Studies on combined radiotherapy and nanoparticles (NPs) have shown that a greater therapeutic ratio can be achieved using gold nanoparticles (GNPs) as radiosensitizing agents within the tumor.1 Recent Monte Carlo studies demonstrated that GNPs enhance the absorbed dose by choroidal melanoma during brachytherapy with sources such as 103Pd and 125I.2 Given that the energy range of these sources is low, NPs with high atomic number can be suitable to yield a higher absorbed dose when used alongside the mentioned sources.3 Although it has been a long time since the merit and priority of using NPs in treating cancer by low-energy sources has been studied, regarding eye cancers a major concern has been the way these NPs would be spread in the ocular tissues. A principal question is the form and method by which the NPs disperse within the intraocular tumor after intralesional injection. Will the NPs be also absorbed by the nearby healthy tissues?
This study focuses on understanding the distribution of GNPs within human choroidal melanoma considering both cancerous and nearby normal cells. Since the fine-needle aspiration biopsy is the current standard of care for diagnosis and prognostication of patients with choroidal melanoma, this method via an ex vivo study was used for intratumoral injection of GNPs in a fresh human whole eye enucleated for choroidal melanoma.4
Under sterile conditions, the enucleated eye was transilluminated with a light source in a dark room to disclose the transillumination defect corresponding to the location of the choroidal melanoma. By using a 30-gauge needle, intratumoral injection of 100 µL of GNPs at the concentration of 2.6 mM was performed. Then, a middle 3-mm-thick calotte including pupil-optic nerve was obtained and kept in an organ culture medium at 32°C for 24 hours, and after fixation in 10% formalin, was processed into paraffin blocks. The rest of the eye fixed in 10% formalin was also subjected to tissue processing. Serial tissue sections in five different tissue levels (250 µm apart) were obtained, stained with hematoxylin and eosin, and examined under a light microscope (Olympus BX41; Olympus Corporation, Tokyo, Japan) for the distribution of GNPs not only in the tumor but also in other parts of the eye.
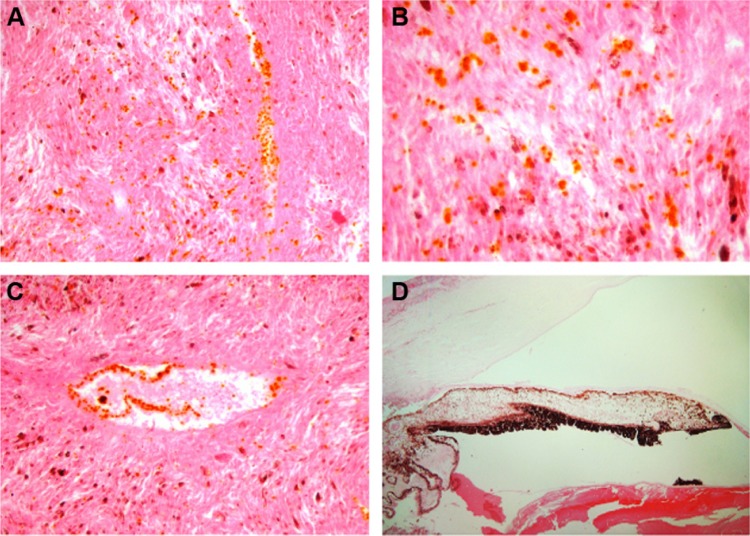
Histopathological examinations disclosed an amelanotic uveal tumor composed of sheets of spindle- and epithelioid-type cells. There was a rather diffuse infiltration of GNPs as orange-colored particles of irregular shapes and sizes within the tumor. There was a focal but high affinity of GNPs for the endothelial lining of the tumor vessels (Figure 1). The GNPs were not observed in extratumoral areas.
Figure 1.
Distribution of gold nanoparticles (GNPs) after intratumoral injection in a human eye enucleated for uveal melanoma. Note rather diffuse infiltration of orange-colored GNPs within the sheets of tumor cells ([A and B], hematoxylin and eosin stain, magnifications ×200 and 400, respectively) and the high affinity of the GNPs for intratumoral vascular endothelial lining ([C], hematoxylin and eosin stain, magnification ×200). Image (D) represents lack of presence of GNPs in extratumoral areas such as the cornea, iris, ciliary body, and lens (hematoxylin and eosin stain, magnification ×200).
Results of this study prove proper distribution of GNPs within choroidal tumor after intralesional injection and under ex vivo conditions. Considering the intrinsic radiosensitization potential of GNPs, accumulation of these NPs within some cancerous cells and their interaction with keV energy radiation via the photoelectric effect, results in the emission of micrometer-range photo/Auger electrons.5 Due to these secondary electrons, dose enhancement has been predicted as far away as 10 µm from the GNP surface which would enhance absorbed radiation dose locally.6 Furthermore, the possibility of increased release of TNF from postradiation apoptotic GNP-loaded tumor cells should be considered.7 Such event may affect the viability of nearby GNP-free tumor cells.
Given the radiation dose needed to halt the growth of melanoma tumor in a specific time interval, brachytherapy of eye tumors using low-energy sources like 125I or 103Pd in combination with NPs results in an increase in the locally absorbed dose by the tumor and a decrease in treatment time. Eventually, a reduction in the treatment time leads to a reduction in the absorbed dose by the surrounding normal tissue.
This research was done through an ex vivo study; however, in vivo study allows us to better understand how NPs disperse within the intraocular tumor after intralesional injection. Animal studies are necessary to enable precise investigation of the effects of GNPs on cancerous and healthy tissues to obtain reliable pretreatment planning for choroidal melanoma therapy. Therefore, future directions should involve a full investigation of the effects of GNPs on the choroidal melanoma dosimetry with a brachytherapy source in an animal model.
The human eye used in this research had been donated for research use. The whole experiment was approved by the ethics committee of the Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The authors alone are responsible for the content and writing of the paper.
References
- 1.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 2.Asadi S, Vaez-zadeh M, Vahidian M, Marghchouei M, Masoudi SF. Ocular brachytherapy dosimetry for 103Pd and 125I in the presence of gold nanoparticles: a Monte Carlo study. J Appl Clin Med Phys. 2016;17(3):90–99. doi: 10.1120/jacmp.v17i3.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79(939):248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Song X, Jia R, et al. Radiation-inducible human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy: a novel treatment for radioresistant uveal melanoma. Pigment Cell Melanoma Res. 2010;23(5):661–674. doi: 10.1111/j.1755-148X.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones BL, Krishnan S, Cho SH. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Med Phys. 2010;37(7):3809–3816. doi: 10.1118/1.3455703. [DOI] [PubMed] [Google Scholar]
- 6.Carter JD, Cheng NN, Qu Y, Suarez GD, Guo T. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B. 2007;111(40):11622–11625. doi: 10.1021/jp075253u. [DOI] [PubMed] [Google Scholar]
- 7.Rübe CE, Wilfert F, Uthe D, et al. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother Oncol. 2002;64(2):177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]