Abstract
Despite the efficacy of a number of first-line treatments, most patients with advanced-stage non-small cell lung cancer (NSCLC) experience disease progression that warrants further treatment. In this review, we examine the role of novel active agents for patients who progress after first-line therapy and who are not candidates for targeted therapies. More therapeutic options are needed for the management of patients with NSCLC after failure of first-line chemotherapy. A PubMed search was performed for articles from January 2012 to May 2015 using the keywords NSCLC, antiangiogenic, immunotherapy, second-line, novel therapies and English language articles only. Relevant papers were reviewed; papers outside that period were considered on a case-by-case basis. A search of oncology congresses was performed to identify relevant abstracts over this period. In recent years, antiangiogenic agents and immune checkpoint inhibitors have been added to our armamentarium to treat patients with advanced NSCLC who have progressed on first-line chemotherapy. These include nintedanib, a triple angiokinase inhibitor; ramucirumab, a vascular endothelial growth factor receptor-2 antibody; and nivolumab, pembrolizumab and atezolizumab, just three of a growing list of antibodies targeting the programmed death receptor-1 (PD-1)/PD ligand-1 pathway. Predictive and prognostic factors in NSCLC treatment will help to optimise treatment with these novel agents. The approval of new treatments for patients with NSCLC after the failure of first-line chemotherapy has increased options after a decade of few advances, and holds promise for future evolution of the management of NSCLC.
Keywords: Nintedanib, Ramucirumab, Antiangiogenesis, Immune checkpoint inhibitors, Prognostic factors
Introduction
Lung cancer incidence, particularly adenocarcinoma,1 is increasing globally and the disease remains the most commonly diagnosed cancer. The majority of patients (85%) are diagnosed with non-small cell lung cancer (NSCLC)2 and, within this population, adenocarcinoma and squamous cell carcinoma are the two major histological subtypes, accounting for ∼45% and 25% of cases, respectively, with large variations according to geographical location.3 4 Up to 45% of patients with advanced NSCLC experience disease progression during first-line chemotherapy,5–7 and all patients with initial disease control will eventually experience progression and require subsequent therapy.
Until 2014, the available agents for the second-line treatment of advanced NSCLC without driver mutations included docetaxel (Taxotere; Sanofi-Aventis, Bridgewater, USA), pemetrexed (Alimta; Eli Lilly, Indianapolis, USA) (non-squamous patients only) and erlotinib (Tarceva, Genentech/OSI Pharmaceuticals/Roche).8 9 In this review, we will examine the role of recently approved novel therapies in the management of patients with NSCLC, with a particular focus on antiangiogenic agents and immune checkpoint inhibitors following first-line chemotherapy.
Tumour angiogenesis: a treatment target
Angiogenesis is widely accepted as a fundamental process for the growth of primary tumours and their subsequent metastases,10 involving multiple receptors and their associated pathways (figure 1).
Figure 1.
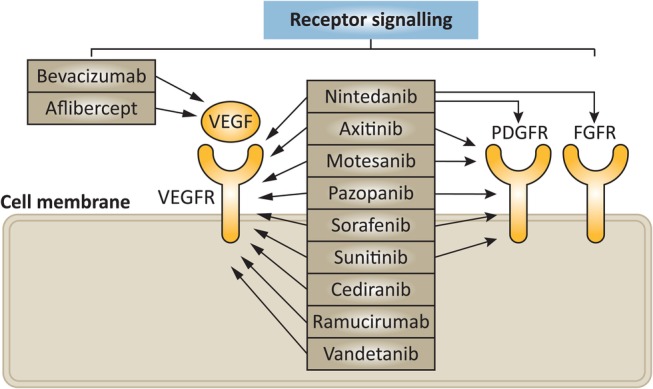
Overview of important signalling pathways in angiogenesis and antiangiogenic agents. Reprinted by permission from Macmillan Publishers: Llovet et al53 copyright 2015. FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Vascular endothelial growth factor (VEGF) has a prominent role in angiogenesis, mediating its effects via endothelial cells; consequently, the VEGF/VEGF receptor (VEGFR) pathway has been a very attractive therapeutic target.10 Proangiogenic pathways have substantial redundancy, allowing tumours to bypass the inhibition of a single pathway and to adapt to the presence of antiangiogenic agents.11 Acquired resistance involves interaction between cells and the tumour microenvironment, and uses various different proangiogenic pathways (including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and other signalling pathways) to recruit vasculature.11 12 The tumour microenvironment—which includes both the malignant transformed cells, and also stromal, immune and endothelial cells—also plays a role in tumour progression.13 It is postulated that non-malignant cells, including immune cells that infiltrate a tumour, acquire tumour-promoting functions, including encouraging the creation of new blood vessels and facilitating rapid expansion and progression towards malignancy.
The two main types of antiangiogenic agents that have been investigated in NSCLC are monoclonal antibodies and small-molecule tyrosine kinase inhibitors (TKIs), both of which target specific angiogenic receptors and pathways (table 1).
Table 1.
Targeted agents influencing angiogenesis evaluated in NSCLC
| Agent | Description | Target |
|---|---|---|
| Bevacizumab | MAb | VEGF-A |
| Ramucirumab | MAb | VEGFR-2 |
| Anlotinib | TKI | VEGFR-2–3 |
| Apatinib | TKI | VEGFR-2 |
| Axitinib | TKI | VEGFR-1–3, PDGFR, c-kit |
| Cediranib | TKI | VEGF-1–3 |
| Fruquintinib | TKI | VEGFR-1–3 |
| Lenvatinib | TKI | VEGFR-1–3, PDGFR-α, FGFR-1–4, RET and c-kit |
| Motesanib | TKI | VEGFR-1–3, PDGFR, kit, RET |
| Nintedanib | TKI | VEGFR-1–3, FGFR-1–3, PDGFR-α/β |
| Pazopanib | TKI | VEGFR, PDGFR and c-kit |
| Sorafenib | TKI | VEGFR-1–3, RET, PDGFR, Flt-3, c-kit |
| Sunitinib | TKI | VEGFR-1/2, PDGFR-α/β, Flt-3 and c-kit |
| Vandetanib | TKI | VEGFR, EGFR, RET |
| Aflibercept | Decoy receptor | All VEGF-A isoforms, VEGF-B, PIGF |
| Endostar | Recombinant human endostatin | VEGF-induced phosphorylation of VEGFR-2, FGF-2 |
EGFR, epidermal growth factor receptor; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; MAb, monoclonal antibody; NSCLC, non-small cell lung cancer; PDGFR, platelet-derived growth factor receptor; PlGF, placental growth factor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Bevacizumab (Avastin; Genentech/Roche, Basel, Switzerland), a humanised monoclonal antibody that binds to VEGF-A, provided proof-of-principle for antiangiogenic therapy in NSCLC in combination with first-line platinum-based chemotherapy and is approved in patients with advanced non-squamous NSCLC.14 Subsequent extensive investigation of other antiangiogenic agents in advanced NSCLC did not result in regulatory approvals in the first-line or maintenance setting.11 However, promising results were reported in previously treated patients with NSCLC.
