Abstract
Background:
Acute exercise does not elicit compensatory changes in appetite parameters in lean individuals; however, less is known about responses in overweight individuals. This study compared the acute effects of moderate-intensity exercise on appetite, energy intake and appetite-regulatory hormones in lean and overweight/obese individuals.
Methods:
Forty-seven healthy lean (n=22, 11 females; mean (s.d.) 37.5 (15.2) years; 22.4 (1.5) kg m−2) and overweight/obese (n=25, 11 females; 45.0 (12.4) years, 29.2 (2.9) kg m−2) individuals completed two, 8 h trials (exercise and control). In the exercise trial, participants completed 60 min treadmill exercise (59 (4)% peak oxygen uptake) at 0–1 h and rested thereafter while participants rested throughout the control trial. Appetite ratings and concentrations of acylated ghrelin, peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) were measured at predetermined intervals. Standardised meals were consumed at 1.5 and 4 h and an ad libitum buffet meal was provided at 7 h.
Results:
Exercise suppressed appetite (95% confidence interval (CI) −3.1 to −0.5 mm, P=0.01), and elevated delta PYY (95% CI 10 to 17 pg ml−1, P<0.001) and GLP-1 (95% CI 7 to 10 pmol l−1, P<0.001) concentrations. Delta acylated ghrelin concentrations (95% CI −5 to 3 pg ml−1, P=0.76) and ad libitum energy intake (95% CI −391 to 346 kJ, P=0.90) were similar between trials. Subjective and hormonal appetite parameters and ad libitum energy intake were similar between lean and overweight/obese individuals (P⩾0.27). The exercise-induced elevation in delta GLP-1 was greater in overweight/obese individuals (trial-by-group interaction P=0.01), whereas lean individuals exhibited a greater exercise-induced increase in delta PYY (trial-by-group interaction P<0.001).
Conclusions:
Acute moderate-intensity exercise transiently suppressed appetite and increased PYY and GLP-1 in the hours after exercise without stimulating compensatory changes in appetite in lean or overweight/obese individuals. These findings underscore the ability of exercise to induce a short-term energy deficit without any compensatory effects on appetite regardless of weight status.
Introduction
The impact of exercise on energy balance is influenced by its effects on appetite regulation and food intake. Evidence suggests that acute moderate- to high-intensity exercise transiently suppresses appetite in healthy, lean individuals, which occurs with commensurate fluctuations in appetite-regulatory hormones, including acylated ghrelin, peptide YY (PYY) and glucagon-like peptide-1 (GLP-1).1, 2 These responses are transient, typically returning to resting control values in the hours after exercise and do not influence energy intake at meals on the day of exercise.1, 2, 3 This lack of compensatory response highlights the ability of exercise to induce a short-term energy deficit and modulate weight control.
Considering obesity is characterised by a chronic surplus of energy intake above expenditure, it is conceivable that appetite responses to exercise may differ between lean and overweight/obese individuals. Previous non-exercise studies have demonstrated that postprandial increases in circulating satiety hormones (PYY and GLP-1) are blunted in obese compared with lean individuals,4, 5 and obese individuals exhibit lower fasting concentrations and reduced postprandial suppression of the orexigenic hormone ghrelin.6 Despite these divergent responses, few studies have investigated the acute effects of exercise on appetite regulation in overweight/obese compared with lean individuals.
Current evidence suggests that acute exercise does not stimulate any immediate compensatory changes in appetite, energy intake or appetite-regulatory hormones in overweight or obese individuals.7, 8, 9, 10 A direct comparison of lean and overweight/obese individuals identified a similar transient elevation in total PYY and GLP-17–36 concentrations up to 1 h after exercise in both groups that occurred without simultaneous changes in acylated ghrelin or appetite.11 Furthermore, ad libitum energy intake in the immediate postexercise period appears unaffected12 or reduced11 in lean and overweight/obese individuals. However, additional research is required to understand and compare the appetite regulation of lean and overweight/obese individuals in response to acute exercise, including examination of responses beyond the immediate postexercise period (>1 h). This information is important for elucidating the impact of exercise on appetite regulation in a clinically relevant population.
Therefore, the aim of this study was to examine the appetite, ad libitum energy intake and appetite-regulatory hormone responses during and for 7 h after an acute bout of moderate-intensity exercise in healthy lean and overweight/obese individuals.
Methods
This study received approval from the NHS East Midlands Research Ethics Committee (13/EM/0290) prior to its initiation and was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki.13
Participants
Sixty participants provided written informed consent to participate in this study. All participants were metabolically healthy, not taking any medications, body mass stable for ⩾3 months (±2 kg) and non-smokers. Participants were ‘inactive’ or ‘moderately active’ according to the International Physical Activity Questionnaire,14 had no abnormal eating behaviour traits15 and had a body mass index (BMI) between 18.5–24.9 kg m−2 and 25.0–39.9 kg m−2. Female participants were either premenopausal or postmenopausal and not pregnant. Thirteen participants withdrew before completing all trial visits. Consequently, data are reported for 47 participants in this manuscript (lean n=22, BMI range 19.6–24.5 kg m−2 (males n=11; females n=11); overweight/obese n=25, BMI range 25.3–35.4 kg m−2 (males n=14; females n=11)). Table 1 details the key participant characteristics.
