Abstract
Objectives:
Temporal changes for intestinal resections for Crohn’s disease (CD) are controversial. We validated administrative database codes for CD diagnosis and surgery in hospitalized patients and then evaluated temporal trends in CD surgical resection rates.
Methods:
First, we validated International Classification of Disease (ICD)-10-CM coding for CD diagnosis in hospitalized patients and Canadian Classification of Health Intervention coding for surgical resections. Second, we used these validated codes to conduct population-based surveillance between fiscal years 2002 and 2010 to identify adult CD patients undergoing intestinal resection (n=981). Annual surgical rate was calculated by dividing incident surgeries by estimated CD prevalence. Time trend analysis was performed and annual percent change (APC) with 95% confidence intervals (CI) in surgical resection rates were calculated using a generalized linear model assuming a Poisson distribution.
Results:
In the validation cohort, 101/104 (97.1%) patients undergoing surgery and 191/200 (95.5%) patients admitted without surgery were confirmed to have CD on chart review. Among the 116 administrative database codes for surgical resection, 97.4% were confirmed intestinal resections on chart review. From 2002 to 2010, the overall CD surgical resection rate was 3.8 resections per 100 person-years. During the study period, rate of surgery decreased by 3.5% per year (95% CI: −1.1%, −5.8%), driven by decreasing emergent operations (−10.1% per year (95% CI: −13.4%, −6.7%)) whereas elective surgeries increased by 3.7% per year (95% CI: 0.1%, 7.3%).
Conclusions:
Overall surgical resection rates in CD are decreasing, but a paradigm shift has occurred whereby elective operations are now more commonly performed than emergent surgeries.
Introduction
Over the past two decades, the medical armamentarium for the treatment of Crohn’s disease (CD) has expanded dramatically (1, 2, 3). However, surgery continues to play a pivotal role in the treatment of CD, particularly for patients with aggressive penetrating phenotype, fibrostenotic obstructions, or disease refractory to medical therapy (4). In a meta-analysis, nearly half of CD patients required a surgical resection within 10 years of diagnosis and 35% needed a second operation within the next decade (5, 6). Approximately one-third of persons undergoing surgery for CD will develop postoperative complications (7, 8). The cost of surgery and associated hospitalizations contributes to the high direct healthcare costs associated with CD management (9).
CD patients typically undergo intestinal resections in one of two settings: (a) elective operations that occur due to lack of efficacy of outpatient medical therapies or (b) emergent operations occurring because of acute or life-threatening complications (e.g., small bowel obstruction) or failure of rescue inpatient medical treatment. This distinction is important because patients undergoing emergency surgery are more likely to experience postoperative complications and postoperative mortality (2, 10).
Temporal changes in the incidence of CD-related surgery are controversial. Although a meta-analysis of population-based studies suggests a decreasing incidence of surgery over the past several decades (5), studies derived from administrative databases have reported conflicting results (11, 12, 13). These differences may be a reflection of study design, particularly because results derived from administrative data rely heavily on the selection and validity of administrative coding (14). In many jurisdictions, the International Classification of Disease (ICD)-10 has now been introduced to more comprehensively capture clinical data. Although this coding system has been expanded from previous editions, it has not yet been validated for hospitalized patients with CD.
Thus, we conducted a population-based study to evaluate temporal trends in surgical rates for CD stratified by the setting of surgery. We first performed a detailed validation of ICD-10-CM coding for CD diagnosis among hospitalized patients and administrative coding for surgical bowel resections. We then used the validated codes to define surgical resection rates over time in this population.
Methods
Data source
The Data Integration, Measurement and Reporting (DIMR) discharge abstract administrative database captures all hospitalizations in the Calgary Health Zone (CHZ) of Alberta Health Services, Canada. The Calgary Health Zone is a population-based health authority under a public, single payer system, with an estimated catchment population of 1.4 million in 2011 (15). Geographically, the CHZ covers over 39,300 km2 and includes a large urban metropolis as well as multiple suburban communities and smaller rural towns and villages. This area is serviced by 56 gastroenterologists and eight colorectal surgeons, working out of four large tertiary care medical centers. The DIMR database captures 42 diagnostic codes (based on International Classification of Disease, 10th Revision, Clinical Modification) and 25 procedural codes (based on Canadian Classification of Health Intervention (CCI) coding). CCI coding is based on an alphanumeric structure with six field codes specifying the type of intervention (e.g., diagnostic vs. therapeutic), anatomy (e.g., large vs. small bowel), type of procedure (e.g., partial vs. total resection), and qualifying conditions (e.g., procedure approach and technique used to perform the intervention). The CHZ transitioned to ICD-10-CM diagnostic coding from ICD-9 in 2002.
Part 1—validation of ICD-10-CM and CCI coding
We first validated ICD-10-CM coding for CD diagnosis among hospitalized patients and CCI coding for surgical resections. Population-based surveillance was conducted in the CHZ between January 1 and December 31, 2011 to identify all adults (≥18 years) admitted to hospital with a diagnostic code for CD (ICD-10-CM K50.X). All patients with CD who underwent an intestinal resection in 2011 were included for the validation study. A negative control population was created by randomly sampling 200 patients with CD who were admitted to hospital but did not undergo an intestinal resection surgery. We validated administrative coding for 2011 because hospital records (including discharge summaries and operative reports) became available electronically after this time; using the electronic records allowed for more accurate capture of surgical procedures and hospitalization details for validation.