Antiangiogenic agents
Over the past decade, numerous clinical trials involving novel agents in patients with NSCLC who progressed on first-line therapy reported modest improvements in progression-free survival (PFS) but no significant improvements in overall survival (OS). These include vandetanib (ZODIAC, ZEAL and ZEST trials),15–17 aflibercept (VITAL),18 bevacizumab (BeTa)19 and sunitinib (SUN1087)20 21 as monotherapy, or in combination with chemotherapy (docetaxel or pemetrexed) or erlotinib.
In 2014, two antiangiogenic agents were approved for patients with advanced NSCLC after first-line chemotherapy. The results of the LUME-Lung 1 trial (NCT00805194, study 1199.13) first led to European Union (EU) approval of nintedanib (Vargatef; Boehringer Ingelheim, Ingelheim, Germany), in combination with docetaxel, for the treatment of patients with locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma histology after first-line chemotherapy.22 Subsequently, results of the REVEL trial (NCT01168973, study 13852) led to the US and EU approvals of ramucirumab (Cyramza; Eli Lilly, Indianapolis, USA) in combination with docetaxel for patients with metastatic NSCLC with disease progression on or after platinum-based chemotherapy.22 23
Nintedanib
Nintedanib is an oral, triple angiokinase inhibitor that inhibits VEGFR-1–3, FGF receptors 1–3 and PDGF receptor-α and PDGF receptor-β.24 Nintedanib also inhibits FLT3 and the Src kinase family.
Two phase III trials assessed the efficacy and safety of nintedanib.25 26 Both studies were similar in design: multinational, randomised, double-blind, placebo-controlled trials conducted in Eastern Cooperative Oncology Group performance score (ECOG PS) 0–1 patients with histologically or cytologically confirmed stage IIIB/IV or recurrent NSCLC after one previous platinum-based chemotherapy.25 26
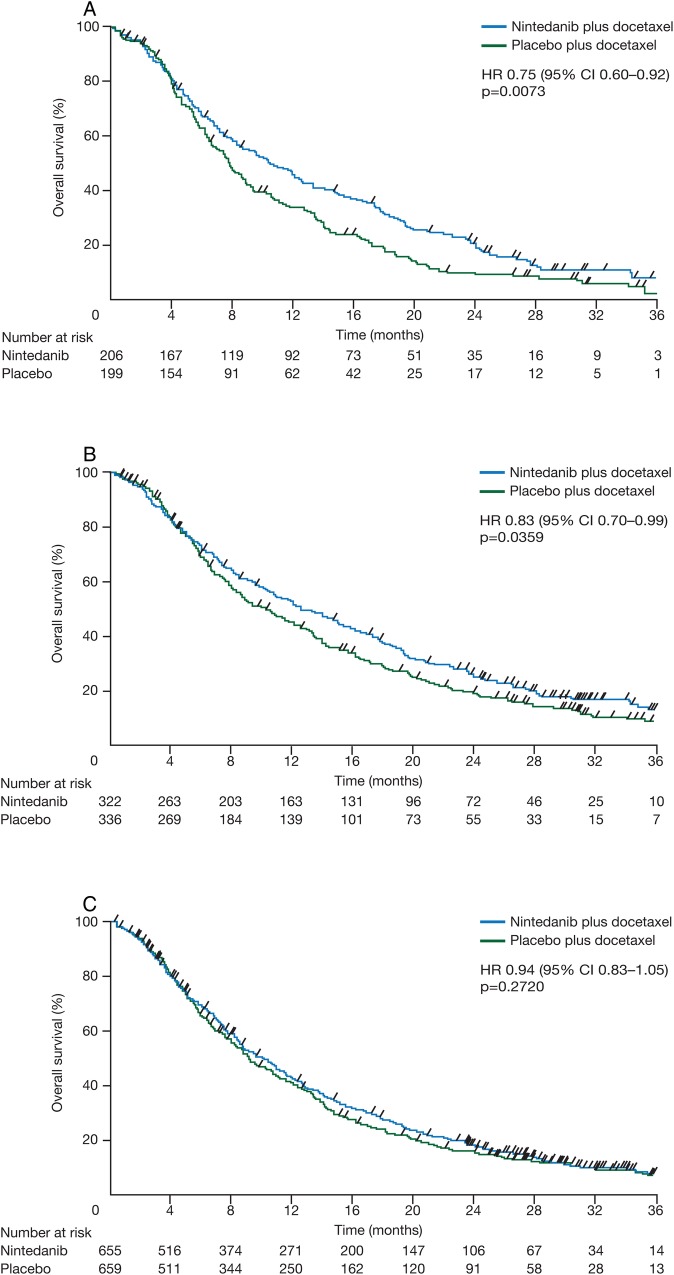
In LUME-Lung 1, 1314 patients were randomised to receive nintedanib (200 mg two times a day, n=655) or placebo (n=659) on days 2–21 in combination with docetaxel (75 mg/m2) on day 1, every 3 weeks. The two major histologies were adenocarcinoma (n=658) and squamous cell carcinoma (n=555). The study met its primary end point of PFS by independent central review, with nintedanib plus docetaxel showing significant improvement in median PFS versus placebo plus docetaxel (3.4 vs 2.7 months, HR 0.79, p=0.002) independent of histology.25 The key secondary end point, OS, was tested in a prespecified stepwise order, maintaining adequate power: first, in patients with adenocarcinoma histology who progressed within 9 months after the start of first-line therapy; then in all adenocarcinoma patients; and then in the overall population. A significant increase in median OS (10.9 vs 7.9 months, HR 0.75, 95% CI 0.60 to 0.92, p=0.007) was observed in the nintedanib arm versus the placebo arm in patients with adenocarcinoma histology who progressed within 9 months after the start of first-line therapy (figure 2A). In the adenocarcinoma population, median OS was longer than 1 year in the nintedanib arm and significantly longer than in the placebo arm (12.6 vs 10.3 months, HR 0.83, 95% CI 0.70 to 0.99, p=0.036) (figure 2B). In the overall population, the 1-month increase in median OS in the nintedanib arm was not statistically significant (HR 0.94, 95% CI 0.83 to 1.05, p=0.272) (figure 2C). In an exploratory analysis of chemorefractory adenocarcinoma patients with disease progression as best response to first-line therapy, treatment with nintedanib also significantly increased survival (median OS 9.8 vs 6.3 months, HR 0.62, 95% CI 0.41 to 0.94 p=0.025). Further exploratory analysis reported decreased tumour burden and decelerated tumour growth over time in the nintedanib arm compared with the placebo arm in adenocarcinoma patients, including patients with the poorest prognosis.27
Figure 2.