Table 1. Participant characteristics.
| Lean (n=22) | Overweight/obese (n=25) | Lean vs overweight/obese 95% CIa | Effect size | |
|---|---|---|---|---|
| Age (years) | 37.5 (15.2) | 45.0 (12.4) | −0.6 to 15.6 | 0.55 |
| Body mass (kg) | 63.7 (7.9) | 86.5 (14.5) | 15.9 to 29.8b | 1.92 |
| Body mass index (kg m−2) | 22.4 (1.5) | 29.2 (2.9) | 5.4 to 8.2b | 2.87 |
| Waist circumference (cm) | 75.7 (7.6) | 91.8 (9.5) | 11.1 to 21.3b | 1.87 |
| Peak V̇O2 (ml kg−1 min−1) | 43.6 (12.2) | 34.7 (8.9) | −15.1 to −2.7b | 0.84 |
| Peak V̇O2 (l min−1) | 2.83 (0.91) | 2.99 (0.86) | −0.36 to 0.68 | 0.18 |
| Fasting glucose (mmol l−1) | 4.72 (0.82) | 4.70 (0.94) | −0.54 to 0.50 | 0.03 |
| Fasting TC (mmol l−1) | 3.89 (1.08) | 4.77 (1.40) | 0.14 to 1.63b | 0.70 |
| Fasting HDL-C (mmol l−1) | 2.00 (0.59) | 1.48 (0.55) | −0.86 to −0.19b | 0.93 |
| Fasting LDL-C (mmol l−1) | 1.62 (1.12) | 2.96 (1.43) | 0.59 to 2.11b | 1.04 |
| Fasting TAG (mmol l−1) | 1.12 (0.86) | 1.65 (1.04) | −0.04 to 1.09 | 0.55 |
| Fasting leptin (pg ml−1) | 8273 (9119) | 15 340 (9984) | 1420 to 12 713b | 0.74 |
Abbreviations: CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TAG, triacylglycerol; TC, total cholesterol; V̇O2, oxygen uptake.
All values are mean (s.d).
95% confidence interval of the mean absolute difference between the groups.
Significant difference between lean and overweight/obese individuals (P<0.05).
Preliminary testing
Participants completed two preliminary visits to confirm eligibility, determine peak oxygen uptake (V̇O2) and familiarise themselves with main trial procedures. The eligibility assessment included a full medical evaluation with blood screen (Table 1) and cardiovascular risk assessment. Eligible participants completed treadmill-based submaximal incremental and peak V̇O2 tests as described previously.16 Oxygen consumption and carbon dioxide production were determined using an online breath-by-breath gas analysis system (Metalyser 3B, Cortex, Biophysik, Germany).
Experimental procedures
Participants completed two, 8 h (0900–1700 hours) trials (control and exercise) in a randomised crossover design. Main trials were separated by at least 7 days for men and 28 days for women to standardise for menstrual cycle phase. In the 48 h before trials, participants standardised their diets using a weighed food diary and did not consume any alcohol or caffeinated drinks. Participants also refrained from strenuous physical activity and consumed a prescribed meal on the evening before each main trial. The meal, consisting of pasta, tomato sauce and chocolate biscuits, provided 3138 kJ for males and 2820 kJ for females (71% carbohydrate, 11% protein, 18% fat). After this meal, only water was permitted until the beginning of main trials.
At the beginning of the exercise trial (0−1 h), participants completed 60 min of treadmill exercise at a speed predicted to elicit 60% peak V̇O2 and then rested within the laboratory for the subsequent 7 h. An online breath-by-breath gas analysis system was used to estimate energy expenditure and maintain exercise intensity, with accompanying measurements of heart rate (Model T31 Polar, Kempele, Finland) and ratings of perceived exertion.17 Identical procedures were undertaken in the control trial except no exercise was performed. Instead, resting expired air samples were collected in a semisupine position to permit the calculation of net energy expenditure during the exercise bout using the equations of Frayn.18
Appetite, food provision and ad libitum energy intake
During each trial, appetite ratings (hunger, satisfaction, fullness and prospective food consumption) were assessed at baseline and every 30 min thereafter using 100 mm visual analogue scales.19 An overall appetite rating was quantified as the mean value of the four appetite ratings after satisfaction and fullness were reverse-scored.20 At 1.5 h (~1030 hours), participants consumed a standardised breakfast consisting of jam sandwiches (brown bread, margarine and strawberry jam), banana and orange juice. The energy content of this meal was 2690 kJ for males and 2418 kJ for females (72% carbohydrate, 10% protein, 18% fat). At 4 h (~1300 hours), participants consumed a standardised lunch consisting of either tuna and mayonnaise or cheese white bread sandwiches, salted crisps, apple and a chocolate muffin. The energy content of this meal was 3138 kJ for males and 2820 kJ for females (43% carbohydrate, 32% protein, 25% fat). Each meal was consumed within 15 min.
Energy and macronutrient intakes were assessed at an ad libitum buffet meal provided at 7 h (~1600 hours) during main trials. The items available were: white bread, brown bread, apples, bananas, margarine, ham, cheese, tuna, mayonnaise, crisps, chocolate rolls, cereal bars, cookies, and chocolate muffins. The buffet foods were presented identically on each occasion in excess of expected consumption. Participants were given 30 min to eat until they were ‘comfortably full’. Participants consumed meals in isolation so that social influence did not affect eating rate, food selection or quantity eaten. Manufacturer values were applied to the weight of foods consumed to determine energy and macronutrient intakes.
