Abstract
Objectives
Malnutrition continues to contribute to a high infant mortality rate. This study aimed to determine the prevalence of malnutrition and its potential association with the time at which complementary feeding was introduced among children aged 0‐5 years in Batouri, Republic of Cameroon.
Methods
Mothers (n=212) were interviewed using a structured questionnaire. Child height or length, and weight measurements were determined and the appropriate Z‐scores calculated. Multiple regression analysis was performed with the values of all nutritional status indicators as dependent variables and the time of commencing complementary feeding, and the child's age and sex, as independent variables.
Results
The prevalence of stunting (height/length for age<−2 standard deviation [SD]), underweight (weight for age<−2SD), and wasting (weight for height/length<−2SD) was 45.8%, 30.2%, and 11.3%, respectively. Even taking into consideration the biological variables, there was a significant association in the effects of time of starting complementary foods on the nutritional status indicators. Furthermore, adding socio‐economic variables did not produce a rise in adjusted R 2 values for all age group models concerned.
Conclusions
Approximately 30% of the children in the study region were underweight, and approximately half of the children exhibited stunting, indicating chronic malnutrition. Commencing complementary feeding at an appropriate time had a positive effect on nutritional status from approximately 2 years of age.
Keywords: Cameroon, children, complementary feeding, malnutrition
1. BACKGROUND
Malnutrition in children leads to weakened immunity and plays a large role in the high mortality rates observed among children in sub‐Saharan Africa.1 The United Nations Children's Fund's State of the World's Children report indicates that rates of wasting (weight for height/length<−2SD), underweight (weight for age<−2SD), and stunting (height/length for age<−2SD) in this group of children are as high as 9%, 21%, and 37%, respectively.2 Malnutrition remains one of the factors contributing to a high infant mortality rate. This child public health issue urgently needs attention and must be addressed with combined global efforts. As a preventative measure against malnutrition and infectious diseases in children in developing countries, the World Health Organization recommends breastfeeding for the first 6 months of a child's life with the introduction of complementary foods between 4 and 6 months.3 However, the practice of breastfeeding in West and Central Africa is only 25%, considerably lower than the world average of 38%.2 Given this low breastfeeding rate, we presume that children are introduced to complementary food early, and that this is one of the factors driving malnutrition.
Several studies have been published on the association between child malnutrition and poverty,4, 5 but few studies to date have focused on the association between the nutritional status of children and practical factors, such as the time at which complementary feeding is introduced to children. The Republic of Cameroon has the 15th highest rate of malnutrition among children <5 years of age globally. The East Region in particular has one of the highest proportions of children in the country who are affected by stunting.6 Batouri is a major city in the East Region, with a population of approximately 204 000.7 Recently, in 2015, a paper was published examining factors associated with nutritional status in children 5‐24 months of age from the Batouri district.8 We extended this further to include children aged 2‐5 years old. Herein, we report on the prevalence of malnutrition in children aged 0‐5 years, and explore the association between malnutrition and the age at which complementary feeding was introduced.
2. METHODS
2.1. Design and sample
We employed a cross‐sectional descriptive design to determine the relationship between child malnutrition and data obtained from interviews with their mothers. The study was conducted at five health centers and two villages in the health district of Batouri, at two different times, using convenience samples. The first part took place between February 18 and March 1, 2013, and included children from 5 months up to 24 months of age and their mothers (n=100). The second part, conducted between August 19 and September 2, 2014, included children from 2 to 5 years of age and their mothers (n=112). Mothers were interviewed using a structured questionnaire.
All study participant mothers received a verbal explanation of the study prior to participation. If they agreed to participate together with their child, informed written consent was obtained. Individual confidentiality was maintained throughout. The study protocol was reviewed and approved by the Human Ethics Committees of our institute's Graduate School in the Faculty of Health Sciences (N0 12‐11, 2012/5/31) and the Ministry of Science, Republic of Cameroon (N0 0000040, 2012/6/27; N0 0000083, 2014/6/26). Research was conducted in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments.
