The World Health Organization (WHO) classification system recognizes four variants of myelofibrosis (MF): primary (PMF), prefibrotic (pre-PMF), post-essential thrombocythemia (post-ET MF) and post-polycythemia vera (post-PV MF).1 Current prognostic models in PMF include the International Prognostic Scoring System (IPSS),2 the dynamic IPSS (DIPSS),3 and DIPSS-plus.4 These prognostic systems utilize up to 8 risk factors: age >65 years, hemoglobin <10 g/dl, leukocyte count >25 × 109/l, circulating blasts ⩾1%, presence of constitutional symptoms, unfavorable karyotype, red cell transfusion need and platelet count <100 × 109/l). When these prognostic systems were applied to 1000 consecutive PMF patients from the Mayo Clinic,5 the application of DIPSS-plus resulted in median survivals of 1.7, 4.7, 8.1 and 19.2 years for high, intermediate-2, intermediate-1 and low-risk patients, respectively; the corresponding median survivals using DIPSS were 1.5, 2.7, 6.3 and 17.5 years and using IPSS 2, 4.6, 6.8 and 17.5 years.
The objectives of the current study were as follows: (i) to determine if the aforementioned eight variables used in IPSS/DIPSS/DIPSS-plus are independently predictive of shortened survival in post-PV/ET MF and (ii) to assess the performance of the IPSS, DIPSS and DIPSS-plus risk stratification in post-PV/ET MF. Study patients were selected from the Mayo Clinic institutional database of myeloproliferative neoplasms (MPN). Diagnoses of ET, PV and post-ET/PV MF were according to WHO and International Working Group for MPN research, and treatment criteria.1, 6 Statistical analyses considered the clinical and laboratory data collected at the time of documented disease transformation from PV to post-PV MF or from ET to post-ET MF. Survival was calculated from the date of disease transformation to the date of death or last contact.
A total of 125 patients with post-PV (n=79) or post-ET (n=46) MF were studied (median age 62 years; 50% females); percentages of patients were 46% for age >65 years, 44% for hemoglobin <10 g/dl, 19% for red cell transfusion need, 19% for leukocyte count >25 × 10(9)/l, 14% for platelet count <100 × 10(9)/l, 45% for circulating blasts ⩾1%, 38% for constitutional symptoms and 17% for unfavorable karyotype. Risk distribution of the 125 patients with post-PV/ET MF, according to IPSS, was high in 39 (31%) patients, intermediate-2 in 40 (32%), intermediate-1 in 30 (24%) and low in 16 (13%); the corresponding percentages for DIPSS were 10, 38, 38 and 13%, and for DIPSS-plus 26, 41, 21 and 12%. Comparison of patients with post-PV and post-ET MF disclosed higher hemoglobin level (P=0.002), higher leukocyte count (P=0.0007) and larger palpable spleen size (P=0.002) in post-PV MF.
After a median follow-up of 3 years, from the date of fibrotic progression, 86 (69%) deaths and 10 (8%) leukemic transformations were documented. Multivariable analysis, which included the 5 aforementioned risk variables used in IPSS or DIPSS, disclosed independent predictive value for shortened survival, for all but constitutional symptoms: HR (95% CI; P-value) were 2.6 (1.6–4.2; P<0.0001) for age >65 years, 2.2 (1.4–3.5; P=0.001) for circulating blasts ⩾1%, 1.8 (1.1–2.8; P=0.01) for hemoglobin <10 g/dl, 1.8 (1.1–3.1; P=0.02) for leukocyte count >25 × 10(9)/l and 1.2 (0.7–1.9; P=0.5) for constitutional symptoms. Similarly, multivariable analysis that included all eight risk variables used in DIPSS-plus disclosed significant predictive value for all except constitutional symptoms (P=0.9) and leukocyte count >25 × 10(9)/l with borderline significance (P=0.06).