Blood sampling and biochemical analysis
Fasting capillary blood samples were collected during the preliminary testing to determine plasma concentrations of glucose (Accutrend Plus, Roche, Switzerland), total cholesterol, high-density lipoprotein cholesterol and triacylglycerol (CardioChek, PTS Diagnostics, Indianapolis, IN, USA). Low-density lipoprotein cholesterol concentration was estimated.21
During main trials, venous blood samples were collected via a cannula (Venflon, Becton Dickinson, Helsingborg, Sweden) inserted into an antecubital vein. Blood samples were collected into prechilled EDTA monovettes (Sarstedt, Leicester, UK) for the determination of plasma acylated and desacylated ghrelin, total PYY, total GLP-1, insulin and glucose at baseline (fasting), 1, 1.5, 2.25, 2.75, 3.25, 4, 4.75, 5.25, 5.75, 7 and 8 h. The monovettes for acylated ghrelin also contained protease inhibitors to prevent the degradation of acylated ghrelin. At each sampling point, haemoglobin and haematocrit were measured to permit the assessment of plasma volume changes.22
Commercially available enzyme-linked immunosorbent assays were used to determine the concentrations of plasma acylated and desacylated ghrelin (SPI BIO, Montigny le Bretonneux, France), total PYY, total GLP-1 (Millipore, Billerica, MA, USA), leptin (R&D Systems, Minneapolis, MN, USA) and insulin (Mercodia, Uppsala, Sweden). Plasma glucose concentrations were determined by enzymatic, colorimetric methods using a benchtop analyser (Pentra 400, HORIBA ABX Diagnostics, Montpellier, France). The within-batch coefficients of variation for the assays were as follows: acylated ghrelin 5.2%, desacylated ghrelin 4.8%, total PYY 6.1%, total GLP-1 10.2%, leptin 3.0%, insulin 4.5%, and glucose 0.7%.
Statistical analyses
Data were analysed using SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA). Plasma hormone and metabolite concentrations are presented relative to baseline concentrations (that is, delta) to minimise the potential influence of day-to-day biological variation.23, 24 Baseline (fasting) plasma hormone and metabolite concentrations are presented in Supplementary Table 1. Correction of blood parameters for plasma volume changes did not alter the results; therefore, unadjusted values are presented. The homeostasis model assessment of insulin resistance was calculated.25
Linear mixed models were used to examine differences in participant characteristics and exercise responses with group (lean or overweight/obese) included as a fixed factor. Baseline (fasting) appetite parameters and energy/macronutrient intakes were analysed using linear mixed models with trial (control and exercise) and group included as fixed factors. Differences in appetite parameters over the 8 h trials were examined using linear mixed models with trial, time and group included as fixed factors. Sex was included as a fixed factor in the model for acylated ghrelin considering that between-sex differences in this hormone have been reported previously.26 No significant between-sex differences were observed for the other plasma hormones or overall appetite (P⩾0.09); therefore, this data is presented without sex included in the model. All linear mixed models included a random effect for each participant.
Area under the curve (AUC) values for appetite and blood parameters were calculated using the trapezoidal rule and are presented in Supplementary Table 2. Bivariate correlations identifying possible determinants of the exercise-induced changes in AUC values were quantified using Pearson’s product moment correlations. Statistical significance was accepted as P<0.05. The 95% confidence intervals (CIs) were calculated for mean absolute pairwise differences between experimental trials and groups. Absolute standardised effect sizes (ES) are provided to supplement important findings, with 0.2 considered the minimum important difference, 0.5 moderate and 0.8 large.27 Results are presented as mean (s.d.) in text and tables and mean (s.e.m.) in figures (for clarity).
Sample size calculations
Based on previous data from our laboratory,28 it was estimated that a sample size of 42 participants would have >95% power at the 0.05 level to detect a 188 pg ml−1 increase in circulating PYY after acute exercise assuming a s.d.diff of 219 pg ml−1. This calculation was conducted using G*Power (v3.1.9.2).29
Results
Exercise responses
Compared with lean individuals, overweight/obese individuals exercised at a lower treadmill speed (7.1 (1.4) vs 6.3 (0.9) km h−1, respectively; ES=0.68, P=0.03) and a marginally lower relative exercise intensity (60 (3) vs 58 (4)% peak V̇O2, respectively; ES=0.56, P=0.06). No other between-group differences were seen in exercise responses (P⩾0.39; Supplementary Table 3).
Appetite
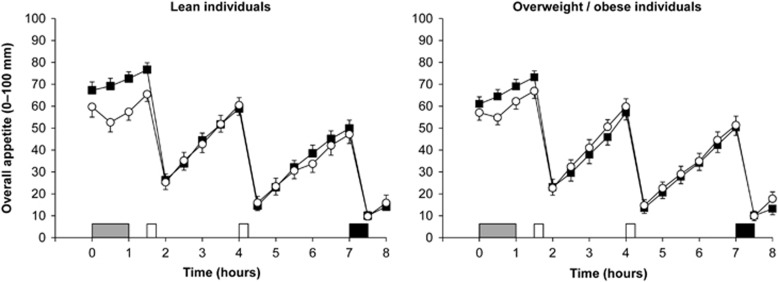
Overall appetite ratings were similar between groups at baseline (95% CI −13.8 to 4.9 mm, P=0.34) but were lower in the exercise than in the control trial (95% CI −11.2 to −0.4 mm, ES=0.34, P=0.04) (Figure 1). Linear mixed models for overall appetite identified a main effect of trial (P=0.01) and time (P<0.001) but not group (95% CI −7.4 to 4.4 mm, P=0.60) (Figure 1). The main effect of trial revealed lower overall appetite in the exercise than in the control trial (95% CI −3.1 to −0.5 mm, ES=0.07, P=0.01). Analysis also revealed a significant trial-by-time interaction (P<0.001), with lower overall appetite in exercise than in control at 0.5, 1.0 and 1.5 h (all ES⩾0.60, P⩽0.001). The magnitude of reduction in overall appetite after exercise was marginally albeit significantly different between the overweight/obese and lean participants (−0.1 mm (ES<0.01) vs −3.5 mm (ES=0.14), respectively; trial-by-group interaction P=0.01).