Kaplan-Meier curves for overall survival in patients with adenocarcinoma and time since first-line therapy of <9 months (A), all patients with adenocarcinoma (B) and the total population (C) from LUME-Lung 1. Patients without documented death were censored at the date of last contact when the patient was known to be alive. Adapted from Reck et al.25 Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. 143–155, Copyright (2014), with permission from Elsevier.
In the overall population, there were higher incidences in the nintedanib arm than in the placebo arm of diarrhoea (all grades: 42% vs 22%; grade ≥3: 7% vs 3%), liver-enzyme elevations (aspartate aminotransferase, all grades: 23% vs 7%; grade ≥3: 3% vs 1%; alanine aminotransferase, all grades: 29% vs 8%; grade ≥3: 8% vs 1%) that were reversible in the majority of patients, nausea (all grades: 24% vs 18%; grade ≥3: 1% vs 1%) and decreased appetite (all grades: 22% vs 16%; grade ≥3: 1% vs 1%).25 These adverse events (AEs, Common Terminology Criteria for Adverse Events (CTCAE) V.3.0) were manageable with supportive treatment or dose reduction. The incidence of AEs associated with VEGF inhibition was generally low; bleeding events and hypertension were slightly higher with nintedanib than with placebo. Similarly, the incidence of AEs associated with docetaxel (such as peripheral neuropathy and mucositis) was slightly higher in the nintedanib arm than in the placebo arm. A similar AE profile was observed in the adenocarcinoma population.
In LUME-Lung 2, patients with non-squamous NSCLC were randomised to either nintedanib (200 mg two times a day, n=353) or placebo (n=360) on days 2–21 plus pemetrexed (500 mg/m2) on day 1, every 3 weeks. An independent data monitoring committee (DMC) conducted a preplanned futility analysis of investigator-assessed PFS and recommended that the study be halted prematurely. Subsequent analysis demonstrated a significant improvement in centrally reviewed PFS favouring the nintedanib arm over the placebo arm (median PFS 4.4 vs 3.6 months, HR 0.83, 95% CI 0.70 to 0.99, p=0.040).26 A retrospective analysis of the futility calculations indicated that the predefined threshold for futility was only crossed at the time of the futility analysis for investigator-assessed PFS, but not for centrally reviewed PFS at any point during the study, suggesting that the single time point for preplanned futility analysis was inadequate. Nintedanib plus pemetrexed had a manageable safety profile with no new or unexpected findings.
Ramucirumab
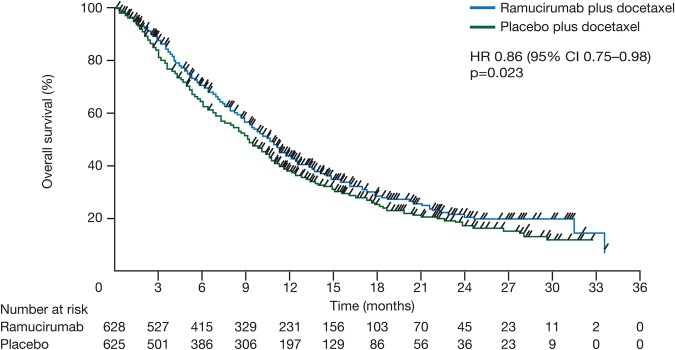
Ramucirumab is an intravenously administered monoclonal antibody that specifically binds to the extracellular domain of VEGFR-2.28 The multicentre, double-blind, randomised phase III REVEL study assessed the efficacy and safety of docetaxel (75 mg/m2) plus ramucirumab (10 mg/kg, n=627) or placebo (n=625) every 3 weeks in ECOG PS 0–1 patients with stage IV NSCLC who progressed during or after first-line platinum-based chemotherapy.28 29 The study met its primary end point of OS, with a longer survival reported for ramucirumab plus docetaxel compared with docetaxel plus placebo (10.5 vs 9.1 months, HR 0.86, 95% CI 0.75 to 0.98, p=0.023) (figure 3). Median PFS, a secondary end point, was also higher in the ramucirumab arm versus the placebo arm (4.5 vs 3.0 months, HR 0.76, 95% CI 0.68 to 0.86; p<0.0001).
Figure 3.
Kaplan-Meier curves for overall survival in the REVEL trial (intent-to-treat population). Reprinted from Garon et al.28 Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. 665–673, Copyright (2014), with permission from Elsevier.
REVEL was not powered for subgroup analysis according to histology; however, longer median OS was observed with ramucirumab plus docetaxel than with placebo plus docetaxel in patients with non-squamous NSCLC (n=912; 11.1 months vs 9.7 months; HR 0.83, 95% CI 0.71 to 0.97, p=0.02),28 including patients with adenocarcinoma histology (n=725; 11.2 months vs 9.8 months; HR 0.83, 95% CI 0.69 to 0.99; p value not reported).29 In the squamous population, a numerically longer median OS in the ramucirumab arm did not reach statistical significance (n=328; 9.5 months vs 8.2 months; HR 0.88, 0.69 to 1.13; p=0.32).28 In a univariate exploratory analysis, patients with time since start of prior therapy of <9 months had a longer OS in the ramucirumab arm versus the placebo arm (HR 0.75, 95% CI 0.64 to 0.88).28
In REVEL, the most frequently observed AEs (CTCAE V.4.0) in the ramucirumab arm were similar to those observed in the placebo arm and included fatigue (all grades: 55% vs 49%; grade ≥3: 14% vs 10%), decreased appetite (all grades: 29% vs 25%; grade ≥3: 2% vs 1%), diarrhoea (all grades: 32% vs 27%; grade ≥3: 5% vs 3%), nausea (all grades: 27% vs 27%; grade ≥3: 1% vs 1%) and alopecia (all grades: 26% vs 25%; grade ≥3: NA).28 AEs observed more frequently in the ramucirumab arm (≥10% difference between treatment arms) were neutropenia (55% vs 45%) and stomatitis (23% vs 13%). Overall, the toxicities observed with ramucirumab were manageable with dose adjustments or supportive care. AEs associated with VEGF inhibition that were higher in the ramucirumab arm included bleeding (29% vs 15%) with most events related to epistaxis (19% vs 6%), and hypertension (11% vs 5%) with more than half of events at grade ≥3 (6% vs 2%).28 The frequency of grade ≥3 pulmonary haemorrhage was comparable between the treatment arms.
