Abstract
Hearing loss is often the only symptom of OMAAV at initial presentation, thus making early diagnosis difficult. We present OMAAV in a 70‐year‐old woman with hearing loss and dry cough. Otoscopy showed otitis media with effusion. Audiometry showed mixed hearing loss, especially in the right ear. Serum myeloperoxidase antineutrophil cytoplasmic antibody was positive. Image analyses showed lung lesion and interstitial pneumonia, while bronchoscopy showed possible microscopic polyangiitis. After starting and tapering prednisolone, respiratory and otologic symptoms improved. When examining patients with acute otologic symptoms and suspected lung and/or renal disease, OMAAV should be included in differential diagnosis.
Keywords: ANCA, hearing loss, MPA, OMAAV, otitis media, vasculitis
1. INTRODUCTION
A 70‐year‐old woman initially complained of hearing loss and then developed dry cough and shortness of breath 1 month later. The patient's primary care physician revealed abnormal chest radiography and referred her to our hospital.
The patient had difficulty hearing, tinnitus, and ear fullness, but denied otalgia or otorrhea. She presented no history of headache, bloody sputum, weight loss, fever, paralysis, paresthesia or gait disturbance. There was no past medical history of asthma or sinusitis. She was not taking medication, she had no allergies, and she denied alcohol or cigarette use. She was married and had been helping her husband who was a farmer, indicating possible exposure to silica.
On physical examination, the patient was alert and oriented. Her blood pressure (102/60 mm Hg), pulse rate (68/minutes), and body temperature (36.0°C) were normal. Her respiratory rate was 12 breaths per min, and oxygen saturation was 97% in room air. Heart examination results were normal. A chest examination revealed volume of decreased breathing sounds in the right upper and middle lung area. There was no arthralgia, rash, superficial lymphadenopathy, or ankle swelling.
Otological examination showed hearing loss, with a Weber test showing lateralization to the right. Rinne test was negative in the right ear and positive in the left ear. These results indicated right ear bone conductive hearing loss. Otoscopy showed otitis media with effusion (Figure 1A‐left). Audiometry showed mixed hearing loss, especially in the right ear (Figure 1B‐left). The four frequency average hearing loss in the right ear was 108 dB, while that in the left was 58 dB.
Figure 1.
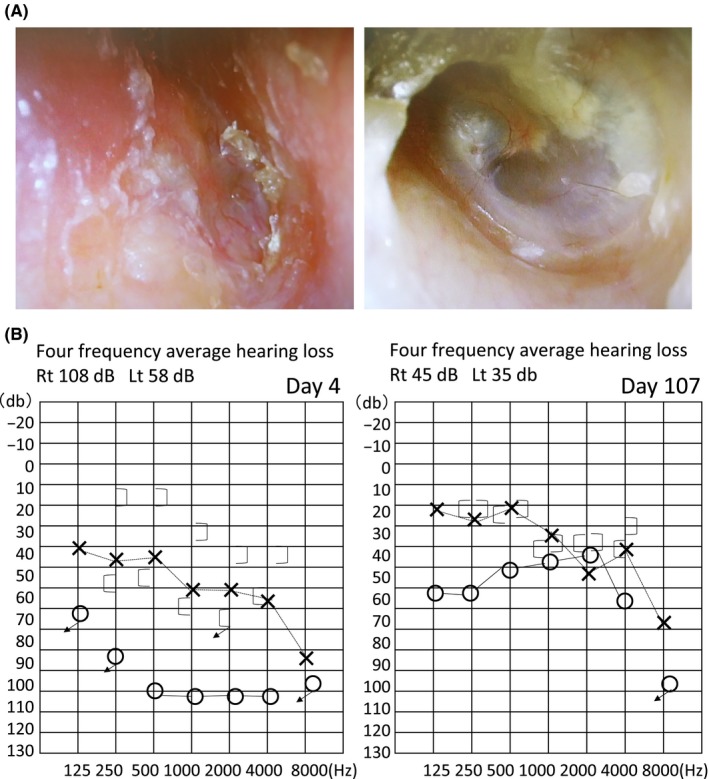
Otoscopy (A) and audiometry (B). Intervention helped improve reddening and thickening of the right tympanic membrane (A‐left) (see auditory ossicles, A‐right), and mixed hearing loss (B‐left) was also recovered (ie. became hearing without difficulty, 1B‐right)
Laboratory data revealed the following (Tables 1 and 2): hemoglobin was 10.3 g/dL; albumin was 2.8 g/dL; serum protein fractions were albumin 44.5% (α‐1 3.9%, α‐2 14.0%, β 10.0%, γ 27.6%); C‐reactive protein was 5.6 mg/dL; proteinase 3 antineutrophil cytoplasmic antibody was <0.5 IU/mL; myeloperoxidase antineutrophil cytoplasmic antibody (MPO‐ANCA) was 18.4 IU/mL; angiotensin‐converting enzyme was 8.7 U/L; interleukin 2 receptor was 1014 U/mL. Urinalysis test was normal with no urinary protein or occult blood. Chest radiography showed a mass lesion in the right upper lung area (Figure 2A‐above). A chest computed tomography (CT) scan revealed multiple consolidations with ground grass opacity (Figure 2B‐above), which was not present 3 months ago. Bronchoscopic examination and transbronchial lung biopsy were performed, revealing a histopathological image of interstitial pneumonia (Figure 2C). There were no granulomatous or malignant lesions. Furthermore, there was no inflammatory cell infiltration surrounding arterioles and capillaries and no necrosis of these blood vessels, which is typical in the lung tissues of microscopic polyangiitis (MPA). Typical findings of the alveolar hemorrhage were not recognized.