Figure 1.
Overall appetite perceptions in the lean (n=22, left panel) and overweight/obese (n=25, right panel) individuals during the control  and exercise (○) trials. Values are mean (s.e.m.). Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
and exercise (○) trials. Values are mean (s.e.m.). Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
Energy and macronutrient intakes
Absolute energy and macronutrient intakes were similar across trials and groups (main effect trial P⩾0.17; main effect group P⩾0.24; trial-by-group interaction P⩾0.20; Table 2). Linear mixed models for relative energy intake (absolute energy intake minus the net energy expenditure of exercise) revealed a main effect of trial (P<0.001) but not group (P=0.41) or trial-by-group interaction (P=0.60) (Table 2). The main effect of trial revealed lower relative energy intake in the exercise than in the control trial (ES=1.26, P<0.001).
Table 2. Ad libitum energy and macronutrient intakes at the buffet meal in the control and exercise trials.
|
Lean (n=22a) |
Overweight/obese (n=25) |
Control vs exercise 95% CIb | Lean vs overweight/obese 95% CIb | |||
|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | |||
| Absolute energy intake (kJ) | 3183 (1389) | 3062 (1730) | 3465 (1572) | 3542 (1791) | −391 to 346 | −507 to 1268 |
| Relative energy intake (kJ) | 3223 (1410) | 1231 (1628) | 3465 (1572) | 1684 (1585) | −2290 to −1483c | −487 to 1182 |
| Fat intake (g) | 35.1 (16.6) | 32.9 (19.6) | 40.4 (23.7) | 39.2 (25.0) | −7.1 to 3.6 | −5.8 to 17.4 |
| Fat intake (%) | 42 (10) | 40 (9) | 42 (12) | 42 (10) | −3 to 1 | −4 to 7 |
| Carbohydrate intake (g) | 88.2 (47.9) | 83.9 (54.9) | 85.9 (39.7) | 94.6 (47.6) | −8.0 to 12.3 | −21.9 to 30.3 |
| Carbohydrate intake (%) | 45 (10) | 47 (11) | 43 (12) | 45 (13) | −1 to 5 | −9 to 4 |
| Protein intake (g) | 22.9 (10.7) | 25.1 (22.4) | 30.2 (14.9) | 28.8 (19.3) | −3.6 to 4.4 | −3.9 to 14.9 |
| Protein intake (%) | 13 (7) | 13 (9) | 15 (6) | 13 (7) | −3 to 1 | −2 to 4 |
Abbreviation: CI, confidence interval. All values are mean (s.d.).
Relative energy intake available for n=21 due to missing data for net energy expenditure.
95% confidence interval of the mean absolute difference between the trials or groups.
Significant difference between control and exercise trials (P<0.001).
Plasma hormone and metabolite concentrations
Owing to problems with venous cannulation, data for concentrations of plasma ghrelin, insulin and glucose are presented for n=43 (lean: n=20; overweight/obese: n=23), total PYY for n=42 (lean: n=20; overweight/obese: n=22) and total GLP-1 for n=40 (lean: n=18; overweight/obese: n=22).
Acylated ghrelin
Fasting acylated ghrelin concentrations at baseline were not different between trials (95% CI −7 to 13 pg ml−1, P=0.61) or between lean and overweight/obese individuals (95% CI −36 to 69 pg ml−1, P=0.53) but were higher in women than in men (95% CI 5 to 110 pg ml−1, ES=0.72, P=0.03). Linear mixed models for delta acylated ghrelin identified a main effect of time (P<0.001) and sex (P=0.02) but no trial, group or interaction effects (P⩾0.37) (Figure 2). The main effect of sex revealed lower delta acylated ghrelin concentrations in women than in men (95% CI −57 to −6 pg ml−1, ES=0.64, P=0.02).
Figure 2.
Plasma concentrations of delta acylated ghrelin in the lean (left panel) and overweight/obese (right panel) individuals during the control ( male;
male;  female) and exercise (□ male; ○ female) trials. Values are mean (s.e.m.), n=10 lean men, n=12 overweight/obese men, n=10 lean women and n=11 overweight/obese women. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
female) and exercise (□ male; ○ female) trials. Values are mean (s.e.m.), n=10 lean men, n=12 overweight/obese men, n=10 lean women and n=11 overweight/obese women. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
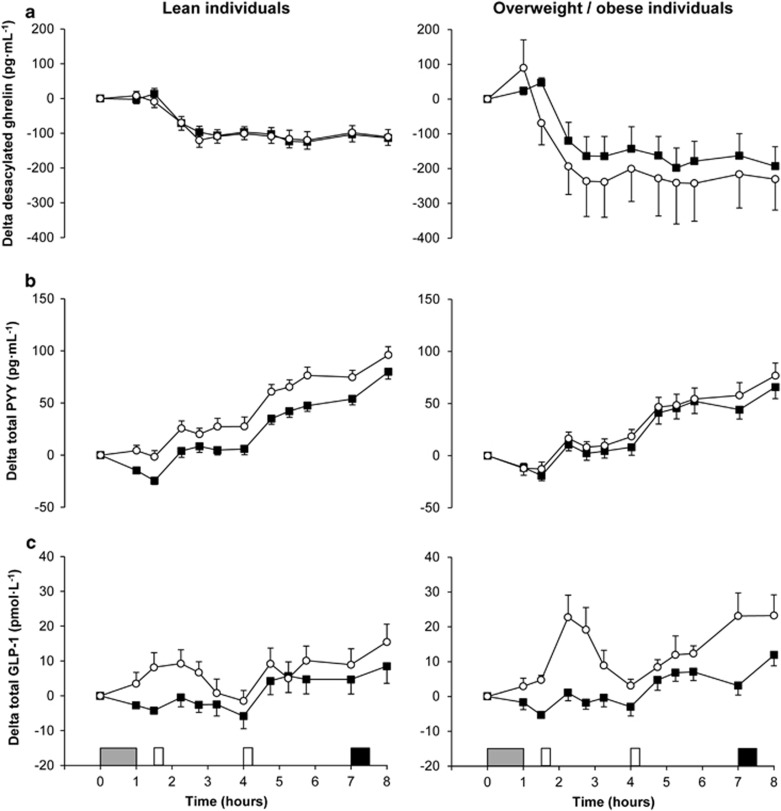
Desacylated ghrelin
Fasting desacylated ghrelin concentrations were similar across trials and groups at baseline (main effect trial P=0.37; main effect group P=0.27; trial-by-group interaction P=0.55). Linear mixed models for delta desacylated ghrelin identified a main effect of trial (P=0.01) and time (P<0.001) but not group (95% CI −201 to 66 pg ml−1, P=0.31) (Figure 3a). The main effect of trial revealed lower delta desacylated ghrelin concentrations in the exercise than in the control trial (95% CI −48 to −6 pg ml−1, ES=0.14, P=0.01). The magnitude of reduction in delta desacylated ghrelin concentrations after exercise was greater in overweight/obese than in lean individuals (−54.1 pg ml−1 (ES=0.21) vs −0.3 pg ml−1 (ES<0.01), respectively; trial-by-group interaction P=0.01).