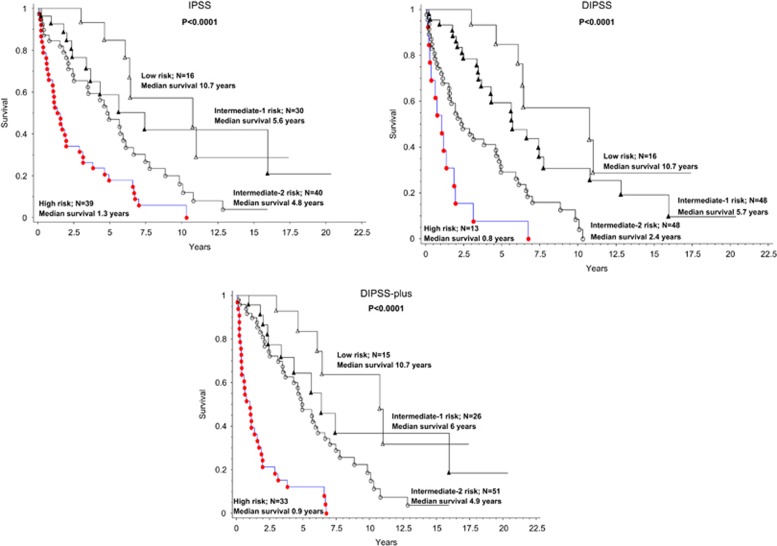
Application of IPSS, DIPSS and DIPSS-plus, to the 125 study patients with post-ET/PV MF is outlined in Figure 1. HR (95% CI) using IPSS were 2.3 (1.4–3.7) for high vs intermediate-2, 4.3 (2.2–8.5) for high vs intermediate-1, 6.6 (2.9–15.3) for high vs low, 2.9 (1.3–6.7) for intermediate-2 vs low, 1.9 (1.0–3.8) for intermediate-2 vs intermediate-1 and 1.5 (0.6–3.9) for intermediate-1 vs low; accordingly, IPSS in this group of patients was effective in delienating high- and intermediate-2-risk patients but was less effective in distinguishing low from intermediate-1-risk patients. Similar analyses using DIPSS and DIPSS-plus produced similar results (Figure 1).
Figure 1.
Survival of 125 patients with post-polycythemia vera or post-essential thrombocythemia. myelofibrosis, stratified by the international prognostic scoring system (IPSS), dynamic IPSS (DIPSS) and DIPSS-plus.
With the exception of constitutional symptoms, the current study confirms the prognostic value of the eight risk variables used in IPSS, DIPSS and DIPSS-plus, in the setting of post-PV/ET MF. The study also validates the adequate performance of the three prognostic models in delineating high- vs intermediate-2- vs low/intermediate-1-risk patients; the lack of significant distinction between intermediate-1- and low-risk patients, as well as the appearance of similar survival data between intermediate-2- and intermediate-1-risk disease (Figure 1; although significantly different), might be related to either the small number of informative cases or the demonstrated loss of significant contribution from constitutional symptoms.
The spirit of our observations is somewhat different than that echoed by our respected colleagues from Italy regarding their recently published new prognostic system for post-ET/PV MF.7 In the particular study, the authors found constitutional symptoms to retain its significance, along with anemia, thrombocytopenia, advanced age, circulating blasts and absence of CALR mutations, and used these variables to devise a newly-proposed prognostic model. Although the effort to include molecular markers is laudable, it might have been truncated in this instance because of the absence of information on other mutations known to affect outcome in PMF, such as ASXL1 and SRSF2.8 On the other hand, considering the fact that virtually all patients with post-PV MF are JAK2-mutated, one can argue the appropriateness of using driver mutational status, as a variable for a risk model, in post-PV MF. Consistent with these reservations, another recent study of 359 patients with post-ET/PV MF did not find a survival impact from either the type of driver mutation or its allele burden;9 instead, the study reported a detrimental effect of triple-negative driver mutational status and SRSF2 mutations in post-ET MF only. From a practical standpoint, our observations provide the evidence to support the use of IPSS/DIPSS/DIPSS-plus in post-PV/ET MF, thus maintaining familiarity and uniformity, until a molecularly more robust system is developed.
Footnotes
The authors declare no conflict of interest.
References
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010; 115: 1703–1708. [DOI] [PubMed] [Google Scholar]
- Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011; 29: 392–397. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Jimma T, Finke CM, Gangat N, Vaidya R et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc 2012; 87: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008; 22: 437–438. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Giorgino T, Mora B, Guglielmelli P, Rumi E, Maffioli M et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia 2017; 31: 2726–2731. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013; 27: 1861–1869. [DOI] [PubMed] [Google Scholar]
- Rotunno G, Pacilli A, Artusi V, Rumi E, Maffioli M, Delaini F et al. Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thrombocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am J Hematol 2016; 91: 681–686. [DOI] [PubMed] [Google Scholar]