Surgical resection codes were defined a priori and stratified by CCI group: partial small intestine excisions including ileocecal resection (1.NK.87), partial large intestine excision (1.NM.87), total large intestine excision including total abdominal colectomy (1.NM.89, 1.NM.91), partial rectal excision including rectosigmoid resection (1.NQ.87), and total rectal excision including proctocolectomy (1.NQ.89). We specifically wanted to capture intestinal resection surgeries. We excluded relatively less invasive procedures with a lower burden of patient morbidity, including endoscopic procedures, “second-step” operations (e.g., pouch formation, completion proctectomy, ileostomy reversal), isolated perianal surgery, and abscess drainage. A complete list of administrative codes included and excluded from this study are found in Supplementary Tables 1 and 2.
Administrative database codes were then validated against comprehensive chart review for CD diagnosis, disease location (small intestine, large intestine, small and large intestine), surgical urgency (elective vs. non-elective), surgical approach (open vs. laparoscopic), and post-surgical anatomy (primary anastomosis vs. stoma). Surgery was defined as elective if the decision to operate was made prior to hospital admission. In contrast, the decision for emergent or urgent surgery occurred during the admission (e.g., after non-response to medical therapy or in response to life-threatening CD complications). Laparoscopic surgery requiring intra-operative conversion to open approach was classified as an open laparotomy.
For validation of administrative coding for CD diagnosis among hospitalized patients and for surgical resection, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence intervals (CIs) were calculated.
Part 2—CD surgical rates
Using validated ICD-10-CM diagnostic codes and CCI surgical resection codes, the DIMR database was used to identify all adults admitted to hospital with a diagnostic code of CD and undergoing surgical bowel resection between fiscal years 1 April 2002 and 31 March 2011. Data beyond 2011 were not available for analysis. Analysis was restricted to patients living within the CHZ to minimize uncaptured surgical resections occurring at hospitals outside the CHZ. Patients with codes for both CD and ulcerative colitis were excluded. Multiple admissions occurring within a 12-month period were counted as a single operation. This restriction was applied to prevent double counting of multi-stage operations. Rate of stricturoplasties was assessed in a sensitivity analysis. Perianal surgery in isolation (i.e., without intestinal resection or partial rectal excisions) and abscess drainage were excluded.
Annual surgical rates were calculated by dividing the annual surgical incident cases by the corresponding prevalence of CD in the CHZ. The prevalence of CD in CHZ from fiscal years 1997 to 2009 was derived from a previous study (16). For fiscal year 2010, the prevalence of CD was forecast using double exponential smoothing of data from fiscal years 1997 to 2009. Time trend analysis was performed on surgical rates by calculating the annual percent change (APC) using a generalized linear model that assumed a Poisson distribution. A negative binomial distribution was assumed when the data was over-dispersed. APCs were not statistically significant when the 95% CI crossed 0. The analysis was subsequently stratified by elective vs. non-elective surgical urgency, age at surgery (18–34, 35–64, and ≥65 years), and type of anatomic resection (small bowel vs. large bowel). Joinpoint regression was used to assess for statistical inflection points in the temporal trends.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Joinpoint Regression Program 4.0.1 (Statistical Research and Applications Branch, National Cancer Institute, USA). The study was approved by the Conjoint Health Research Ethics Board of the University of Calgary and was conducted in accordance with the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplementary Table 3) (17).
Results
Validity of ICD-10-CM CD diagnostic coding
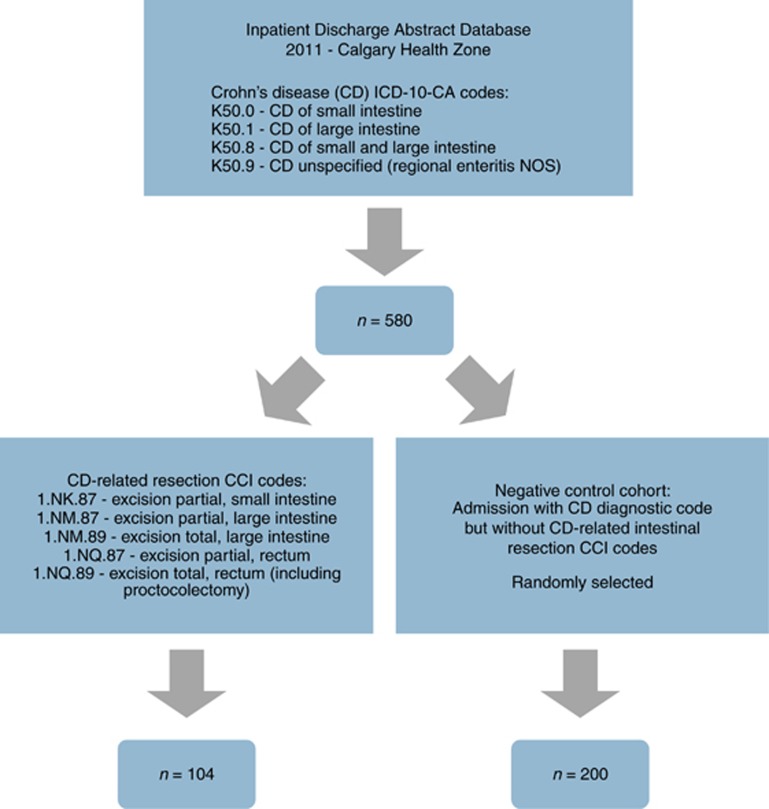
In our validation cohort, we identified 580 admissions to hospital with a diagnostic code for CD (K50.X) in 2011. Among these 580 admissions, we identified 104 patients with codes for both CD and surgical bowel resection. Among those without surgical bowel resection, we randomly sampled 200 negative control CD patients (Figure 1). CD was confirmed in 101/104 (97.1%) patients undergoing surgical resection compared to 191/200 (95.5%) patients admitted but not requiring surgery. Common reasons for misclassification were ischemic colitis or other inflammatory bowel disease (e.g., ulcerative colitis).