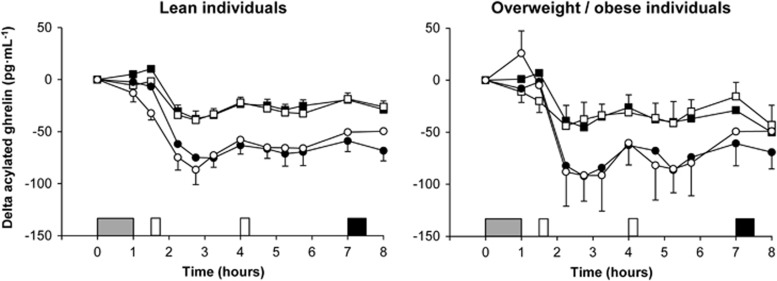
Figure 3.
Plasma concentrations of delta desacylated ghrelin (a, top panels), delta total PYY (b, middle panels) and delta total GLP-1 (c, bottom panels) in the lean (left panels) and overweight/obese (right panels) individuals during the control  and exercise (○) trials. Values are mean (s.e.m.), n=43 for desacylated ghrelin, n=42 for total PYY and n=40 for total GLP-1. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
and exercise (○) trials. Values are mean (s.e.m.), n=43 for desacylated ghrelin, n=42 for total PYY and n=40 for total GLP-1. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
Total PYY
Fasting total PYY concentrations were similar across trials and groups at baseline (main effect trial P=0.12; main effect group P=0.56; trial-by-group interaction P=0.39). Linear mixed models for delta total PYY identified a main effect of trial (P<0.001) and time (P<0.001) but not group (95% CI −22 to 9 pg ml−1, P=0.40) (Figure 3b). The main effect of trial revealed higher delta total PYY concentrations in the exercise than in the control trial (95% CI 10 to 17 pg ml−1, ES=0.32, P<0.001). The magnitude of increase in delta total PYY concentrations after exercise was lower in overweight/obese than in lean individuals (6.4 pg ml−1 (ES=0.14) vs 21.3 pg ml−1 (ES=0.56), respectively; trial-by-group interaction P<0.001).
Total GLP-1
Fasting total GLP-1 concentrations were similar across trials and groups at baseline (main effect trial P=0.33; main effect group P=0.80; trial-by-group interaction P=0.62). Linear mixed models for delta total GLP-1 identified a main effect of trial (P<0.001) and time (P<0.001) but not group (95% CI –3 to 11 pmol l−1, P=0.27) (Figure 3c). The main effect of trial revealed higher delta total GLP-1 concentrations in the exercise than in the control trial (95% CI 7 to 10 pmol l−1, ES=0.63, P<0.001). Analysis also revealed a significant trial-by-time interaction (P=0.001), with higher delta total GLP-1 concentrations in exercise than in control at 1.5, 2.25, 2.75, 3.25, 7 and 8 h (all ES⩾0.55, P⩽0.008). The magnitude of increase in delta total GLP-1 concentrations after exercise was greater in overweight/obese than in lean individuals (10.6 pmol l−1 (ES=0.88) vs 6.1 pmol l−1 (ES=0.41), respectively; trial-by-group interaction P=0.01).