Immune checkpoint inhibitors
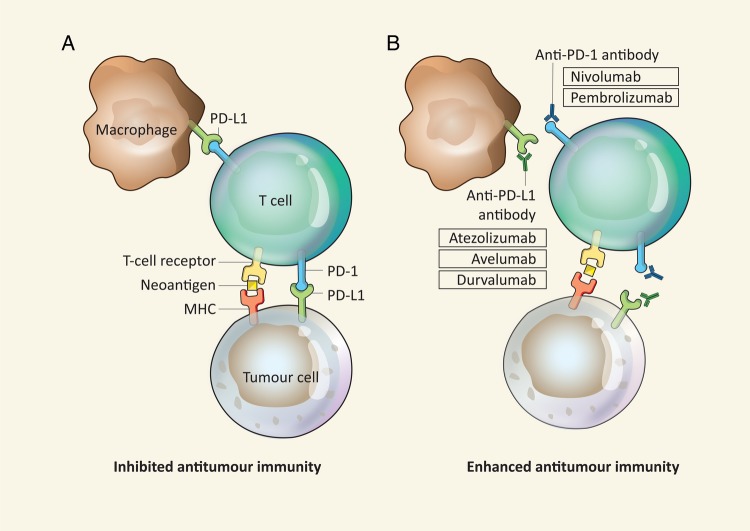
Results with programmed death receptor-1 (PD-1) inhibitors and PD ligand-1 (PD-L1) inhibitors were encouraging in early clinical trials, leading to several large, randomised phase III trials in previously treated patients with NSCLC.30 PD-1 is expressed on several immune cells, including T cells, B cells and natural killer cells, whereas PD-L1 is expressed on tumour cells, as well as a range of immune effector cells. The PD-1/PD-L1 interaction has a strong immunosuppressive effect in downregulating T-cell function, and blockade of the PD-1/PD-L1 pathway using antagonistic monoclonal antibodies has been shown to increase the number and functionality of tumour-specific T cells (figure 4).31
Figure 4.
Overview of checkpoint blockade activating antitumour immunity and checkpoint immune inhibitors in development in NSCLC. Reprinted by permission from Macmillan Publishers : Wolchok et al.54 MHC, major histocompatibility complex; PD-1, programmed death receptor-1; PD-L1, programmed death receptor ligand-1.
Nivolumab
Nivolumab is a fully human immunoglobulin (Ig) G4 antibody that disrupts PD-1-mediated signalling and has the potential to restore antitumour immunity.32 Phase III trials for nivolumab versus docetaxel in patients with squamous (CheckMate-017, NCT01642004) and non-squamous (CheckMate-057, NCT01673867) histology after the failure of platinum-based doublet chemotherapy were stopped early following planned interim analyses and a DMC assessment that concluded that both studies met their primary end point, demonstrating superior OS in patients receiving nivolumab when compared to the control arm.32 33 Nivolumab (Opdivo; Bristol-Myers Squibb, New York, USA) has received US and EU approval for the treatment of locally advanced or metastatic NSCLC with progression on or after chemotherapy.34
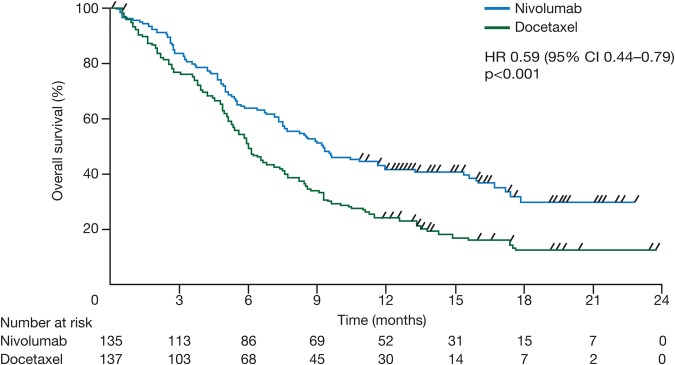
In the CheckMate-017 study (n=272), patients with stage IIIB/IV, squamous NSCLC and ECOG 0–1 were randomised to receive nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks); patients were required to submit pretreatment (archival or recent) tumour-tissue specimen for retrospective biomarker analyses.33 Treatment with nivolumab improved median OS by 3.2 months from 6.0 months for docetaxel to 9.2 months for nivolumab in patients with squamous NSCLC (HR 0.59, 95% CI 0.44 to 0.79, p<0.001) (figure 5). PFS also significantly improved (HR 0.62, 95% CI 0.47 to 0.81, p<0.001). OS and PFS were similar among the subgroups of patients with differing levels of PD-L1 expression.
Figure 5.
Kaplan-Meier curves for overall survival in the CheckMate-017 trial (intent-to-treat population). Reprinted from Brahmer et al.33
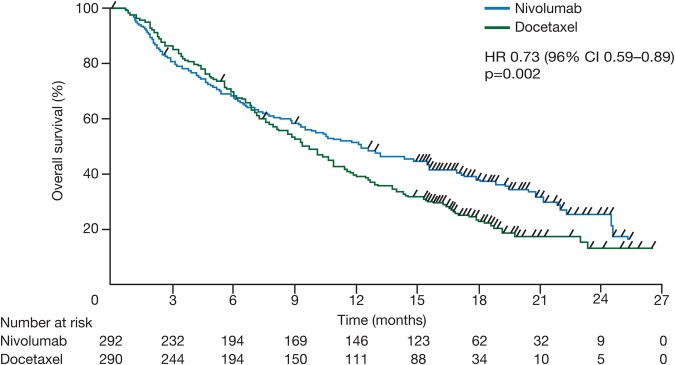
The similarly designed CheckMate-057 study (n=582) also demonstrated a superior OS (median OS 12.2 months vs 9.4 months, HR 0.73, 96% CI 0.59 to 0.89, p=0.002) with nivolumab in patients with non-squamous NSCLC (figure 6).32 There was no significant improvement in PFS (HR 0.92, 95% CI 0.77 to 1.11, p=0.39). In terms of PD-L1 expression, median OS was higher with nivolumab than with docetaxel in patients with ≥1% PD-L1 expression (17.7 months vs 9.0 months, HR 0.58, 95% CI 0.43 to 0.79) but similar in patients with <1% PD-L1 expression (10.5 months vs 10.1 months, HR 0.87, 95% CI 0.63 to 1.19). Nivolumab appeared to be more effective in patients with a longer time from completion of the most recent regimen to randomisation, with an HR for OS of 0.46 (95% CI 0.27 to 0.79) in patients with more than 6 months from completion of most recent regimen to randomisation versus 0.85 (95% CI 0.67 to 1.08) in patients with <3 months to randomisation. The most frequently observed any-grade AEs were similar in the two treatment arms (98% vs 99%). Any grade treatment-related AEs were lower with nivolumab than with docetaxel (69% vs 88%) as were grade ≥3 AEs (10% vs 54%). Frequent all-causality AEs (CTCAE V.4.0) with nivolumab included fatigue (all grades: 32% vs 38%; grade 3–4: 3% vs 7%), decreased appetite (all grades: 29% vs 22%; grade 3–4: 2% vs 1%), cough (all grades: 26% vs 23%; grade 3–4: <1% vs 0), constipation (all grades: 23% vs 17%; grade 3–4: 1% vs 1%), dyspnoea (all grades: 23% vs 24%; grade 3–4: 5% vs 4%), nausea (all grades: 22% vs 30%; grade 3–4: 2% vs 1%) and asthenia (all grades: 21% vs 23%; grade 3–4: 3% vs 4%). Safety profiles were similar between the subgroups of patients with PD-L1 expression of <1% and ≥1%. Immune-modulating agents were administered to resolve AEs such as rash and pruritus. Safety profiles were comparable in the CheckMate-057 and CheckMate-017 studies.32 33
Figure 6.