Table 1.
Laboratory data
| Variable | Units |
|---|---|
| RBC | 3.46×106/μL |
| Hb | 10.3 g/dL |
| Ht | 31.5% |
| MCV | 91 fL |
| WBC | 8.21×103/μL |
| Neutro | 67% |
| Lympho | 26% |
| Eosino | 5% |
| Mono | 1% |
| Baso | 0% |
| PLT | 180×103/μL |
| PT‐time | 14.3 s |
| PT‐activity | 71.7% |
| PT‐INR | 1.15 |
| APTT | 44.5 s |
| TP | 7.4 g/dL |
| Alb | 2.8 g/dL |
| T‐Bil | 0.34 mg/dL |
| AST | 17 IU/L |
| ALT | 15 IU/L |
| LDH | 190 IU/L |
| γ‐GTP | 90 IU/L |
| BUN | 12.8 mg/dL |
| Cr | 0.34 mg/dL |
| Na | 140 mEq/L |
| K | 4.4 mEq/L |
| Cl | 104 mEq/L |
| Ca | 8.5 mg/L |
| CRP | 5.6 mg/L |
Table 2.
Additional laboratory data
| Variable | Units | Normal range |
|---|---|---|
| Protein fraction (Alb) | 44.5% | 60.2‐71.4 |
| α‐1 | 3.9% | 1.9‐3.2 |
| α‐2 | 14% | 5.8‐9.6 |
| β | 10% | 7‐10.5 |
| γ | 27.6% | 10.6‐20.5 |
| IgG | 2064 mg/dL | 870‐1700 |
| IgA | 415 mg/dL | 110‐410 |
| IgM | 228 mg/dL | 40‐260 |
| C3 | 146 mg/dL | 65‐135 |
| C4 | 31.1 mg/dL | 13‐35 |
| ANA | <40 | 0‐80 |
| PR3‐ANCA | <0.5 IU/mL | 0‐2 |
| MPO‐ANCA | 18.4 IU/mL | 0‐3.5 |
| ACE | 8.7 U/L | 6.6‐21.4 |
| IL‐2R | 1014 U/mL | 122‐496 |
Figure 2.

Radiological study (A, B) and histological image (C). Multiple consolidations with ground grass opacity were at the apical–posterior segment of the right superior lobe, the superior segment of the right inferior lobe, the apicoposterior segment of the left superior lobe, and the anterior segment next to the left hilar area (B) ‐above Lung biopsy showed interstitial fibrosis, fibrous thickening of the alveolar wall, and mild inflammatory cell infiltration (C). Abnormal shadows disappeared 2 months after treatment (A, B‐below)
Head CT scan showed thickening of the right tympanic membrane and soft tissue lesion in the tympanum. Gadolinium‐enhanced MRI was performed, revealing enhanced soft tissue in the right tympanum. Dura mater was normal and not hypertrophic.
We diagnosed the patient as probable MPA because of interstitial pneumonia and positive findings for MPO‐ANCA and CRP,1 and considered OMAAV as most likely diagnosis for this patient's otitis media.