2.2. Measures
Anthropometric measurements (including height, length, and weight) were recorded by the principal researcher for all 212 mother‐child pairs enrolled in this study, using a previously defined standard protocol.9 For children ≤24 months of age, the length of the child was measured with the child lying supine. For children ≥25 months of age, the height of the child was measured with the child standing. For weight measurements, if the child was unable to be measured alone, the mother held the child and the combined weight was recorded; the mother's weight was subtracted to determine the difference as the weight of the child. The child wore only thin clothes during measurement. Weight measurements were determined using a weighing scale accurate to 0.1 kg (BC‐751 for children ≤24 months of age and HD‐386‐BK for children ≥25 months of age [Tanita Corporation, Tokyo, Japan]). Height and length measurements were determined using a height meter accurate to 0.1 cm (213 for measurements of height and 210 for measurements of length [Seca GmbH & Co. KG, Hamburg, Germany]).
For the questionnaire, the principal researcher interviewed the mothers in French, provided they understood the language. For mothers who preferred using the local language, District Health Office staff conducted the interview and the mothers’ responses were translated into French for the researchers. The questionnaire was based on the nutrition strategy of the United Nations Children's Fund and focused on “underlying cause at the household/family level” for “causes of child malnutrition.” Interview topics were as follows: (i) information concerning the mother (including age, religion, educational level, place of residence, ethnic group, husband's occupation, and number of pregnancies/deliveries); (ii) information concerning the child, including birth data (eg, age, sex, date of birth, gestational status, length, and weight) and nutritional data (eg, breastfeeding status, and the age at which the child was weaned and complementary feeding was introduced); (iii) information concerning vaccinations (including diphtheria, whooping cough, tetanus, poliomyelitis, hepatitis B, Haemophilus influenzae type b, and Bacille de Calmette et Guérin); and (iv) information concerning tap water (including its source and method of treatment). The time at which complementary feeding was introduced was dichotomized into commencing at (i) ≤4 months or ≥8 months of age, and (b) 5‐7 months of age.
Nutritional status was evaluated using the Child Growth Standards of the World Health Organization to calculate Z‐scores of height/length for age (H/LAZ), weight for age (WAZ), and weight for height/length (WH/LZ).10 Z‐scores were computed using WHO AnthropacR, version 3.2.2. Indicators of malnutrition were defined as follows: stunting, H/LAZ<−2 SD; underweight, WAZ<−2 SD; and wasting, WH/LZ<−2 SD. As no reference data are available from nutritional assessments of populations in Benin and neighboring regions, we used WHO anthropometric data for reference.
2.3. Analytical strategy
Descriptive statistics were calculated by categorizing the interview items into basic attributes, food, and living environment/vaccinations. Correlations between values of H/LAZ, WAZ, and WH/LZ, and the child's age, sex, time at which complementary feeding was introduced, and each item on the questionnaire were analyzed using Student's t tests and analysis of variance. In terms of dichotomizing the time at complementary feeding data, children 5‐7 months of age who had not yet commenced complementary feeding (n=8) were excluded from the classification, and those ≥8 months of age who had not commenced complementary feeding were categorized into the group commencing at ≤4 months or ≥8 months of age. Multiple regression analysis was subsequently performed with the values of H/LAZ, WAZ, and WH/LZ as dependent variables and the time at which complementary feeding was introduced, and the child's age and sex as independent variables. Analyses were performed for groups from 0‐5, 0‐2, and 2‐5 years of age using JMP Pro software version 11.2 (SAS Institute Inc., Cary, NC, USA). Probability (P)‐values below .05 were considered statistically significant.
3. RESULTS
3.1. Participant characteristics
The mean age±standard deviation (SD) of the mothers enrolled in this study was 25.8±6.73 years (range, 17‐50 years). One hundred and sixteen mothers (54.8%) were Christians. Of the 148 mothers (69.8%) who attended elementary school, 95 (44.8%) graduated; the remaining 53 mothers (25.0%) did not attend elementary school. Ninety‐nine mothers (46.8%) resided in Batouri Central, while the remaining 113 mothers (53.2%) resided in outlying rural areas. One hundred and six mothers (50.0%) belonged to the Kako ethnic group. With respect to the husband's occupation, 113 (53.3%) were unemployed or in agriculture, 12 (5.7%) were in retail, nine (4.3%) were in the gold mining industry, three (1.4%) were in teaching, and a further three (1.4%) were in nursing. The mean number±SD of pregnancies and deliveries was 4.0±2.57 and 3.9±2.51, respectively.