Figure 1.
Identification of surgical intestinal resection validation cohort and negative control cohort from the Data Integration, Measurement and Reporting Discharge Database (2011).
Validity of individual CD diagnostic codes is summarized in Table 1. When stratified by disease location, sensitivity for small bowel, large bowel, and ileocolonic CD was variable (range 0.30–0.95) but highly specific (range 0.87–0.99). Administrative database coding was highly predictive of small bowel (PPV 0.90 (95% CI: 0.8, 0.95)) and large bowel CD (PPV 0.90 (95% CI: 0.76, 0.97)). The administrative database identified 127 patients with a diagnostic code of K50.9 (CD, unspecified); this was more commonly identified in the negative control cohort (109/127, 85.9%) whereas only 18 (14.2%) CD patients undergoing surgery had unspecified disease location. When K50.8 and K50.9 codes are pooled, the combined sensitivity for ileocolonic disease increases (0.91 (95% CI: 0.83, 0.94)) but becomes less specific (0.59 (95% CI: 0.51, 0.67)).
Table 1. Validation of diagnostic codes by location of disease for hospitalized patients with Crohn’s disease.
| Diagnostic codesa | Definition | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| CD patients undergoing surgical resection | |||||
| K50.0 | Crohn’s disease of the small intestine | 0.70 (0.54, 0.82) | 0.90 (0.78, 0.96) | 0.84 (0.68, 0.93) | 0.79 (0.67, 0.88) |
| K50.1 | Crohn’s disease of the large intestine | 0.95 (0.74, 0.99) | 0.99 (0.93, 1.00) | 0.95 (0.74, 1.00) | 0.99 (0.93, 1.00) |
| K50.8 | Crohn’s disease of the small and large intestine | 0.53 (0.35, 0.70) | 0.87 (0.76, 0.94) | 0.67 (0.46, 0.83) | 0.79 (0.68, 0.87) |
| CD patients admitted without surgery | |||||
| K50.0 | Crohn’s disease of the small intestine | 0.53 (0.41, 0.65) | 0.98 (0.93, 0.99) | 0.92 (0.79, 0.98) | 0.78 (0.71, 0.84) |
| K50.1 | Crohn’s disease of the large intestine | 0.43 (0.28, 0.59) | 0.97 (0.93, 0.99) | 0.82 (0.59, 0.94) | 0.87 (0.80, 0.91) |
| K50.8 | Crohn’s disease of the small and large intestine | 0.30 (0.20, 0.41) | 0.97 (0.92, 0.99) | 0.89 (0.69, 0.97) | 0.66 (0.59, 0.73) |
CD, Crohn’s disease; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
K50.9 (Crohn’s disease, unspecified) not included because this diagnostic code does not specify disease location.
Validity of CCI CD surgical coding
On chart review, 113 resections were confirmed among 116 surgical resection codes (97.4%), performed in 104 separate patients in 2011 (Table 2). Predominantly, these were partial small bowel resections (58/113, 51.3%). Overall, procedural coding was highly accurate for identifying patients undergoing partial small bowel resections (PPV 0.87 (95% CI: 0.75, 0.94)) and partial large bowel resections (PPV 0.81 (95% CI: 0.64, 0.91)). Procedural coding also accurately identified patients undergoing total abdominal colectomy (PPV 1.00 (95% CI: 0.40, 1.00)), total proctocolectomy (PPV 0.86 (95% CI: 0.42, 0.99)), and partial rectal resections (PPV 0.80 (95% CI: 0.30, 0.99)), although this was based on limited sample size.
Table 2. Validation of surgical resection procedural codes for patients with Crohn’s disease by site and extent of resection.
| Procedural codes | Definition | Procedures Performed (n, %) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| 1.NK.87 | Excision partial, small intestine | 58 (51.3) | 0.83 (0.70, 0.91) | 0.96 (0.91, 0.98) | 0.87 (0.75, 0.94) | 0.94 (0.89, 0.96) |
| 1.NM.87 | Excision partial, large intestine | 38 (33.6) | 0.79 (0.62, 0.90) | 0.96 (0.92, 0.98) | 0.81 (0.64, 0.91) | 0.96 (0.91, 0.98) |
| 1.NM.89 | Excision total, large intestine | 5 (4.4) | 0.80 (0.30, 0.99) | 1.00 (0.98, 1.00) | 1.00 (0.40, 1.00) | 1.00 (0.97, 1.00) |
| 1.NQ.87 | Excision partial, rectum | 5 (4.4) | 0.80 (0.30, 0.99) | 0.99 (0.97, 1.00) | 0.80 (0.30, 0.99) | 1.00 (0.97, 1.00) |
| 1.NQ.89 | Excision total, rectum (including proctocolectomy) | 7 (6.2) | 0.86 (0.42, 0.99) | 1.00 (0.97, 1.00) | 0.86 (0.42, 0.99) | 1.00 (0.97, 1.00) |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Procedural qualifier codes were validated for surgical approach and post-surgical anatomy (Table 3). The sensitivity of administrative coding for elective vs. non-elective surgery and open vs. laparoscopic surgical approach exceeded 85%. Six laparoscopic procedures were misclassified: in 2/6 cases (33.3%) misclassification was due to conversion from initial laparoscopic approach to open laparotomy. The administrative database accurately identified post-surgical anastomosis (PPV 0.98 (95% CI: 0.92, 1.00)) and stoma formation (PPV 0.86 (95% CI: 0.64, 0.96)).