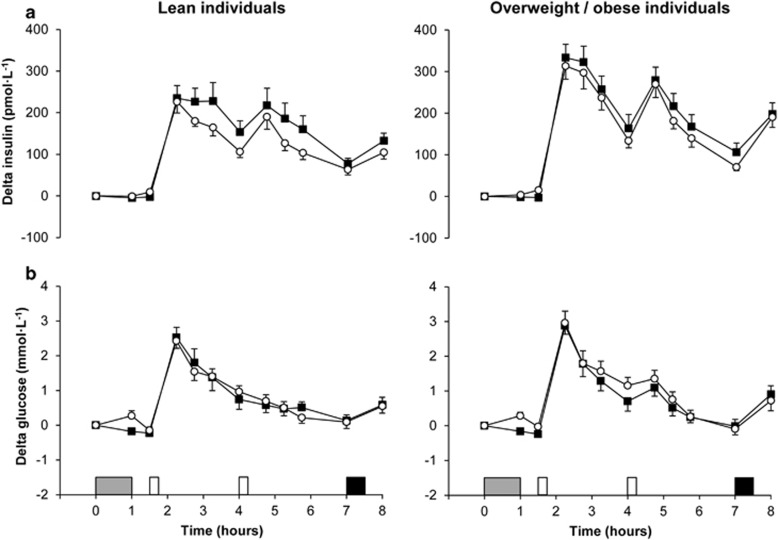
Insulin
Fasting insulin concentrations were similar across trials and groups at baseline (main effect trial P=0.92; main effect group P=0.23; trial-by-group interaction P=0.70). Linear mixed models for delta insulin identified a main effect of trial (P<0.001), time (P<0.001), group (P=0.03) and group-by-time interaction (P=0.002) (Figure 4a). The main effects of trial and group revealed that delta insulin concentrations were higher in the control than in the exercise trial (95% CI 12 to 37 pmol l−1, ES=0.15, P<0.001) and in overweight/obese than in lean individuals (95% CI 6 to 85 pmol l−1, ES=0.28, P=0.03). Post hoc analysis of the group-by-time interaction revealed higher delta insulin concentrations in overweight/obese than in lean individuals at 2.25, 2.75, 4.75 and 8 h (all ES⩾0.43, P⩽0.05).
Figure 4.
Plasma concentrations of delta insulin (a, top panels) and delta glucose (b, bottom panels) in the lean (left panels) and overweight/obese (right panels) individuals during the control  and exercise (○) trials. Values are mean (s.e.m.), n=43 for insulin and glucose. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
and exercise (○) trials. Values are mean (s.e.m.), n=43 for insulin and glucose. Grey rectangle indicates exercise, open rectangles indicate standardised meals and black rectangle indicates ad libitum buffet meal.
Glucose
Fasting glucose concentrations at baseline were similar between trials (95% CI −0.17 to 0.05 mmol l−1, P=0.27) but were higher in overweight/obese than in lean individuals (95% CI 0.03 to 0.60 mmol l−1, ES=0.56, P=0.03). Delta glucose concentrations were similar across trials and groups (main effect trial P=0.11; main effect group P=0.46; trial-by-group interaction P=0.15) (Figure 4b).
Correlations
A positive correlation was identified between exercise-induced changes in AUC values for delta acylated and desacylated ghrelin concentrations (r=0.55, P<0.001) and delta insulin and glucose concentrations (r=0.48, P=0.001). There were no significant correlations between exercise-induced changes in AUC values for delta appetite-regulatory hormone concentrations and overall appetite or ad libitum energy intake (P⩾0.14).
Discussion
The primary findings from this study were that a single bout of moderate-intensity exercise transiently suppressed appetite and increased concentrations of total PYY and GLP-1 but did not stimulate compensatory changes in appetite or energy intake on the same day in lean and overweight/obese individuals. There was remarkably little difference in appetite, ad libitum energy intake and appetite-regulatory hormone concentrations between lean and overweight/obese individuals. Subtle differences in appetite hormone responses to exercise were, however, detected between the groups, with overweight/obese individuals experiencing a greater exercise-induced suppression in desacylated ghrelin and elevation in total GLP-1, whereas lean individuals experienced a greater increase in total PYY after exercise. These findings refute the hypothesis that overweight/obese individuals exhibit greater compensatory changes in subjective and homeostatic mediators of appetite in response to acute exercise-induced energy deficits.
To the authors’ knowledge, only two previous studies have directly compared appetite-regulatory hormone responses to acute exercise between lean and overweight/obese individuals.11, 30 In line with the findings of Ueda et al.,11 appetite-regulatory hormone profiles were largely similar between the groups in this study with the exception of the anticipated greater meal-induced perturbations in insulin concentrations in overweight/obese individuals. This contrasts the findings of Marzullo et al.30 who observed lower fasting concentrations of total and acylated ghrelin in obese compared with lean individuals. Furthermore, previous non-exercise studies have demonstrated that obese individuals exhibit a diminished meal-induced increase in PYY and GLP-1 concentrations4, 5 and reduced postprandial suppression of acylated ghrelin6 compared with healthy, lean individuals. This inconsistency in findings is most likely explained by differences in the range of BMI values adopted to distinguish adiposity status coupled with key variations in study design. Specifically, appetite-regulatory hormone concentrations are influenced by factors such as diet standardisation, meal energy content and/or macronutrient composition, sample processing and the form of circulating gut peptide quantified.31
Despite the comparable appetite-regulatory hormone concentrations between groups, subtle differences in the magnitude of change in the hormone profiles after exercise emerged and represent a novel finding from this study. Specifically, the exercise-induced suppression of circulating desacylated ghrelin and elevation in total GLP-1 were greater in overweight/obese individuals, whereas lean individuals experienced a greater increase in total PYY after exercise. This contrasts previous findings suggesting that acute exercise elicits a similar increase in total PYY and GLP-17–36 in lean and overweight/obese men.11 However, it is possible that the short follow-up after exercise in the study by Ueda et al.11 (1 vs 7 h in this study) precluded any between-group differences in the response of these appetite-regulatory hormones to exercise becoming apparent.
