramidal neurons and approximately one quarter of GABAergic interneurons in cortex (Willins et al., 1997; Santana et al., 2004). The diminished response to DOI was particularly striking in magnitude. These results provide evidence for altered cortical processing related to 5-HT2A.
In addition to altered effects of 5-HT2A receptor ligands, the 5-HT2C receptor antagonist had an unusual behavioral effect in Del mice. Del mice appeared very sensitive to a locomotor inhibition produced by RS10221, while this drug did not significantly change swim coping strategy. This observation might suggest additional hypotheses regarding a potential contribution of 5-HT2C receptors to other elements of the phenotype of Del mice and by extension humans. 5-HT2C receptors are involved in feeding (Simansky, 1996), which is altered in both species although in opposite directions (mice are underweight while humans tend to be overweight). In addition, the findings might lead to speculation on a potential involvement of 5-HT2C receptors in epilepsy or seizure phenotype where 5-HT2C receptors are known to play a role (Heisler et al., 1998). Epilepsy or seizure is the most frequent neurological disorder observed in deletion carriers, present in about 25% of cases (Zufferey et al., 2012; Steinman et al., 2016).
Conclusions
We discover abnormalities in serotonin signaling in Del mice most notably involving altered action of a 5-HT2A-receptor antagonist. Blocking 5-HT2A receptors in fact reversed the perseverative active coping response Del mice exhibit in the forced swim. There is a wealth of literature on the contribution of 5-HT2A receptors to arousal, attention, working memory, cognitive flexibility and inhibitory control (reviewed by: (Aznar and Hervig Mel, 2016)) and deficits in all of these functions could come into play in contributing to the susceptibility of human Del carriers to ASD or attention deficit disorders. Likewise observations regarding altered 5-HT2C antagonist effects suggest this may also be a therapeutic target of interest for additional characteristics of the disorder. These findings strengthen the basis for the hypothesis that abnormalities in endogenous serotonin may contribute to a common neural circuit deficit in ASD. Thus even though antidepressants do not appear useful to treat ASD, other serotonergic targets such as 5-HT2A and 5-HT2C receptors may prove substantially more fruitful, particularly in the case of 16p11.2 deletion syndrome.
Supplementary Material
Acknowledgments
Thanks to Jeremy Kamari for technical assistance. Thanks to Drs. Benlian Gao and Ginger Milne at the Neurochemistry Core at Vanderbilt University for HPLC analysis. Funding provided by the National Institutes of Health grants DA021801 and HD036379, the Brain and Behavior Foundation NARSAD Independent Investigator Award, and the Sara Page Mayo Foundation for Pediatric Pain Research.
List of Abbreviations
- ASD
autism spectrum disorder
- Del mouse
16p11.2 deletion syndrome mouse model or B6129S-Del(7Slx1b-spet1)4Aam/J
- WT
wild-type
- 5-HT
5-hydroxytriptamine or serotonin
- SERT
serotonin transporter
- HTR
head twitch response
- ESR
ear scratch response
- TPH
tryptophan hydroxylase
- 5-HIAA
5-hydroxyindoleacetic acid
- DOI
2,5,-dimethyoxy-4-iodoamphetamine
- PLC
prelimbic cortex
- DS
dorsal striatum
- FMR1
Fragile-X Mental Retardation 1
- TS2-neo
Timothy Syndrome Type 2
Footnotes
Conflict of Interest Disclosure: Dr. Commons/Consultant for Zogenixs, Inc.