Table 3. Validation of surgical resection procedural codes for patients with Crohn’s disease by approach, urgency, and anastomosis type.
| Procedure type | Procedures Performed (n, %) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Surgical approach | |||||
| Open Approach | 78 (69.0) | 0.86 (0.76, 0.92) | 0.98 (0.95, 0.99) | 0.93 (0.84, 0.97) | 0.92 (0.86, 0.96) |
| Laparoscopic | 35 (30.1) | 0.86 (0.69, 0.95) | 0.98 (0.96, 1.00) | 0.88 (0.72, 0.96) | 0.97 (0.93, 0.99) |
| Surgical urgency | |||||
| Elective surgery | 73 (64.6) | 0.85 (0.74, 0.92) | 0.98 (0.95, 0.99) | 0.94 (0.84, 0.98) | 0.93 (0.87, 0.96) |
| Non-elective surgery | 40 (35.4) | 0.88 (0.72, 0.95) | 0.96 (0.92, 0.98) | 0.76 (0.61, 0.87) | 0.97 (0.93, 0.99) |
| Post-surgical anatomya | |||||
| Anastamosis | 90 (80.4) | 0.89 (0.80, 0.94) | 0.99 (0.97, 1.00) | 0.98 (0.92, 1.00) | 0.99 (0.97, 1.00) |
| Stoma | 22 (19.4) | 0.86 (0.64, 0.96) | 0.99 (0.97, 1.00) | 0.86 (0.64, 0.96) | 0.98 (0.95, 1.00) |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Post-surgical anatomy not defined in one case by chart review.
Surgical rates
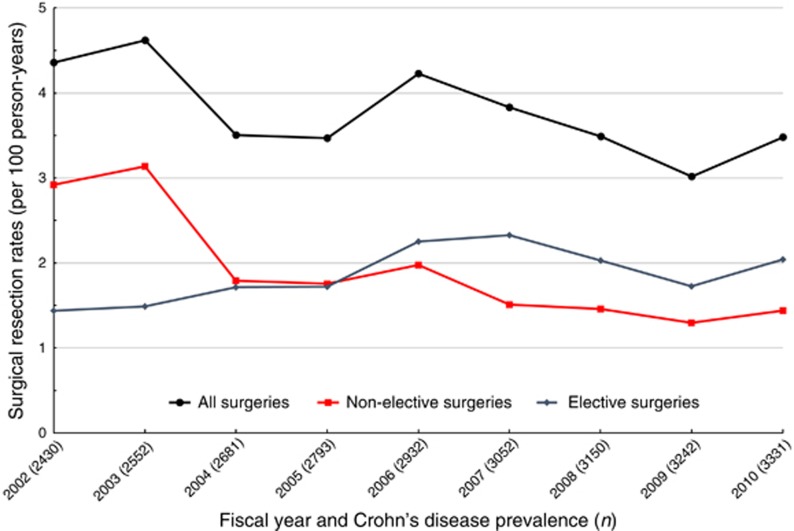
There were 981 surgical resections performed between fiscal years 2002 and 2010 across the CHZ, with an average surgical incidence of 3.8 operations per 100 person-years. The overall rate of surgery decreased by 3.5% per year (95% CI: −5.8%, −1.0%), from 4.4/100 person-years in 2002 to 3.5/100 person-years in 2010 (Figure 2).
Figure 2.
Decreasing annual surgical resection rates for Crohn’s disease from fiscal years 2002 to 2010 in the Calgary Health Zone. Resection rates stratified by elective (blue) vs. non-elective surgeries (red).
When stratified by surgical setting, non-elective surgeries decreased significantly by 10.1% per year (95% CI: −13.4%, −6.7%). Indeed, the rate of non-elective surgery was halved between fiscal years 2002 and 2010 (2.9 vs. 1.4 per 100 person-years). In contrast, there was a statistically significant increase in incidence of elective surgeries by 3.7% per year (95% CI: 0.1%, 7.3%). On joinpoint regression, this trend was driven by increasing rates of elective surgeries from 2002 to 2007 (APC 11.3% (95% CI: 9.3%, 13.2%, P<0.001)), after which time elective surgery incidence stabilized (APC −5.4% (95% CI: −12.6, 2.4%, P=0.17)). There was no statistically significant joinpoint for emergent operations. At the start of the study period, the non-elective surgery rate was more than double that of elective surgeries (2.9 vs. 1.4 per 100 person-years). However, these rates equalized by 2005 and in the final year, rate of elective surgery exceeded that of non-elective operations (2.0 vs. 1.4 per 100 person-years).
The incidence of partial small bowel resection (includes ileocecal resection), was stable during the study period (APC −2.2% (95% CI: −5.3%, 1.0%)). However, partial colonic resections significantly decreased by 8.5% per year (95% CI: −13.2%, −3.5%). Total abdominal colectomy or proctocolectomy rates were stable (APC 1.1% (95% CI: −7.1%, 10.1%)). Surgery was most commonly performed in patients with CD between the ages of 18 to 34 years (annual incidence 5.0 per 100 person-years) and surgical rates were stable in this age group (APC −2.2% (95% CI: −6.7%, 2.4%)). In contrast, patients with CD≥65 years experienced the greatest decline in annual surgical incidence rates (APC −12.8% (95% CI: −20.0%, −5.1%)) (Table 4).