The children enrolled in this study included 107 male children (50.5%) and 105 female children (49.5%), with a mean age±SD of 1.9±1.60 years (range, 5 months to 5 years). One hundred and forty‐four children (67.9%) were breastfed during the first 6 months of life. Owing to insufficient quantities of breastmilk, 12 mothers (5.6%) provided their children with sugar water immediately after birth; all of these mothers also stated that this was a custom. There was no significant association between exclusive breastfeeding and child nutritional status.
The mean age at which complementary feeding was introduced was 7.3±3.29 months (range, 1‐24 months). Regarding complementary feeding, it was introduced to the diet of 17 children (8.0%) at ≤4 months of age, and to a further 48 children (22.6%) at ≥8 months of age; 48.1% of these mothers’ explanatory reasons included “no comment” and “it is the way everyone does it.” One mother indicated that she had not introduced complementary feeding until 24 months after birth “because the child did not want it.” Only 66 children (31.1%) were fed complementary dairy products.
3.2. Nutritional status and correlations with the child's age, sex, and time at which complementary feeding was introduced
The prevalence of malnutrition in the children enrolled in this study was as follows: stunting, 45.8% (n=97); underweight, 30.2% (n=64); and wasting, 11.3% (n=24) (Table 1).
Table 1.
Children's nutritional status
| Indicator | Time of starting complementary foods | |||||
|---|---|---|---|---|---|---|
| Total (n=212) | ≤4 M (n=17) | 5‐7 M (n=136) | ≥8 M (n=48) | Not commenced (n=11) | Total (n=212) | |
| Mean (SD) | Z‐score<−2 SD (%) | |||||
| H/LAZ | −1.8 (1.71) | −1.6 (0.40) | −1.7 (0.14) | −2.6 (1.86) | −0.6 (1.31) | 45.8 |
| WAZ | −1.4 (1.39) | −0.9 (0.32) | −1.2 (0.11) | −2.2 (1.62) | −0.7 (1.33) | 30.2 |
| WH/LZ | −0.5 (1.37) | 0.2 (0.32) | −0.3 (0.11) | −1.1 (1.57) | −0.5 (1.43) | 11.3 |
SD, standard deviation; H/LAZ, Z‐score for height/length for age; WAZ, Z‐score for weight for age; WH/LZ, Z‐score for weight for height/length.
The proportion of children with Z‐score values of <−2 SD increased at 2 years of age for all indicators, but recovered after 3 years of age (Figure 1). A significant association was observed between the child's age and H/LAZ, WAZ, and WH/LZ (all P<.05) values. A significant association was also observed between the child's sex in the 0‐ to 5‐ and 2‐ to 5‐year‐old age groups and H/LAZ and WAZ values (P<.05), but WH/LZ values were not associated with any age group. Further significant associations were observed between the times at which complementary feeding was introduced in the 0‐5 and 2‐5 years of age groups and the values of all three nutritional status indicators. The number of children with malnutrition increased when complementary feeding was introduced at a later date. In contrast, no associations were observed between the types of complementary foods introduced or vaccination status and H/LAZ, WAZ, and WH/LZ values.
Figure 1.
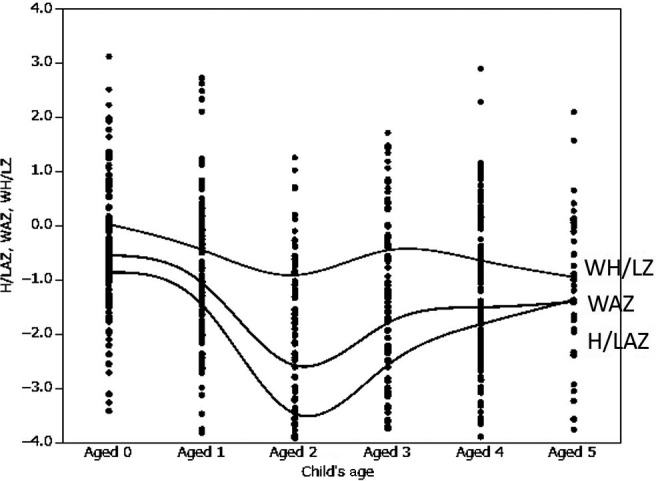
H/LAZ, WAZ, and WH/LZ values of each age. H/LAZ, Z‐score of height (length) for age; WAZ, Z‐score of weight for age; WH/LZ, Z‐score of weight for height (length)
3.3. Effect of time of commencing complementary feeding on H/LAZ, WAZ, and WH/LZ values
The results of the multiple regression analysis of H/LAZ values, adjusted for the child's age, sex, and the dichotomized variable of time at which complementary feeding was introduced, are displayed in Table 2. For the 2‐ to 5‐year‐old age group, the freely adjusted contribution ratio was the highest of all the age group models (adjusted R 2=.281, P<.001). In addition, variables of children's age, sex, and the time at which complementary feeding was introduced significantly predicted H/LAZ values in the model for ages 0‐5 and 2‐5 years.