A potential explanation for the divergent appetite hormone response to exercise between groups in this study may reflect differences in body composition and insulin resistance. Along with regulating glucose metabolism, insulin acts as a chronic mediator of energy homeostasis and has also been implicated in short-term appetite regulation.32, 33 The lower insulin concentrations after exercise in the absence of a change in circulating glucose points to an acute improvement in insulin sensitivity in both lean and overweight/obese individuals, which represents a well-established effect of acute exercise.34 Insulin resistance increases with adiposity and has been associated previously with disrupted postprandial satiety signalling,35 lower acylated ghrelin concentrations independent of weight status36 and impaired meal-induced incretin hormone secretion (for example, GLP-1).37 GLP-1 exerts anorexigenic effects via central appetite-regulating pathways and augments the insulin response to nutrient intake to regulate postprandial glucose excursions.33, 38, 39 Therefore, the greater exercise-induced elevation in total GLP-1 in overweight/obese individuals may point to enhanced postprandial satiety signalling and nutrient sensing in these individuals. However, PYY is co-secreted with GLP-1 from the intestinal L-cells,40 yet circulating total PYY concentrations were increased to a greater extent after exercise in lean individuals. Furthermore, no meaningful associations emerged between exercise-induced changes in appetite, energy intake or appetite-regulatory hormones that may explain these differences. Consequently, the implications of these findings remain unclear and further work is required to ascertain whether appetite hormone responses to exercise differ according to adiposity status.
Consistent with previous research, there was a marked suppression of appetite during and immediately after exercise that coincided with elevated total PYY and GLP-1 concentrations in the hours after exercise.1, 2 These outcomes occurred with a concomitant exercise-induced suppression in desacylated, but not in acylated, ghrelin concentrations. This finding is surprising considering the consensus of evidence suggests that acylated ghrelin concentrations are transiently suppressed during and immediately after single bouts of exercise.2, 41 It is possible that the exercise intensity in this study (59% peak V̇O2) was insufficient to elicit the transient suppression in acylated ghrelin typically reported elsewhere in the literature when the exercise intensity exceeds 65% peak V̇O2.24, 28, 42, 43 In this regard, low-intensity cycling and walking (~50% peak V̇O2) does not influence acylated ghrelin concentrations,11, 44 and exercise intensity has been identified as an important determinant of the acylated ghrelin response to exercise.45, 46 Nevertheless, the exercise protocol adopted in this study failed to provoke any compensatory changes in appetite or appetite-regulatory hormones in the hours after exercise, supporting previous studies in lean23, 24, 28, 42, 43 and overweight/obese8, 9, 10, 11 individuals.
Despite exercise inducing transient changes in subjective and hormonal appetite parameters, ad libitum absolute energy and macronutrient intakes were similar between trials and groups 6 h after exercise cessation. This corroborates previous findings demonstrating no change in energy intake at meals provided on the day of exercise in lean23, 24, 28, 43 and overweight/obese7, 10, 47 individuals. Furthermore, direct comparisons of lean and overweight/obese individuals have reported that energy intake in the immediate postexercise period (⩽1 h postexercise) is unchanged12 or reduced11 in both groups. Collectively, these findings suggest that exercise can induce a short-term energy deficit in lean and overweight/obese individuals. This refutes the hypothesis that overweight/obese individuals are more likely to exhibit physiological compensatory responses to acute exercise-induced energy deficits and may have important implications regarding the use of exercise in weight control and energy homeostasis.
Exploratory analysis of potential sex-based differences revealed higher fasting acylated ghrelin concentrations in women than in men, a finding that has been replicated previously and shown to persist across the 7 h experimental trial.26 In contrast, acylated ghrelin concentrations across the 8 h trial were lower in women than in men in this study. This disparity most likely reflects the differing statistical approaches adopted with concentrations analysed relative to baseline levels (delta) in this study but as absolute concentrations in the study by Alajmi et al.26 Furthermore, a closer examination of our data identified higher acylated ghrelin concentrations in women than in men when analysed as absolute concentrations (data not shown). It has been suggested previously that women may exhibit greater exercise-induced compensatory responses in the appetite control system than men to preserve body fat stores, energy balance and reproductive function.48 However, in contrast to this hypothesis, men and women exhibited a similar acylated ghrelin response to exercise in this study that is consistent with previous evidence demonstrating equivalent physiological responses in the homeostatic regulation of appetite to acute and chronic exercise-induced energy deficits between the sexes.26, 49
This investigation is limited by the provision of the ad libitum buffet meal 3 h after the standardised lunch before appetite values had returned to baseline levels. Although similar to previous study designs,24, 28 this pattern of meal provision may influence energy intake through non-homeostatic drivers of appetite. Additionally, participants were not familiarised with the ad libitum buffet meal before the experimental trials. The provision of a wide selection of foods may promote overconsumption and reduce the ability to identify exercise-induced changes in energy/macronutrient intakes. A further limitation concerns the use of BMI to define weight status, which does not account for differences in fat and fat-free mass,50 and combining the data for overweight and obese individuals may reduce the ability to detect between-group differences. However, further statistical analyses stratifying weight status according to waist circumference or body fat percentage and comparing distinct groups using the bottom and top tertiles of BMI, waist circumference and body fat percentage revealed comparable findings to those presented for the lean and overweight/obese individuals defined using BMI cut points. Nevertheless, despite these limitations, our findings provide important insights into appetite responses to acute exercise in lean and overweight/obese individuals.
In conclusion, a single session of moderate-intensity exercise transiently suppressed appetite and increased total PYY and GLP-1 concentrations after exercise in lean and overweight/obese individuals. Furthermore, exercise did not provoke any compensatory perturbations in energy intake or subjective and hormonal appetite regulation in either group, thereby maintaining the exercise-induced energy deficit on the day of exercise. These findings support the promotion of exercise as a method for inducing a short-term negative energy balance in lean and overweight/obese individuals.
Acknowledgments
We thank Professor Rachel Batterham for assistance with study design. We also thank the volunteers for participating in this study. The research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
The authors declare no conflict of interest.