Table 4. Trends in Crohn’s disease related bowel resection rates.
| Surgery subgroup | Average incidence of surgery (per 100 person-years) | APC (95% CI) | P-value |
|---|---|---|---|
| All surgeries (n=981)a | 3.8 | −3.5% (−5.8%, −1.1%) | 0.004 |
| Non-elective surgeries (n=489) | 1.9 | −10.1% (−13.4%, −6.7%) | <0.001 |
| Elective surgeries (n=492) | 1.9 | 3.7% (0.1%, 7.3%) | 0.042 |
| Partial small bowel resection (n=572) | 2.2 | −2.2% (−5.3%, 1.0%) | 0.169 |
| Partial colonic resection (n=349) | 1.4 | −8.5% (−13.2%, −3.5%) | 0.001 |
| Partial rectal resection (n=35) | 0.1 | 12.7 (−8.4, 38.7) | 0.258 |
| Total abdominal colectomy (n=37) | 0.1 | −4.3% (−15.7%, 8.7%) | 0.499 |
| Total abdominal colectomy or total proctocolectomy (n=88) | 0.3 | 1.1% (−7.1%, 10.1%) | 0.798 |
| Age 18–34 (all surgeries) (n=346) | 5.0 | −2.2% (−6.7%, 2.4%) | 0.342 |
| Age 35–64 (all surgeries) (n=548) | 3.4 | −2.1% (−5.3%, 1.1%) | 0.193 |
| Age 65+ (all surgeries) (n=86) | 3.3 | −12.8% (−20.0%, −5.1%) | 0.002 |
APC, annual percent change; CI, confidence interval.
Sum of surgical resections performed exceeds total surgeries (n=981) because some patients were coded for more than one procedure. This was counted as a single surgery in the total cohort for trend analysis.
In a sensitivity analysis, 59 stricturoplasty procedures (47/59, 79.6% small bowel) were identified in the administrative data between 2002 and 2010. The rate of stricturoplasties decreased by 7.1% per year, although this trend did not meet statistical significance (95% CI: −18.8%, 6.4%, P=0.20) with no identifiable joinpoint.
Discussion
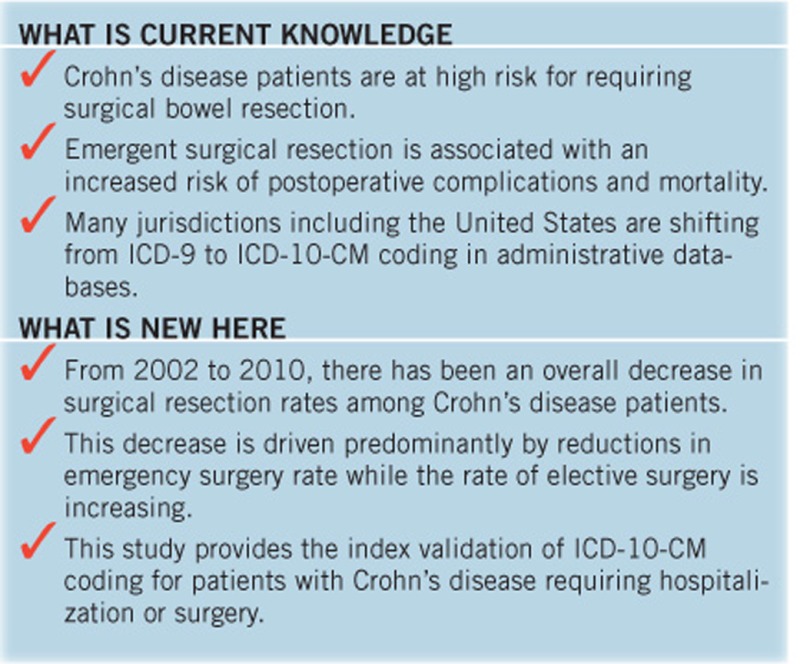
In this large population-based cohort study, we first validated administrative database coding in hospitalized CD patients: specifically, ICD-10-CM coding is highly predictive of CD diagnosis among patients requiring hospitalization or surgery, while procedural coding accurately identifies surgical resections and surgical setting. These validated codes can serve as a reference for future administrative database research in this field. We subsequently demonstrate using validated administrative coding that the rate of intestinal resection for CD decreased by 3.5% per year from 2002 to 2010. Interestingly, although there was a dramatic decrease in emergent surgeries by 10.1% per year, the rate of elective surgeries actually increased significantly by 3.7% per year. These findings highlight a fundamental paradigm shift in the surgical management of CD during the first decade of the 21st century, with a greater emphasis towards an ambulatory model of care.
A reduction in emergent operations is ideal because CD patients who require an emergent procedure have an increased risk of in-hospital postoperative morbidity (18), 90-day readmission (19), and most importantly, postoperative mortality (2, 10). In a meta-analysis of 75,971 CD patients, Singh et al. found a 6-fold difference in postoperative mortality for patients undergoing elective as compared to emergent surgery (0.6 vs. 3.6%) (2). Thus, the evolution of surgical management towards more elective procedures is an important achievement in the care of CD patients.