Table 2.
Multiple regression analysis between H/LAZ values and child's age and sex variables
| Independent variable | Dependent variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H/LAZ (aged 0‐5) n=212 | LAZ (aged 0‐2) n=100 | HAZ (aged 2‐5) n=112 | |||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Time of starting from 5 to 7 Mb | 0.173 | 0.070 to 0.552 | .012a | −0.019 | −0.304 to 0.252 | .852 | 0.189 | 0.046 to 0.641 | .024a |
| Child's age | −0.175 | −0.332 to −0.045 | .011a | −0.211 | −1.052 to −0.009 | .046a | 0.412 | 0.432 to 1.003 | <.001a |
| Child's sex (female) | 0.164 | 0.051 to 0.505 | .017a | 0.127 | −0.099 to 0.470 | .225 | 0.246 | 0.147 to 0.710 | .003a |
| Adjusted R 2 (P‐value) | .071 (<.001a) | .050 (.173) | .281 (<.001a) | ||||||
P<.05.
Except for children not starting complementary foods at age 5‐7 mo (aged 0‐5 y, n=204; aged 0‐2 y, n=92).
H/LAZ, Z‐score for height/length for age; LAZ, Z‐score for length for age (age 2 indicates 24 mo of age); HAZ, Z‐score for height for age (age 2 indicates 25‐35 mo of age).
The results of the multiple regression analysis of WAZ values in relation to other variables are displayed in Table 3. Findings were similar to those reported for H/LAZ values (Table 2). For standardized partial regression coefficients, the time at which complementary feeding was introduced was highest in the model for children aged 2‐5 years (β=.299).
Table 3.
Multiple regression analysis between WAZ values and child's age and sex variables
| Independent variable | Dependent variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| WAZ (aged 0‐5) n=212 | WAZ (aged 0‐2) n=100 | WAZ (aged 2‐5) n=112 | |||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Time of starting from 5 to 7 Mb | 0.226 | 0.141 to 0.522 | <.001a | 0.020 | −0.213 to 0.259 | .845 | 0.299 | 0.194 to 0.705 | <.001a |
| Child's age | −0.241 | −0.325 to −0.100 | <.001a | −0.238 | −0.954 to −0.069 | .024a | 0.264 | 0.135 to 0.626 | .003a |
| Child's sex (female) | 0.170 | 0.055 to 0.420 | .011a | 0.170 | −0.037 to 0.401 | .103 | 0.219 | 0.073 to 0.557 | .011a |
| Adjusted R 2 (P‐value) | .120 (<.001a) | .043 (.075) | .225 (<.001a) | ||||||
P<.05.
Except for children not starting complementary foods at age 5‐7 mo (aged 0‐5 y, n=204; aged 0‐2 y, n=92).
WAZ, Z‐score for weight for age.
The results of the multiple regression analysis of WH/LZ values in relation to other variables are displayed in Table 4. The time at which complementary feeding was introduced significantly predicted WH/LZ values in the models for children aged 0‐5 and 2‐5 years. For standardized partial regression coefficients, the time at which complementary feeding was introduced was highest in the model for children aged 2‐5 years (β=0.265).
Table 4.