Supplementary Material
References
- Deighton K, Stensel DJ. Creating an acute energy deficit without stimulating compensatory increases in appetite: is there an optimal exercise protocol? Proc Nutr Soc 2014; 73: 352–358. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Sabapathy S, Leveritt M, Desbrow B. Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med 2014; 44: 387–403. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Desbrow B, Sabapathy S, Leveritt M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013; 63: 92–104. [DOI] [PubMed] [Google Scholar]
- Verdich C, Toubro S, Buemann B, Madsen JL, Holst JJ, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety – effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001; 25: 1206–1214. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006; 147: 3–8. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab 2005; 90: 1068–1071. [DOI] [PubMed] [Google Scholar]
- Unick JL, Otto AD, Goodpaster BH, Helsel DL, Pellegrini CA, Jakicic JM. Acute effect of walking on energy intake in overweight/obese women. Appetite 2010; 55: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrup ME, Fairchild TJ, Keslacy S, Weinstock RS, Kanaley JA. Satiety, but not total PYY, is increased with continuous and intermittent exercise. Obesity 2013; 21: 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AY, Wallman KE, Fairchild TJ, Guelfi KJ. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond) 2014; 38: 417–422. [DOI] [PubMed] [Google Scholar]
- Martins C, Stensvold D, Finlayson G, Holst J, Wisloff U, Kulseng B et al. Effect of moderate- and high-intensity acute exercise on appetite in obese individuals. Med Sci Sports Exerc 2015; 47: 40–48. [DOI] [PubMed] [Google Scholar]
- Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol 2009; 201: 151–159. [DOI] [PubMed] [Google Scholar]
- George VA, Morganstein A. Effect of moderate intensity exercise on acute energy intake in normal and overweight females. Appetite 2003; 40: 43–46. [DOI] [PubMed] [Google Scholar]
- World Medical Association (WMA). Declaration of Helsinki: ethical principles for medical research involving human subjects, 2013. Available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [DOI] [PubMed]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- Deighton K, Zahra JC, Stensel DJ. Appetite, energy intake and resting metabolic responses to 60 min treadmill running performed in a fasted versus a postprandial state. Appetite 2012; 58: 946–954. [DOI] [PubMed] [Google Scholar]
- Borg GA. Perceived exertion: a note on ‘history’ and methods. Med Sci Sports 1973; 5: 90–93. [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983; 55: 628–634. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000; 24: 38–48. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000; 84: 405–415. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974; 37: 247–248. [DOI] [PubMed] [Google Scholar]
- Deighton K, Karra E, Batterham RL, Stensel DJ. Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl Physiol Nutr Metab 2013; 38: 947–952. [DOI] [PubMed] [Google Scholar]
- Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite 2014; 81: 52–59. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- Alajmi N, Deighton K, King JA, Reischak-Oliveira A, Wasse LK, Jones J et al. Appetite and energy intake responses to acute energy deficits in females versus males. Med Sci Sports Exerc 2016; 48: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioural Sciences, 2nd edn. Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- King JA, Wasse LK, Ewens J, Crystallis K, Emmanuel J, Batterham RL et al. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J Clin Endocrinol Metab 2011; 96: 1114–1121. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Marzullo P, Salvadori A, Brunani A, Verti B, Walker GE, Fanari P et al. Acylated ghrelin decreases during acute exercise in the lean and obese state. Clin Endocrinol 2008; 69: 970–971. [DOI] [PubMed] [Google Scholar]
- Chandarana K, Drew ME, Emmanuel J, Karra E, Gelegen C, Chan P et al. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology 2009; 136: 2115–2126. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1219–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 2007; 117: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol 2008; 192: 127–135. [DOI] [PubMed] [Google Scholar]
- Flint A, Gregersen NT, Gluud LL, Møller BK, Raben A, Tetens I et al. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr 2007; 98: 17–25. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 2004; 89: 1630–1635. [DOI] [PubMed] [Google Scholar]
- Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001; 24: 1640–1645. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007; 117: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409–1439. [DOI] [PubMed] [Google Scholar]
- Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013; 56: 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Wasse LK, Stensel DJ, Nimmo MA. Exercise and ghrelin. A narrative overview of research. Appetite 2013; 68: 83–91. [DOI] [PubMed] [Google Scholar]
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol 2007; 102: 2165–2171. [DOI] [PubMed] [Google Scholar]
- King JA, Miyashita M, Wasse LK, Stensel DJ. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite 2010; 54: 492–498. [DOI] [PubMed] [Google Scholar]
- King JA, Wasse LK, Broom DR, Stensel DJ. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc 2010; 42: 485–492. [DOI] [PubMed] [Google Scholar]
- Hazell TJ, Islam H, Townsend LK, Schmale MS, Copeland JL. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: potential mechanisms. Appetite 2016; 98: 80–88. [DOI] [PubMed] [Google Scholar]
- Broom DR, Miyashita M, Wasse LK, Pulsford R, King JA, Thackray AE et al. Acute effect of exercise intensity and duration on acylated ghrelin and hunger in men. J Endocrinol 2017; 232: 411–422. [DOI] [PubMed] [Google Scholar]
- Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med 2014; 48: 1472–1476. [DOI] [PubMed] [Google Scholar]
- Hagobian TA, Braun B. Physical activity and hormonal regulation of appetite: sex differences and weight control. Exerc Sport Sci Rev 2010; 38: 25–30. [DOI] [PubMed] [Google Scholar]
- Caudwell P, Gibbons C, Hopkins M, King N, Finlayson G, Blundell J. No sex difference in body fat in response to supervised and measured exercise. Med Sci Sports Exerc 2013; 45: 351–358. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 2008; 32: S56–S59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.