Despite the increase in elective operations, there was an overall decrease in the surgical resection rates in our cohort that mirrors the findings from other population- and cohort-based studies (12, 20, 21, 22). Our study was not designed to determine factors leading to the decrease in surgical incidence. Importantly, our administrative database lacked medication data that would allow us to correlate drug utilization to surgical rates. However, several authors have previously attributed the decrease in surgical rates to increased adoption of immunosuppressive agents (12, 20, 23) and in randomized controlled trials, anti-TNF therapy is associated with decreased risk of surgery in CD (24, 25).
Non-medication related factors may also explain the observed reduction in surgery. The management of CD has become increasingly complex. However, evolving practice patterns have led to earlier disease detection, closer endoscopic and radiographic follow-up, targeting of complete mucosal healing as a treatment outcome, and improved access to tertiary level IBD care. For example, Nguyen et al. demonstrated reduced 5-year surgical risk in CD patients in association with increased access to specialist gastroenterologists (21). Improved access to gastroenterology consultation may result in improved therapeutic decision-making early in the disease course or during hospitalizations, preventing emergency surgery. Furthermore, the implementation of clinical decision tools and treatment algorithms have streamlined the management of complex CD and highlighted important quality indicators for treatment (26). These non-medication related factors play an important role in improving the care of CD patients and potentially, reducing the long-term risk of surgery.
The increase in incidence of elective resections could be explained by changes in surgical practice patterns or indications for resection. For instance, concerns regarding poor postoperative wound healing and infection have resulted in hesitancy among some surgeons for operating on CD patients actively treated with biologics (27). This may result in postponing surgery to allow drug washout and increased rates of planned or staged elective operations. Additionally, we observed the greatest decline in surgical incidence among elderly patients. Traditionally, this is a group of patients with milder inflammatory disease activity but a higher burden of comorbidities and operative risk (28). This may discourage surgery as a therapeutic option in this cohort, particularly for smoldering disease.
Administrative databases provide a cost- and time-efficient method for studying epidemiologic and health system trends compared to retrospective cohort studies. However, results must be interpreted in the context of the validity of administrative coding. Many coding algorithms are utilized by different administrative databases but ICD-10-CM coding has recently been adopted in the United States. In Canada, ICD-10-CM coding has been in use for over a decade and in the Calgary Health Zone, discharge administrative data has been using ICD-10-CM coding since 2002. This study provides the index validation for ICD-10-CM coding for CD diagnosis among hospitalized patients. It can be also used as a reference for both diagnostic and procedural codes for researchers studying this population in the future.
In our cohort, over 95% of patients with K50.X diagnostic coding were confirmed as having CD by chart review. In comparison, Thirmurthi et al. previously validated ICD-9 coding for CD in the Department of Veterans Affairs National Patient Care Database, demonstrating a PPV of 88–100% (29). Our point estimates of administrative data accuracy may be higher due to selection bias because we only evaluated hospitalized CD patients: this subset of patients is more likely to have a confirmed diagnosis of CD prior to invasive surgery and the diagnosis can be verified on operative pathology. Accuracy of ICD-10-CM coding for CD is likely higher than that for ulcerative colitis: in a similar cohort, we have demonstrated that patients coded as having ulcerative colitis often are misclassified on the basis of having colonic CD, but the converse misclassification bias of CD as ulcerative colitis is less likely, particularly for Crohn’s ileitis (30).
However, the accuracy of predicting CD location based on ICD-10-CM coding alone remains limited due to low sensitivity. We speculate that this is due to incomplete reporting in the primary medical record that may be influenced by the complex nature of the disease or due to progression of disease extent over time. Nevertheless, frequent utilization of the K50.9 administrative code (CD, unspecified location) highlights a gap in the quality of current diagnostic reporting. Emphasizing the precise description of CD extent and location using a standardized classification system such as the Montreal Classification (31) may improve diagnostic accuracy moving forwards.
Interestingly, other authors studying the trend in incident surgeries for CD using administrative data have reported conflicting results (11, 13, 32, 33, 34, 35). For instance, in evaluating the Nationwide Inpatient Sample, Geltzeiler et al. reported a 26% increase in Crohn’s-related operations from 1988 to 2011 (13). Although precise estimates for the comparable time period from 2002 to 2010 are not specified, the authors present a linear regression model assuming a constant increase of approximately 1% per year in the number of surgical procedures performed. However, the authors report only the crude number of operations performed and do not account for the increase in incidence of IBD (36). Further, the Nationwide Inpatient Sample is a discharge abstract database that cannot differentiate repeat admissions of the same patient, which could lead to overinflating surgical rates. Data from Europe is also available for comparison: Burke et al. evaluated Crohn’s-related surgeries between 2000 to 2010 in the Republic of Ireland, demonstrating stable rates of small bowel and right colon procedures, decreasing rates of left colon surgeries, but increasing rates of total colectomy (32). However, these rates were calculated using the total estimated adult Irish population from census data as the denominator and do not account for the higher relative increase in IBD prevalence. Further, differences in biologic use, surgical and medical care, or patient preferences for treatment may also account for the differences observed compared to our population.