Multiple regression analysis between WH/LZ values and the child's age and sex variables
| Independent variable | Dependent variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| WH/LZ (aged 0‐5) n=212 | WLZ (aged 0‐2) n=100 | WHZ (aged 2‐5) n=112 | |||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Time of starting from 5 to 7 Mb | 0.154 | 0.028 to 0.430 | .026a | −0.019 | −0.304 to 0.252 | .852 | 0.265 | 0.119 to 0.680 | .005a |
| Child's age | −0.170 | −0.266 to −0.030 | .014a | −0.211 | −1.052 to −0.009 | .046a | −0.022 | −0.301 to 0.236 | .812 |
| Child's sex (female) | 0.102 | −0.046 to 0.326 | .140 | 0.127 | −0.099 to 0.417 | .224 | 0.106 | −0.111 to 0.419 | .251 |
| Adjusted R 2 (P‐value) | .050 (.006a) | .054 (.173) | .078 (.031a) | ||||||
P<.05.
Except for children not starting complementary foods at age 5‐7 mo (aged 0‐5 y, n=204; aged 0‐2 y, n=92).
WH/LZ, Z‐score for weight for height/length; WLZ, Z‐score for weight for length (age 2 indicates 24 mo of age); WHZ, Z‐score for weight for height (age 2 indicates 25‐35 mo of age).
Even taking into consideration biological sex and age, there was still a significant association between the time at which complementary feeding was introduced and H/LAZ, WAZ, and WH/LZ values.
In the model for 2‐ to 5‐year‐olds, the addition of covariates such as ethnic group (Kako, Mbororo, etc.), educational level (primary school level or lower, junior high school level or higher), occupation of husband/partner (agriculture or unemployed, other), and place of residence (central or rural area), decreased the adjusted R 2 for HAZ (.269); the variable “time of starting complementary feeding” was not significant. Meanwhile, the adjusted R 2 for WAZ (.315) and WHZ (.147) increased, and the time of starting complementary feeding was significant. WAZ and WHZ models showed no change in regression coefficient (β) and significance, even when adding covariates; the importance of the time of starting complementary feeding did not change.
4. DISCUSSION
4.1. Prevalence of child malnutrition
The prevalence malnutrition among children in the Batouri district was 45.8%, 30.2%, and 11.3% for stunting, underweight, and wasting, respectively. According to the reports of the United Nations Children's Fund,2 the overall prevalence of malnutrition among children <5 years of age in the Republic of Cameroon is 33.0%, 15.0%, and 6.0% for stunting, underweight, and wasting, respectively. Moreover, Cameroonian statistics have demonstrated the prevalence of malnutrition among children <5 years of age in the East Region of the Republic of Cameroon to be 37.3%, 15.4%, and 5.9% for stunting, underweight, and wasting, respectively.6 In comparison, the present prevalence rates of malnutrition in our study far exceed those of previously published reports. The prevalence of malnutrition in children aged 0‐2 years exhibited a large increase from approximately 2 years of age. An increase in stunting in children >2 years of age has also been reported in previous studies.11
The prevalence of malnutrition increased from 2 years of age; hence, we can infer that complementary foods and breastfeeding do not provide adequate nutrition.12 In addition, until the age of 2‐3 years, immune function is immature, making children susceptible to infection. This results in poor nutritional status and slow recovery. As immune function increases beyond 3 years of age, nutritional indicators seem to improve.13
4.2. Associations between nutritional status and the time at which complementary feeding was introduced
The results of this study suggest that there is an association between a child's nutritional status and the time at which complementary feeding was introduced. Commencing at approximately 6 months of age (5‐7 months of age in this present survey), as recommended by the World Health Organization,3 has a particularly positive influence on the nutritional status of children ≥2 years of age. Concerning the association between nutritional status and the time at which complementary feeding was introduced, Ergin et al. stated that “there is a correlation between time of starting complementary food and stunting, and beginning before four months of life is a factor in malnutrition due to lack of development in the digestive and immune systems.”14 In addition, previous studies suggest that a late introduction to complementary foods is a risk factor for malnutrition.15
If complementary feeding is commenced too early, it is not possible for the child to absorb the necessary nutrients, and if it is introduced too late, the mother's milk will not be able to supply the nutrients required for optimal continued growth. It is recommended, therefore, that complementary feeding be introduced at approximately 6 months of age. In contrast, the authors of prior research in Senegal reported that “no correlation was found between an early start to complementary food of no later than four months and the nutritional status of children.”16 Additionally, prior research in Kenya reported that “a correlation was found with the number of ingredients in children's food rather than the starting time of weaning food.”17 Consequently, not only the time at which complementary feeding is first introduced, but also the quantity and balance of the food given are thought to influence a child's nutritional state. Bouillie, made by kneading and liquefying grains such as corn and cassava, is somewhat similar to Japanese rice gruel. It is widely consumed in Africa, both as a complementary feed for children and as breakfast for adults.