Kaplan-Meier curves for overall survival in the CheckMate-057 trial (intent-to-treat population). Reprinted from Borghaei et al.32
Pembrolizumab
Pembrolizumab is a monoclonal IgG4 anti-PD-1 antibody that disrupts the engagement of PD-1 with its ligand.35 The KEYNOTE-001 phase I study (NCT01295827) enrolled 495 patients of whom 101 were treatment-naïve and 394 previously treated for advanced or metastatic NSCLC and with ECOG status 0–1. Overall, 401 (81%) patients had non-squamous histology, 85 (17%) had squamous NSCLC and 9 (2%) had adenosquamous or unknown histology. Treatment with pembrolizumab (2 mg/kg or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks) demonstrated antitumour activity (objective response rate (ORR) 19.4%, mean response duration: 12.5 months) and an acceptable side-effect profile in the overall population.35 Biomarker analysis was performed, with results being reported as the percentage of neoplastic cells showing PD-L1 staining (tumour proportion score (TPS)). The biomarker-evaluable population included 73 patients with TPS ≥50% and 131 patients with TPS <50%. ORR was significantly higher (43.9% vs 14.1%; p<0.001) for previously treated patients with TPS ≥50% (n=57) than those with TPS <50% (n=99). Median PFS among patients with a TPS ≥50% was 6.1 months for previously treated patients. At a follow-up analysis with a data cut-off 6 months after the primary data cut-off, among 124 previously treated patients with PD-L1 TPS ≥50%, median PFS was 5.8 months and median OS was 14.0 months (HR not reported).36
Based on results from the KEYNOTE-001 study, pembrolizumab (Keytruda; Merck & Co, Kenilworth, USA) was granted accelerated approval in the USA for the treatment of patients with metastatic NSCLC whose tumours express PD-L1, as determined by a Food and Drug Administration (FDA)-approved test and who have disease progression on or after platinum-containing chemotherapy.37 The PD-L1 immunohistochemistry (IHC) 22C3 pharmDx test is designed to detect PD-L1 expression, with tumour samples considered to be PD-L1-positive if ≥50% of viable cells exhibit membrane staining. Based on this cut-off, KEYNOTE-001 reported the estimated prevalence of PD-L1-positive patients as 22.7% of the previously treated patient population.35
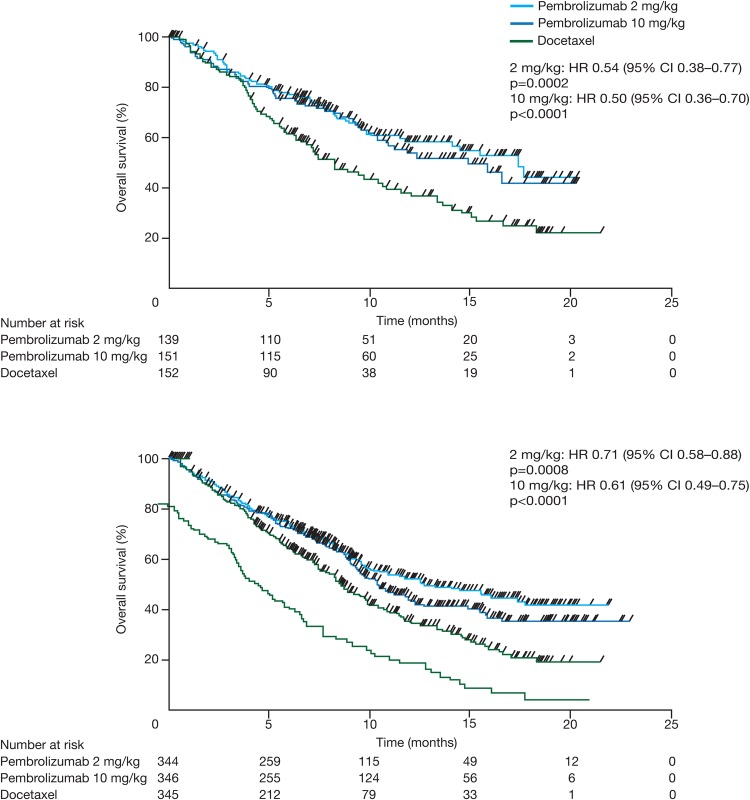
In the KEYNOTE-010 phase II/III study (NCT01905657), previously treated PD-L1-positive (TPS ≥1%) patients with advanced NSCLC were randomised in a 1:1:1 ratio to receive either pembrolizumab 2 mg/kg every 3 weeks (n=344), pembrolizumab 10 mg/kg every 3 weeks (n=346) or docetaxel 75 mg/m2 every 3 weeks (n=343).38 Primary end points were OS and PFS in the total population and in patients with TPS ≥50%. A total of 724 patients (70.1%) had non-squamous disease, 222 patients (21.5%) had squamous disease and 87 patients (8.4%) had other/unknown histology. In patients with PD-L1 TPS ≥50%, median OS was 14.9 months for the 2 mg/kg group (HR vs docetaxel 0.54, 95% CI 0.38 to 0.77, p=0.0002), 17.3 months for the 10 mg/kg group (HR vs docetaxel 0.50, 95% CI 0.36 to 0.70, p<0.0001) and 8.2 months for the docetaxel group (figure 7A). In the total population, median OS was 10.4 months (HR vs docetaxel 0.71, 95% CI 0.58 to 0.88, p=0.0008) for patients treated with pembrolizumab 2 mg/kg, 12.7 months (HR vs docetaxel 0.61, 95% CI 0.49 to 0.75, p<0.0001) for the pembrolizumab 10 mg/kg arm and 8.5 months for the docetaxel arm (figure 7B). Patients with adenocarcinoma (n=708) had greater OS benefit with pembrolizumab compared with docetaxel treatment (HR 0.63, 95% CI 0.50 to 0.79). PFS improvement was not statistically significant for either pembrolizumab arm versus docetaxel. Treatment-related AEs (CTCAE V.4.0) at any grade and at grade ≥3 were more frequent with docetaxel with either dose of pembrolizumab (all grades: pembrolizumab 2 mg/kg 63%, pembrolizumab 10 mg/kg 66%, docetaxel 81%; grade ≥3: pembrolizumab 2 mg/kg 13%, pembrolizumab 10 mg/kg 16%, docetaxel 35%). Treatment-related AEs that were frequent with pembrolizumab included decreased appetite (all grades: 14% vs 10% vs 16%; grade ≥3: 1% vs <1% vs 1%), fatigue (all grades: 14% vs 14% vs 25%; grade ≥3: 1% vs 2% vs 4%), nausea (all grades: 11% vs 9% vs 15%; grade ≥3: <1% vs 1% vs <1%) and rash (all grades: 9% vs 13% vs 5%; grade ≥3: <1% vs <1% vs 0). Immune-mediated AEs occurred at manageable rates, although three (<1%) of the 682 pembrolizumab-treated patients died of pneumonitis. Safety profiles were comparable in the KEYNOTE-001 and KEYNOTE-010 studies.35 38
Figure 7.