There are several limitations to our study. First, administrative databases lack information on important factors that may have influenced surgical rates such as tobacco use and symptom burden. We were also unable to classify disease behavior to determine whether changes in surgical rates were specific to stricturing or penetrating disease phenotypes and the administrative database did not capture CD duration. Second, all studies performed using administrative data are subject to misclassification bias. Although we minimized this by validating our diagnostic and procedural coding, there may be temporal trends in database coding patterns and validity that could not be accounted for, particularly if there were early errors made when the administrative coding switched to ICD-10-CM in 2002. Reassuringly though, there are no early joinpoints in surgical rates after 2002 that could potentially indicate discrepancies due to coding inexperience. Additionally, although we sampled negative control patients for the validation cohort, this control cohort may still be undersized in relation to CD resection prevalence. Thirdly, we lacked data on drug utilization to determine the influence of immunomodulators and biologics on surgical rates for CD. Even if medication data were available, theses correlations would still be subject to ecological fallacy. Finally, re-validation of the codes should be undertaken in other regions to determine whether our findings can be generalized outside of a large Canadian health region, particularly in jurisdictions where surgical procedures are performed outside of the hospital setting in dedicated ambulatory surgery centers. Additionally, our validation of ICD-10 codes is specifically applicable to hospitalized patients but may not be generalizable to the outpatient setting.
While gastroenterologists and CD patients often strive to manage disease medically and avoid intestinal resection, surgery is crucial in certain clinical settings to achieving disease control. The optimal clinical setting for minimizing postoperative morbidity and mortality is a planned elective surgery after consultation between the patient, surgeon, and gastroenterologist, working together as a multidisciplinary team. This large population-based cohort study serves as the index study for validating ICD-10-CM coding for CD in hospitalized patients and supports the transition to ICD-10-CM coding in many jurisdictions. Using these validated codes, we demonstrate an overall decline in surgical resection rates for CD, but more importantly we highlight a fundamental paradigm shift whereby emergent surgeries have dramatically decreased with a parallel increase in operations occurring in the elective setting. Future studies are necessary to delineate medical and non-medical factors that have contributed to this change in surgical management and to further elucidate the continuing evolution of these trends. Surgical rates after 2010 will likely be influenced not only by the continually expanding medical armamentarium for CD treatment, but also new concepts in management including the integration of primary care networks, rigorous therapeutic drug monitoring, and treatment-to-target of both symptoms and possibly objective endoscopic, radiographic, histologic, and biomarker outcomes.
Study Highlights
Acknowledgments
This study is based in part on data provided by Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the Government nor Alberta Health Services express any opinion in relation to this study.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Gilaad Kaplan, MD, MPH, FRCPC.
Specific author contributions: C.M. contributed to study design, data collection, data analysis, and manuscript drafting. G.W.M. contributed to study design and manuscript drafting. E.I.B. contributed to study design and manuscript editing. J.N.H. contributed to data analysis. L.T., S.J.H., C.H.S., K.L.N., S.G., and R.P. contributed to manuscript editing. G.G.K. contributed to study design, data analysis, and manuscript editing.
Financial support: Dr G. Kaplan is a Canadian Institutes of Health Research Embedded Clinician Research Chair. Dr E. Benchimol was supported by a New Investigator Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology and Crohn’s and Colitis Canada. He was also supported by the Canadian Child Health Clinician Scientist Program Career Enhancement Program.
Potential competing interests: G.M. has received consultancy fees from AbbVie, Takeda, Janssen, and Dr Falk; speaker fees from Merck Sharp, Dohme Ltd, AbbVie, Ferring, Janssen, and Takeda; and financial support for educational activities from AbbVie, Merck Sharp, Dohme Ltd, Ferring, and Dr Falk. L.T. has served on the advisory boards for Pfizer, Takeda, AbbVie, and Janssen and received speaking fees from Janssen, Takeda, and Pfizer. She has received grant support from Pfizer and AbbVie. C.S. has participated in advisory board meetings for Janssen, AbbVie, Shire, Takeda, and Actavis. She has received speaker fees from Janssen and AbbVie. K.N. has served as a speaker for AbbVie and Janssen. She has participated in advisory board meetings for AbbVie, Janssen, Pfizer, and Ferring. She has received research support from AbbVie and Janssen. S.G. has participated in advisory board meetings for Centocor, Abbott, Merck, Schering Plough, Proctor & Gamble, Shire, UCB Pharma, Pfizer, and Millennium. He has received research support from Proctor & Gamble, Merck, Schering-Plough and speaking fees from Merck, Schering-Plough, Centocor, Abbott, UCB Pharma, Pfizer, Ferring, and Proctor & Gamble. R.P. has participated in advisory board meetings for Abbott/AbbVie, Amgen, Janssen, Merck, Pfizer, Prometheus Laboratories, Salix Pharma, Shire, Takeda, and Warner Chilcott. He has received consulting fees from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Elan/Biogen, Eisai, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus Therapeutics and Diagnostics, Schering-Plough, Shire, Takeda, UCB Pharma, and Warner Chilcott. Dr R.P. has received research support from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Eisai, Elan/Biogen, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus, Shire, Schering-Plough, Takeda, UCB Pharma, and Warner Chilcott. He has received speaking fees from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Eisai, Elan/Biogen, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus, Schering-Plough, Shire, Takeda, UCB Pharma, and Warner Chilcott. G.K. has served as a speaker for Janssen, Merck, Schering-Plough, Abbvie, and UCB Pharma. He has participated in advisory board meetings for Janssen, Abbvie, Merck, Schering-Plough, Shire, and UCB Pharma. Dr G.K. has received research support from Merck, Abbvie, GlaxoSmith Kline, and Shire. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Hazlewood GS, Rezaie A, Borman M et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology 2015;148:344–354. [DOI] [PubMed] [Google Scholar]