References
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Risperidone and the 5-HT2A receptor antagonist M100907 improve probabilistic reversal learning in BTBR T + tf/J mice. Autism research : official journal of the International Society for Autism Research. 2014;7:555–567. doi: 10.1002/aur.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakos CC, Watson AJ, O’Brien WT, Krainock KS, Nickl-Jockschat T, Abel T. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism research : official journal of the International Society for Autism Research. 2016 doi: 10.1002/aur.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast T, Ouagazzal AM, Chevalier C, Kopanitsa M, Afinowi N, Migliavacca E, Cowling BS, Birling MC, Champy MF, Reymond A, Herault Y. Reciprocal Effects on Neurocognitive and Metabolic Phenotypes in Mouse Models of 16p11.2 Deletion and Duplication Syndromes. PLoS Genet. 2016;12:e1005709. doi: 10.1371/journal.pgen.1005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Hervig Mel S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci Biobehav Rev. 2016;64:63–82. doi: 10.1016/j.neubiorev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Brunner D, Kabitzke P, He D, Cox K, Thiede L, Hanania T, Sabath E, Alexandrov V, Saxe M, Peles E, Mills A, Spooren W, Ghosh A, Feliciano P, Benedetti M, Luo Clayton A, Biemans B. Comprehensive Analysis of the 16p11.2 Deletion and Null Cntnap2 Mouse Models of Autism Spectrum Disorder. PloS one. 2015;10:e0134572. doi: 10.1371/journal.pone.0134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Commons KG. Ascending serotonin neuron diversity under two umbrellas. Brain Struct Funct. 2016 doi: 10.1007/s00429-015-1176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS chemical neuroscience. 2017 doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural plasticity. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DJ, Panzini C, Commons KG. Disrupted Cav1.2 L-type calcium channel function and expression alters behavior and ascending serotonin system activity. Society for Neuroscience Abstracts 2016 [Google Scholar]
- Franklin JM, Carrasco GA. Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT(2A)) receptor activity via ERK1/2 signaling. Synapse. 2013;67:145–159. doi: 10.1002/syn.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Villamisar D, Rojahn J. Comorbid psychopathology and stress mediate the relationship between autistic traits and repetitive behaviours in adults with autism. J Intellect Disabil Res. 2015;59:116–124. doi: 10.1111/jir.12083. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, NcKee S, Schoch H, Bowman N, Havekes R, O’Brien W, HMahrt E, Commons K, Portfors C, Nickl-Jockschat T, Rayes T, Abel T. Male-specific deficits in natural reward learning in a mouse model of 16p11.2 hemideletion. Society for Neuroscience Abstracts 460.05. 2016 doi: 10.1038/mp.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YP, Commons KG. Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism research : official journal of the International Society for Autism Research. 2016 doi: 10.1002/aur.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YP, Commons KG. Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism research : official journal of the International Society for Autism Research. 2017;10:66–77. doi: 10.1002/aur.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effect of Hallucinogens on Unconditioned Behavior. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2016_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, et al. The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry. 2015;77:785–793. doi: 10.1016/j.biopsych.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Tecott LH. Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann N Y Acad Sci. 1998;861:74–78. doi: 10.1111/j.1749-6632.1998.tb10175.x. [DOI] [PubMed] [Google Scholar]
- Hervig ME, Jensen NC, Rasmussen NB, Rydbirk R, Olesen MV, Hay-Schmidt A, Pakkenberg B, Aznar S. Involvement of serotonin 2A receptor activation in modulating medial prefrontal cortex and amygdala neuronal activation during novelty-exposure. Behav Brain Res. 2017;326:1–12. doi: 10.1016/j.bbr.2017.02.050. [DOI] [PubMed] [Google Scholar]
- Hippolyte L, et al. The Number of Genomic Copies at the 16p11.2 Locus Modulates Language, Verbal Memory, and Inhibition. Biol Psychiatry. 2016;80:129–139. doi: 10.1016/j.biopsych.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, Krieger AM, Buja A, Henkelman RM, Wigler M, Mills AA. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci U S A. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J. Cortical interneuron dysfunction in epilepsy associated with autism spectrum disorders. Epilepsia. 2016;57:182–193. doi: 10.1111/epi.13272. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience. 2010;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CL, Anacker AM, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016;321:24–41. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Lena C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Portmann T, et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell reports. 2014;7:1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesseveur G, Reperant C, David DJ, Gardier AM, Sanchez C, Guiard BP. 5-HT(2)A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Experimental brain research. 2013;226:285–295. doi: 10.1007/s00221-013-3434-3. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA, Furlanetto RW, Charles JM. Enhanced cortisol response to stress in children in autism. Journal of autism and developmental disorders. 2012;42:75–81. doi: 10.1007/s10803-011-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman KJ, Spence SJ, Ramocki MB, Proud MB, Kessler SK, Marco EJ, Green Snyder L, D’Angelo D, Chen Q, Chung WK, Sherr EH, Simons VIPC. 16p11.2 deletion and duplication: Characterizing neurologic phenotypes in a large clinically ascertained cohort. American journal of medical genetics Part A. 2016;170:2943–2955. doi: 10.1002/ajmg.a.37820. [DOI] [PubMed] [Google Scholar]
- Strachan RT, Sciaky N, Cronan MR, Kroeze WK, Roth BL. Genetic deletion of p90 ribosomal S6 kinase 2 alters patterns of 5-hydroxytryptamine 2A serotonin receptor functional selectivity. Mol Pharmacol. 2010;77:327–338. doi: 10.1124/mol.109.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan RT, Sheffler DJ, Willard B, Kinter M, Kiselar JG, Roth BL. Ribosomal S6 kinase 2 directly phosphorylates the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor, thereby modulating 5-HT2A signaling. J Biol Chem. 2009;284:5557–5573. doi: 10.1074/jbc.M805705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. American journal of human genetics. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49:207–228. doi: 10.1016/j.psyneuen.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uutela M, Lindholm J, Rantamaki T, Umemori J, Hunter K, Voikar V, Castren ML. Distinctive behavioral and cellular responses to fluoxetine in the mouse model for Fragile X syndrome. Front Cell Neurosci. 2014;8:150. doi: 10.3389/fncel.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zufferey F, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. Journal of medical genetics. 2012;49:660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.