Kaplan-Meier curves for overall survival in patients with PD-L1 tumour proportion score of ≥50% (A) and all patients from KEYNOTE-010 (B). Adapted from Herbst et al.38 Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. 1540–1550, Copyright (2015), with permission from Elsevier.
Other checkpoint inhibitors
In this rapidly advancing area of clinical research other PD-L1 checkpoint inhibitors are being intensively investigated including atezolizumab (MPDL3280A, Roche), durvalumab (MEDI4736, AstraZeneca) and avelumab (MSB0010718C, Merck KGaA/Pfizer). Of these investigational agents atezolizumab has recently received Breakthrough Therapy Designation from the FDA, based on results in PD-L1-positive NSCLC.39 Results from an open-label, phase 2 randomised controlled trial, in patients with advanced NSCLC who progressed on postplatinum chemotherapy showed that atezolizumab significantly improved survival compared with docetaxel (12.6 months, 95% CI 9.7 to 16.4 vs 9.7 months 95% CI 8.6 to 12.0; HR=0.73, 95% CI 0.53 to 0.99; p=0.04). Increasing improvement in OS was also noted in patients with higher levels of PD-L1 expression treated with atezolizumab compared with docetaxel.40 Although the clinical programme is not as advanced as atezolizumab, treatment with durvalumab in combination with the cytotoxic T-lymphocyte-associated protein 4 inhibitor tremelimumab in patients with locally advanced or metastatic NSCLC showed evidence of clinical activity in patients with PD-L1-positive tumours and in those with PD-L1-negative tumours. The response in PD-L1 negative tumours represents a potential therapeutic option for a group of patients who have not benefited as much from the use of other checkpoint inhibitors in trials undertaken to date.41 Other studies are being undertaken as summarised in table 2 and in general checkpoint inhibitors have also shown encouraging trends across other solid tumour types, in phase I development.42–46
Table 2.
Overview of phase III trials of antiprogrammed death receptor-1 (PD-1)/programmed death receptor ligand-1 (PD-L1) inhibitors completed or ongoing in previously treated patients with advanced NSCLC
| Agent | Target | Trial name, identifier | Design |
|---|---|---|---|
| Nivolumab | PD-1 | CheckMate-057, NCT01673867 | Nivolumab in previously treated patients with NSCLC vs docetaxel alone in patients with non-squamous histology |
| CheckMate-017, NCT01642004 | Nivolumab in previously treated patients with NSCLC vs docetaxel alone in patients with squamous histology | ||
| Pembrolizumab | PD-1 | KEYNOTE-010, NCT01905657 | Pembrolizumab vs docetaxel in patients with NSCLC who have experienced disease progression after platinum-containing therapy |
| Atezolizumab (MPDL3280A) | PD-L1 | OAK, NCT02008227 | MPDL3280A vs docetaxel in patients with locally advanced or metastatic NSCLC who have failed platinum therapy |
| Durvalumab (MEDI4736) | PD-L1 | ARCTIC, NCT02352948 | MEDI4736, given as monotherapy or in combination with tremelimumab, determined by PD-L1 expression vs standard of care in patients with locally advanced or metastatic NSCLC |
| Avelumab (MSB0010718C) | PD-L1 | JAVELIN Lung 200, NCT02395172 | MSB0010718C vs docetaxel in patients with PD-L1-positive, advanced NSCLC after failure of a platinum-containing doublet |
NSCLC, non-small cell lung cancer; PD-1, programmed death receptor-1; PD-L1, programmed death receptor ligand-1.
Predictive and prognostic factors
Approaches to identify biomarkers/clinical markers include genotyping and simpler assessment of tissue samples, such as pathological and molecular tumour features, grade and pathology of the tumour, as well as plasma/serum markers. Characterising tumours according to histological subtype and genetic composition has resulted in significant progress in the identification of response to certain drugs, for example, the epidermal growth factor receptor (EGFR)-activating mutations.47
Antiangiogenic agents
Despite progress in the identification of predictive biomarkers in patients with differing tumour mutational status, none have been identified for those who receive antiangiogenic agents.48 49 A major challenge in identifying potential biomarkers to antiangiogenic therapy is the complex nature of the angiogenic signalling process, which is characterised by multiple overlapping pathways.50 The activation of compensatory bypass angiogenic pathways after initial treatment response is a significant roadblock as it results in the development of resistance.50 Consequently, there remains an urgent need for biomarkers to angiogenesis inhibitors in the treatment of cancer, including NSCLC.51
Analyses of the LUME-Lung 1 study have been conducted to identify a prognostic and/or predictive factor for the OS improvement observed in adenocarcinoma patients who received nintedanib plus docetaxel after first-line therapy.52 The analysis also used data from patients in the LUME-Lung 2 trial. These analyses suggest that: (1) time since the start of first-line therapy was a prognostic and predictive clinical biomarker for the treatment effect of nintedanib, combined with either docetaxel or pemetrexed, for patients with advanced non-squamous NSCLC, progressing after platinum-based chemotherapy; and (2) a treatment benefit was evident in those non-squamous patients with a particularly poor prognosis who progressed during or shortly after first-line treatment. Results from the REVEL study also confirm time since the start of first-line therapy as a potential clinical marker for ramucirumab.28
PD-1/PD-L1 inhibition
PD-L1 is upregulated in many cancer types and contributes to malignancy by inhibiting T-cell activation, limiting tumour cell killing by the immune system; PD-L1 expression assessed by IHC is being investigated as a marker for many anti-PD-1/PD-L1 agents.22 23 PD-L1 positivity may indicate that an immune-active tumour may be sensitive to anti-PD-1 or PD-L1 therapy because of a correlation with PD-L1 expression and poor prognosis in cancers, including lung adenocarcinoma.22 However, the predictive and/or prognostic uses of PD-L1 expression remain unclear. Several studies have shown improved responses to anti-PD-1 or anti-PD-L1 therapy in ‘PD-L1-positive’ cases; however, most studies also report significant response rates (3–30%) in PD-L1-negative tumours. There is variable definition of PD-L1 positivity in tumours, ranging from ≥1% to ≥50% of cells assessed showing PD-L1 expression. PD-L1 has dynamic expression, which could, together with heterogeneous expression, confound the determination of a positive or negative assay result. Despite the different approaches to PD-L1 assessment for each of the drugs in this class, an association between expression and response has been reasonably consistent. The presence of response in patients deemed to be PD-L1-negative has called into question the validity of this biomarker. Undoubtedly, the PD-L1 biomarker does not show the predictive performance of EGFR mutation or ALK fusion for targeted therapy with TKIs. However, the biology of the immune system, the action of the therapy and the nature of the IHC test all mean that lesser predictive power is inevitable.