Treatment was initiated with 60 mg of oral prednisolone at a dose of 1 mg per kilogram per day, and tapered until 20 mg weekly. Up to 30 days later, respiratory symptoms disappeared. Up to 60‐90 days later, otologic symptoms were improved, and the patient could talk at normal volume. A follow‐up examination showed that MPO‐ANCA titer was negative within 20 weeks after the onset of treatment and that abnormal chest shadows had disappeared (Figures 2A, B‐below). The right tympanic membrane also improved (Figure 1A‐right). Audiometry showed that mixed hearing loss had recovered (Figure 1B‐right). These findings of clinical course suggested that the probability of diagnosis as OMAAV was very high.
2. DISCUSSION
AAV is defined by the medium and small vessel vasculitis that often detects ANCA, including MPA, granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis.2 Some AAVs are complicated with otitis media, which was recognized as OMAAV.
Approximately 75% of OMAAV patients visited primary care or an otolaryngology clinic, complaining of hearing loss, and time from initial hospital or clinic visit until OMAAV diagnosis was approximately 3 months.3 In our case, the patient had visited an otolaryngology clinic 2 months earlier, but could not diagnose the cause for hearing loss (treated as an infectious otitis media). Past unexplained hearing disorder cases might also be OMAAV.
OMAAV was diagnosed based on three findings: first, otitis media with effusion or granulation that was resistant to antibiotics and insertion of tympanic ventilation tubes; second, serum and/or histopathological and/or clinical signs/symptoms of AAV; third, other types of otitis media were excluded.4 Our case fulfilled these criteria, but eardrum biopsy was not performed. A definitive diagnosis rate of OMAAV by biopsy is 16% (8/50 cases),3 which is not high considering its invasiveness.
OMAAV also includes lung, kidney, head (hypertrophic pachymeningitis), and eye symptoms.5, 6, 7 Our case showed ear and lung lesions, but not hypertrophic pachymeningitis. In 32 single cases of pachymeningitis with AAV, all patients had severe headache.6 Our case had no headache. It is also important to check for head and eye symptoms if OMAAV is suspected.
Older age is a predictor for treatment resistance, and PR3 ANCA and lung involvement are predictors for relapse.8 Our case corresponded to older age and lung involvement. Treatment was effective, but we advise care be taken to avoid relapse.
3. CONCLUSION
Acute clinical courses of otologic symptoms with suspected lung and/or renal disease should list OMAAV as a possible diagnosis.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Hisata Y, Sasaki E, Ishimaru K, et al. Do you know otitis media with ANCA‐Associated Vasculitis (OMAAV)?. J Gen Fam Med. 2017;18:428–431. https://doi.org/10.1002/jgf2.112
REFERENCES
- 1. Yoshida M, Kobayashi S, Sueishi K, et al. Tyuukogata kekkanen no rinsyou ni kansuru syouiinkai houkoku. Kouseisyou Tokuteisikkan Tyousakenkyuuhan. Nantiseikekkanenbunkakai. 1999;10:239–46. [Google Scholar]
- 2. Bernhard H, Oliver F, Wolfgang L, et al. Eular recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti‐neutrophil cytoplasm antibody‐associated vasculitis. Ann Rheum Dis 2007;66:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida N, Kishibe K, Tateyama K, et al. Clinical features of 90 cases of otitis media with ANCA associated vasculitis. Otol Jpn 2014;24:53–61. [Google Scholar]
- 4. Harabuchi Y, Kishibe K, Tateyama K, et al. Clinical features and treatment outcomes of otitis media with antineutrophil cytoplasmic antibody (ANCA)‐ associated vasculitis (OMAAV): a retrospective analysis of 235 patients from a nationwide survey in Japan. Mod Rheumatol 2017;27:87–94. [DOI] [PubMed] [Google Scholar]
- 5. Iruku P, Karanth P, Tiu H, Kankam C, Shaheen K. Hearing Loss and Kidney Dysfunction: finding a Unifying Diagnosis. Case Rep Med 2013; Article ID 858963:1‐3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S, Tang H, Rong X, Huang X, Li Q. Pachymeningitis as a manifestation of ANCA‐associated vasculitis. Int J Clin Exp Med 2015;8:6352–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Isse N, Nagamatsu Y, Yoshimatsu N, Obata T, Takahara N. Granulomatosis with polyangiitis presenting as an orbital inflammatory pseudotumor: a case report. J Med Case Rep 2013;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pagnoux C, Hogan Susan L, Chin H, et al. Predictors of Treatment Resistance and Relapse in Antineutrophil Cytoplasmic Antibody–Associated Small‐Vessel Vasculitis. Arthritis Rheum 2008;58:2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