In this present study, only 8.0% of mothers provided their children with complementary foods at ≤4 months of age. As the analysis also included the group that commenced at ≥8 months of age, it was not possible to demonstrate clearly the effects of early introduction to complementary foods. However, the importance of an appropriate time of commencing complementary feeding has been suggested. In the present study, 48.1% of the mothers’ reasons for deciding when to commence complementary feeding included “this is the way this is always done,” “everyone does it this way,” and “no special reason,” suggesting that local customs are influential. Prior research suggests that those who decide the time at which to introduce complementary feeding include mothers‐in‐law and other relatives.4 In the present study as well, the person determining the mother's parenting behavior thought not to be the mother herself. Therefore, when delivering education on the importance of timing to start weaning food, it is important to consider the influences of both the family and the region, so as to target the most appropriate audience.
Children aged 5‐7 months were excluded because of the possibility that they started complementary foods at the appropriate time (ie, 5‐7 months). There were no major differences in the distribution of the excluded children compared with the included children; thus, this exclusion was unlikely to have affected the results of the analysis. Infants who had not started intake of complementary foods by the age of 8 months, which is the appropriate time, were also assigned to this group. As the sample size of this population (n=3) was small, this was unlikely to have affected the results of the analysis.
4.3. Associations between nutritional status and the child's age and sex
Studies in Uganda have observed a significantly larger proportion of older children with stunting.18 Regarding age and malnutrition, Cameroonian statistics have demonstrated a correlation between age and stunting, with the highest proportions at 42.4%, 42.3%, and 38.7% for children 18‐23 months of age, 24‐35 months of age, and 36‐47 months of age, respectively.6 With respect to underweight, no significant differences were observed among the different age groups and with respect to wasting, the proportion declined from 18 months and older. Therefore, there may be a strong relevance between stunting and the observed correlation between age and malnutrition.
Cameroonian statistics have also shown that a higher proportion of male children exhibit signs of malnutrition (stunting, underweight, and wasting) in comparison with female children.6 This may be because male children require more energy and nutrition than female children and are, thus, more strongly influenced by dietary conditions.6 Additionally, male children are more prone to infection than female children owing to the fact that they possess only one X chromosome, which contains genes related to the immune system. This is inferable from data that suggest that the under‐5 mortality rate is higher in male children than in female children.2, 6 It is suspected that children lose weight while suffering from infectious diseases and that malnutrition ensues without recovery.
4.4. Limitations
This study was conducted in only one district of Batouri. Therefore, generalization of the results is difficult owing to the influence of regional characteristics on all aspects of the study. In addition, the questions concerning “complementary foods” for mothers of children >2 years of age are likely to result in bias due to difficulties associated with recollection and memory. To make the results more generalizable, a larger sample size is needed in future studies to expand this research area. Additionally, we only analyzed the time of complementary feeding initiation; the data regarding complementary feeding behavior in this study are not adequate. Moreover, data regarding infants who started complementary foods at ≤4 months of age were scarce; hence, further studies are needed.
We discussed the classification of subjects into age groups at the time of complementary feeding. Although we attempted to perform the analysis first with four‐state variables and then with three‐state variables, owing to the small sample size, there was no coherency in the results obtained. Hence, we decided to perform the analysis with binary variables (ie, complementary feeding initiation at the appropriate time or not); that is, children aged 5‐7 months who had not yet commenced complementary feeding were excluded from the classification, and those aged ≥8 months who had not commenced complementary feeding were categorized into the group commencing at ≤4 months or ≥8 months of age.