To date, concordance between the different IHC antibodies used in the trials has not been reported. The assays available vary in their ability to detect PD-L1, and although some assays consider tumour cell expression, others also score immune-cell PD-L1 expression.22 Low staining thresholds, such as 1% or 5%, reflect the fact that PD-L1 expression is heterogeneous; however, they carry a greater risk that scoring will be inconsistent and an inaccurate representation of a patient's tumour burden.23 Small sample sizes in these studies may also play a role.
The variability in PD-L1 testing used and validated in trials poses serious challenges for pathologists in delivering this biomarker test, assuming it will be requested. An international effort for a standardised approach could enable the use of PD-L1 expression as a reliable biomarker for anti-PD-1/PD-L1 therapy.23
Plasma and circulating tumour cells have also been proposed as alternative forms of non-invasive, blood-based biomarker analysis.22 Plasma PD-L1 protein could provide a method for monitoring PD-1/PD-L1 interaction in NSCLC.
Future directions
Defining ‘clinically meaningful’ benefit is complex and a balance is needed between ‘objective’ end points (which have value in benchmarking) versus ‘subjective’ end points, including patient-oriented factors such as symptom relief, quality of life and toxicity reduction. Median OS remains an important outcome for benchmarking of clinical practice, but other measures should be considered, including survival HR, 1-year and 2-year survival rates, and patient-related factors.
It is important to continue the search for clinical and molecular prognostic and predictive factors. Patient selection through the use of biomarkers is important in choosing the correct treatment option. A shorter time since start of first-line therapy is a potential clinical marker for the antiangiogenic agents, considering the lower OS benefit from immunotherapy in patients with shorter time since completion of their most recent chemotherapy regimen. Collection of tumour and blood samples is essential in future studies to help identify biomarkers in order to select patients who will benefit the most. In addition, a standardised approach to measuring PD-L1 expression is needed to be a consistent biomarker for anti-PD-1/PD-L1 therapy.
Conclusions
In the past few years, new treatment options have become available for patients with NSCLC whose disease has progressed after or during first-line chemotherapy. This has come after a decade of only few advances in this setting, and holds promise for the future evolution of the management of NSCLC.
Footnotes
Funding: This work was supported financially by Boehringer Ingelheim Pharma GmbH & Co KG. Medical writing assistance, supported financially by Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim, Germany, was provided by Sam Kew (inVentiv Medical Communications, UK) during the preparation of this report.
Competing interests: FC has received advisory board and/or lecture fees from Boehringer Ingelheim, Roche, AstraZeneca, Pfizer, Novartis, Bristol-Myers Squibb and Clovis Oncology. LC has received honoraria from Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb and AstraZeneca. RD has received advisory board and/or lecture fees from Boehringer Ingelheim, Roche, AstraZeneca, Pfizer, Novartis and Clovis Oncology. DE is a consultant for ARIAD Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Eli Lilly, EMD Serono, Genentech, Golden Biotech, Helsinn Therapeutics and Sandoz. DF has received consultant fees from Eli Lilly and speaker bureau fees from Bristol-Myers Squibb. KK has received advisory board and/or lecture fees from Eli Lilly, Roche, AstraZeneca, Pfizer, Novartis, Clovis Oncology, Bristol-Myers Squibb and Merck Sharpe & Dohme. TLC has received advisory board and/or lecture fees from Boehringer Ingelheim, Sanofi and Synta. NL is a researcher for the University Health Network, which received a research grant from Novartis. CM has received honoraria for expert study advice and travel support from Boehringer Ingelheim. MP has received research grants from Novartis and honoraria from Eli Lilly, Boehringer Ingelheim, Pfizer and Clovis Oncology. LP-A has given scientific advice to Pfizer, Clovis Oncology, Roche, Boehringer Ingelheim, AstraZeneca, Eli Lilly and Bristol-Myers Squibb. MP has received honoraria for advisory boards from Eli Lilly, Roche and Boehringer Ingelheim. RP has received speaker's fees and honoraria for advisory boards from Boehringer Ingelheim, and honoraria for data safety monitoring board membership from Synta Pharmaceuticals, and Merck Sharpe & Dohme. EQ participated in clinical trials involving nivolumab, pembrolizumab, nintedanib, ramucirumab and ganetespib. MR has received compensated consultant fees from Hoffmann-La Roche, Eli Lilly, Bristol-Myers Squibb, Merck Sharpe & Dohme, AstraZeneca, Pfizer, Novartis, Boehringer Ingelheim and Samsung, and honoraria for lectures from Hoffmann-La Roche, Eli Lilly, Bristol-Myers Squibb, Merck Sharpe & Dohme, AstraZeneca, Pfizer and Boehringer Ingelheim. ES has received honoraria for participating in advisory boards and lecture fees from Boehringer Ingelheim. EV has received consultant/advisory fees from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Clovis Oncology, Eisai, Eli Lilly, GeneCentric, Genentech, Merck, Synta, Transgene and VentiRx. CZ has received honoraria from Hoffmann-La Roche, Eli Lilly, Boehringer Ingelheim and AstraZeneca.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13–22. 10.1016/j.lungcan.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7):vii56–64. 10.1093/annonc/mds226 [DOI] [PubMed] [Google Scholar]
- 3.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 2005;128:452–62. 10.1378/chest.128.1.452 [DOI] [PubMed] [Google Scholar]
- 4.Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41:245–58. 10.1016/S0169-5002(03)00230-7 [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med 2002;346:92–8. 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–55. 10.1016/S1470-2045(12)70063-3 [DOI] [PubMed] [Google Scholar]
- 7.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J Clin Oncol 2009;27:591–8. 10.1200/JCO.2008.17.1405 [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer; Version 4.2014, 2014. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Last accessed 21 Dec 2014.