- Singh S, Al-Darmaki A, Frolkis AD et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology 2015;149:928–937. [DOI] [PubMed] [Google Scholar]
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2016;152:313–321.e2. [DOI] [PubMed] [Google Scholar]
- Fichera A, Michelassi F. Surgical treatment of Crohn's disease. J Gastrointest Surg 2007;11:791–803. [DOI] [PubMed] [Google Scholar]
- Frolkis AD, Dykeman J, Negron ME et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- Frolkis AD, Lipton DS, Fiest KM et al. Cumulative incidence of second intestinal resection in Crohn's disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109:1739–1748. [DOI] [PubMed] [Google Scholar]
- Muller KR, Prosser R, Bampton P et al. Female gender and surgery impair relationships, body image, and sexuality in inflammatory bowel disease: patient perceptions. Inflamm Bowel Dis 2010;16:657–663. [DOI] [PubMed] [Google Scholar]
- Alves A, Panis Y, Mathieu P et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278–283 discussion 284. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Nabalamba A. Hospitalization, surgery, and readmission rates of IBD in Canada: a population-based study. Am J Gastroenterol 2006;101:110–118. [DOI] [PubMed] [Google Scholar]
- Kennedy NA, Clark DN, Bauer J et al. Nationwide linkage analysis in Scotland to assess mortality following hospital admission for Crohn's disease: 1998-2000. Aliment Pharmacol Ther 2012;35:142–153. [DOI] [PubMed] [Google Scholar]
- Jones DW, Finlayson SR. Trends in surgery for Crohn's disease in the era of infliximab. Ann Surg 2010;252:307–312. [DOI] [PubMed] [Google Scholar]
- Rungoe C, Langholz E, Andersson M et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut 2014;63:1607–1616. [DOI] [PubMed] [Google Scholar]
- Geltzeiler CB, Hart KD, Lu KC et al. Trends in the Surgical Management of Crohn's Disease. J Gastrointest Surg 2015;19:1862–1868. [DOI] [PubMed] [Google Scholar]
- Kaplan GG. Administrative database studies in IBD: a cautionary tale. Am J Gastroenterol 2010;105:1808–1810. [DOI] [PubMed] [Google Scholar]
- AHS Alberta Health Services Annual Report 2011-2012. 2012 [cited November 27, 2016] available at http://www.albertahealthservices.ca/Publications/ahs-pub-annual-rpt-2012.pdf.
- Rezaie A, Panaccione R, Fedorak RN et al. Time trends in prevalence and incidence of inflammatory bowel disease in Alberta: A population-based study. Can J Gastroenterol 2012;26:A133. [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GG, Hubbard J, Panaccione R et al. Risk of comorbidities on postoperative outcomes in patients with inflammatory bowel disease. Arch Surg 2011;146:959–964. [DOI] [PubMed] [Google Scholar]
- Frolkis A, Kaplan GG, Patel AB et al. Postoperative complications and emergent readmission in children and adults with inflammatory bowel disease who undergo intestinal resection: a population-based study. Inflamm Bowel Dis 2014;20:1316–1323. [DOI] [PubMed] [Google Scholar]
- Chatu S, Saxena S, Subramanian V et al. The impact of timing and duration of thiopurine treatment on first intestinal resection in Crohn's disease: national UK population-based study 1989-2010. Am J Gastroenterol 2014;109:409–416. [DOI] [PubMed] [Google Scholar]
- Nguyen GC, Nugent Z, Shaw S et al. Outcomes of patients with Crohn's disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141:90–97. [DOI] [PubMed] [Google Scholar]
- Zhulina Y, Udumyan R, Tysk C et al. The changing face of Crohn's disease: a population-based study of the natural history of Crohn's disease in Orebro, Sweden 1963-2005. Scand J Gastroenterol 2016;51:304–313. [DOI] [PubMed] [Google Scholar]
- Lakatos PL, Golovics PA, David G et al. Has there been a change in the natural history of Crohn's disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol 2012;107:579–588. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Yan S, Bala M et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology 2005;128:862–869. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ. Crohn's disease evaluation and treatment: clinical decision tool. Gastroenterology 2014;147:702–705. [DOI] [PubMed] [Google Scholar]
- Oresland T, Faerden AE. Surgery in the age of biological treatment. Scand J Gastroenterol 2015;50:121–127. [DOI] [PubMed] [Google Scholar]
- Ha C, Katz S. Elderly-onset IBD: a milder disease? Nat Rev Gastroenterol Hepatol 2013;10:264–265. [DOI] [PubMed] [Google Scholar]
- Thirumurthi S, Chowdhury R, Richardson P et al. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci 2010;55:2592–2598. [DOI] [PubMed] [Google Scholar]
- Ma C, Crespin M, Proulx MC et al. Postoperative complications following colectomy for ulcerative colitis: a validation study. BMC Gastroenterol 2012;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J, Silverberg MS, Vermeire S et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Velupillai Y, O'Connell PR et al. National trends in intestinal resection for Crohn's disease in the post-biologic era. Int J Colorectal Dis 2013;28:1401–1406. [DOI] [PubMed] [Google Scholar]
- Stallmach A, Dennler U, Marschall U et al. Patient-relevant endpoints in inflammatory bowel diseases—have changes occurred in Germany over the past twelve years? J Crohns Colitis 2015;9:390–397. [DOI] [PubMed] [Google Scholar]
- Herrinton LJ, Liu L, Fireman B et al. Time trends in therapies and outcomes for adult inflammatory bowel disease, Northern California, 1998-2005. Gastroenterology 2009;137:502–511. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Harmsen WS, Tremaine WJ et al. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol 2012;107:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54 e42 quiz e30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.