5. CONCLUSIONS
This study considered the association between nutritional status and the time at which complementary feeding was introduced. The findings of the study are not unexpected and have been detailed globally. We do, however, provide information on the situation in a specific area of Cameroon, finding that: (i) approximately 30% of the children in the study region were underweight, and approximately half of the children exhibited stunting, indicating chronic malnutrition; and (ii) commencing complementary feeding at an appropriate time (5‐7 months of age) had a positive effect on the nutritional status of children from approximately 2 years of age.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGEMENTS
This work was supported by a Univers Foundation research grant 2012, a grant‐in‐aid from the Uruma Fund for the Promotion of Science 2014, and Japanese Society for the Promotion of Science KAKENHI Grant Number 25650151.
Nagahori C, Kinjo Y, Tchuani JP, Yamauchi T. Malnutrition among vaccinated children aged 0‐5 years in Batouri, Republic of Cameroon. J Gen Fam Med. 2017;18:365–371. https://doi.org/10.1002/jgf2.104
REFERENCES
- 1. Black R, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. [DOI] [PubMed] [Google Scholar]
- 2. UNICEF . The state of the world's children 2015. New York, NY: UNICEF; 2015: pp. 43–47. [Google Scholar]
- 3. World Health Organization . Complementary feeding. Geneva: World Health Organization; 2000: pp. 1–2. [Google Scholar]
- 4. Heltberg R. Malnutrition, poverty, and economic growth. Health Econ. 2009;18:577–88. [DOI] [PubMed] [Google Scholar]
- 5. Siponen SM, Ahonen RS, Savolainen PH, Hämeen‐Anttila KP. Children's health and parental socioeconomic factors: a population‐based survey in Finland. BMC Public Health. 2011;11:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ministère de la Santé Publique au Cameroun . Enquête Démographique et de Santé et à Indicateurs Multiples (EDS‐MICS) 2011. Yaoundé. 2011;1:160–5. [Google Scholar]
- 7. Institute National de la Statistique . Annuaire. Statistique du Cameroun 2010. Yaoundé. 2011;1:25–6. [Google Scholar]
- 8. Nagahori C, Tchuani JP, Yamauchi T. Factors associated with nutritional status in children aged 5‐24 months in Cameroon. Nurs Health Sci. 2015;17:229–35. [DOI] [PubMed] [Google Scholar]
- 9. Weiner JS, Lourie JA. Practical human biology. San Francisco, CA: Academic Press; 1981: pp. 32–51. [Google Scholar]
- 10. World Health Organization . WHO child growth standards. Geneva: World Health Organization; 2006: pp. xvii–xx. [Google Scholar]
- 11. Ramli, Agho KE, Inder KJ, Bowe SJ, Jacobs J, Dibley MJ. Prevalence and risk factors for stunting and severe stunting among under‐fives in North Maluku Region of Indonesia. BMC Pediatr. 2009;9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FAO , WHO , United Nations University . Human energy requirements: Report of Joint FAO/WHO/UNU Expert Consultation, Rome, 17‐24 October 2001. 2004; 21–22. Retrieved from Food and Agriculture Organization of the United Nations website: http://www.fao.org/docrep/007/y5686e/y5686e00.htm#Contents [accessed Jan 30 2015].
- 13. Sabina I, Erika M, Susanne L, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ. 2001;322:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ergin F, Okyay P, Atasoylu G, Beşer E. Nutritional status and risk factors of chronic malnutrition in children under five years of age in Aydin, a western city of Turkey. Turkish J Pediatr. 2007;49:283–9. [PubMed] [Google Scholar]
- 15. Amsalu S, Tigabu Z. Risk factors for ever acute malnutrition in children under the age of five: a case‐control study. Ethiop J Health Dev. 2008;22:21–5. [Google Scholar]
- 16. Gupta N, Gehri M, Stettler N. Early introduction of water and complementary feeding and nutritional status of children in northern Senegal. Pub Health Nutr. 2007;10:1299–304. [DOI] [PubMed] [Google Scholar]
- 17. Onyango A, Koski KG, Tucker KL. Food diversity versus breastfeeding choice in determining anthropometric status in Kenyan toddlers. Int J Epidemiol. 1998;27:484–9. [DOI] [PubMed] [Google Scholar]
- 18. Kikafunda KJ, Walker FA, Collet D, Tumwine JK. Risk factors for early childhood malnutrition in Uganda. Pediatrics. 1998;102:E45 Retrieved from http://pediatrics.aappublications.org/content/102/4/e45.full.pdf+html [accessed Jan 30 2015] [DOI] [PubMed] [Google Scholar]