- 9.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii27–39. 10.1093/annonc/mdu199 [DOI] [PubMed] [Google Scholar]
- 10.Kono SA, Heasley LE, Doebele RC, et al. Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets 2012;12:107–23. 10.2174/156800912799095144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crinò L, Metro G. Therapeutic options targeting angiogenesis in non-small cell lung cancer. Eur Respir Rev 2014;23:79–91. 10.1183/09059180.00008913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballas MS, Chachoua A. Rationale for targeting VEGF, FGF, and PDGF for the treatment of NSCLC. Onco Targets Ther 2011;4:43–58. 10.2147/OTT.S18155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno A, Pagani A, Pulze L, et al. Orchestration of angiogenesis by immune cells. Front Oncol 2014;4:131 10.3389/fonc.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genentech. Avastin prescribing information, 2014. https://www.gene.com/download/pdf/avastin_prescribing.pdf Last accessed 21 Dec 2016.
- 15.de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011;29:1067–74. 10.1200/JCO.2010.29.5717 [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619–26. 10.1016/S1470-2045(10)70132-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:1059–66. 10.1200/JCO.2010.28.5981 [DOI] [PubMed] [Google Scholar]
- 18.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640–7. 10.1200/JCO.2012.42.6932 [DOI] [PubMed] [Google Scholar]
- 19.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846–54. 10.1016/S0140-6736(11)60545-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 2012;30:2070–8. 10.1200/JCO.2011.39.2993 [DOI] [PubMed] [Google Scholar]
- 21.Heist RS, Wang X, Hodgson L, et al. CALGB 30704 (Alliance): a randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:214–21. 10.1097/JTO.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixidó C, Karachaliou N, González-Cao M, et al. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med 2015;12:87–95. 10.7497/j.issn.2095-3941.2015.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr KM, Tsao MS, Nicholson AG, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 2015;10:985–9. 10.1097/JTO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 24.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774–82. 10.1158/0008-5472.CAN-07-6307 [DOI] [PubMed] [Google Scholar]
- 25.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143–55. 10.1016/S1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 26.Hanna NH, Kaiser R, Sullivan RN, et al. Lume-Lung 2: a multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy. J Clin Oncol 2013;31(Suppl 15):Abstract 8034. [Google Scholar]
- 27.Reck M, Buchner H, Gottfried M, et al. Tumor growth over time in patients with non-small cell lung cancer (NSCLC) of adenocarcinoma histology (ACH) treated with nintedanib and docetaxel or placebo and docetaxel: analysis of data from the LUME-Lung 1 (LL1) study. J Clin Oncol 2015;33(Suppl):Abstract e19021. [Google Scholar]
- 28.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665–73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 29.Paz-Ares L, Perol M, Ciuleanu T-E, et al. Exploratory analysis of safety by histology and efficacy in a nonsquamous NSCLC subgroup in REVEL: a randomized phase III study of ramucirumab (RAM) plus docetaxel (DOC) vs DOC for second-line treatment of stage IV non-small-cell lung cancer (NSCLC). J Clin Oncol 2015;33(Suppl):Abstract 8055. [Google Scholar]
- 30.Rolfo C, Sortino G, Smits E, et al. Immunotherapy: is a minor god yet in the pantheon of treatments for lung cancer? Expert Rev Anticancer Ther 2014;14:1173–87. 10.1586/14737140.2014.952287 [DOI] [PubMed] [Google Scholar]
- 31.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res 2015;4:177–90. 10.3978/j.issn.2218-6751.2015.01.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bristol-Myers Squibb. Nivolumab BMS summary of product characteristics, 2015. https://www.medicines.org.uk/emc/medicine/30476 Last accessed 22 Oct 2016.
- 35.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 36.Soria JC, Fløtten Ø, Horn L, et al. Efficacy and safety of pembrolizumab (pembro; MK3475) for patients (pts) with previously treated advanced non-small cell lung cancer (NSCLC) enrolled in KEYNOTE-001. Vienna, Austria: Presented at the European Cancer Congress 2015, 2015. Abstract 33LBA. [Google Scholar]
- 37.Merck & Co. Keytruda prescribing information, 2015. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf Last accessed 22 Oct 2016.
- 38.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 39.Roche. Press release: U.S. FDA grants Breakthrough Therapy Designation for Roche's investigational cancer immunotherapy MPDL3280A (anti-PDL1) in non-small cell lung cancer, 2015. http://www.roche.com/media/store/releases/med-cor-2015-02-02.htm Last accessed 22 Oct 2016.
- 40.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 41.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. 10.1016/S1470-2045(15)00544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khleif S, Lutzky J, Segal NH, et al. MEDI4736, an anti-PD-L1 antibody with modified Fc domain: preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors. Eur J Cancer 2013;49(Suppl 2):Abstract 802:S161. [Google Scholar]
- 43.Herbst RS, Gordon RA, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol 2013;31(Suppl):Abstract 3000. [Google Scholar]
- 44.Rizvi N, Chow LQ, Dirix LY, et al. Clinical trials of MPDL3280A (antiPDL1) in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32(Suppl 5):Abstract TPS8123. [Google Scholar]
- 45.Kelly K, Patel MR, Infante JR, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or locally advanced solid tumors: assessment of safety and tolerability in a phase I, open-label expansion study. J Clin Oncol 2015;33(Suppl 15):Abstract 3044. [Google Scholar]
- 46.Gulley JL, Spigel DR, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: a phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33(Suppl 15):Abstract 8034. [Google Scholar]
- 47.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175–80. 10.1016/S1470-2045(10)70087-5 [DOI] [PubMed] [Google Scholar]
- 48.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 2010;11:1172–83. 10.1016/S1470-2045(10)70232-1 [DOI] [PubMed] [Google Scholar]
- 49.Schwaederle M, Lazar V, Validire P, et al. VEGF-A expression correlates with TP53 mutations in non-small cell lung cancer: implications for antiangiogenesis therapy. Cancer Res 2015;75:1187–90. 10.1158/0008-5472.CAN-14-2305 [DOI] [PubMed] [Google Scholar]
- 50.Pilotto S, Bonomi M, Massari F, et al. Anti-angiogenic drugs and biomarkers in non-small-cell lung cancer: a ‘hard day's night’. Curr Pharm Des 2014;20:3958–72. 10.2174/13816128113196660757 [DOI] [PubMed] [Google Scholar]
- 51.Salgia R. Prognostic significance of angiogenesis and angiogenic growth factors in non-small cell lung cancer. Cancer 2011;117:3889–99. 10.1002/cncr.25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaiser R, Barrueco JR, Reck M, et al. Identification of a clinical biomarker for 2nd line combination with nintedanib in adenocarcinoma non-small cell lung cancer (NSCLC) patients in two phase III trials. Eur J Cancer 2013;49(Suppl 2):Abstract 3479:S822. [Google Scholar]
- 53.Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:408–24. 10.1038/nrclinonc.2015.103 [DOI] [PubMed] [Google Scholar]
- 54.Wolchok JD, Chan TA. Cancer: Antitumour immunity gets a boost. Nature 2014;515:496–8. 10.1038/515496a [DOI] [PMC free article] [PubMed] [Google Scholar]