Abstract
Preeclampsia is a pregnancy-specific disorder of new-onset hypertension and proteinuria after 20 weeks' gestation, often resulting in poor outcome. Previous studies demonstrated that apelin is an endogenous active peptide with visodilation and anti-oxidative stress capabilities. The present study investigated the effects of apelin in a rat model of preeclampsia induced by reduced uterine perfusion pressure (RUPP). Rats with RUPP displayed hypertension and poor pregnancy outcomes, such as decreased fetal and placental weight. Of note, apelin treatment significantly ameliorated the symptoms of preeclampsia, improved the impaired endothelial nitric oxide synthase/nitric oxide signaling and attenuated activation of oxidative stress in RUPP rats. Apelin may be a potential agent for preventing and treating preeclampsia.
Keywords: apelin, preeclampsia, endothelial nitric oxide synthase, nitric oxide, oxidative stress
Introduction
Preeclampsia is a pregnancy-specific multisystem disorder featuring new-onset hypertension [systolic blood pressure (SBP), ≥140 mmHg and/or diastolic blood pressure (DBP), ≥90 mmHg] and impaired organ(s), including impaired liver function, thrombocytopenia, pulmonary edema, cerebral/visual symptoms after 20 weeks of gestation. Proteinuria (≥300 mg/24 h or dipstick reading >+1) is generally the most associated additional symptom (1–4). It occurs in 5–7% of all pregnancies worldwide.
Preeclampsia has adverse outcomes for maternal as well as neonatal health: It causes an estimate of 50,000–100,000 deaths annually worldwide, along with severe fetal and neonatal morbidity and mortality such as enhanced risk of fetal growth restriction and still birth. The cost of preeclampsia is great and includes not only the direct expense of monitoring pregnancies and the morbidity and mortality directly caused by preeclampsia but also the cost of associated complications such as prematurity and growth restriction (5).
Despite the serious health, social and economic costs of preeclampsia, no therapies are available to prevent, stabilize or cure the disease. The only treatment is to terminate pregnancy by delivery of the baby and placenta, which itself is associated with a risk of premature birth (6). Therefore, an effective treatment for preeclampsia is required.
Possible therapeutic agents for preeclampsia include those targeting pro-angiogenic factors, vasodilators and factors preventing inflammation and oxidative stress (6). Apelin, encoded by the APLN gene, is an endogenous ligand of G protein-coupled receptor APJ, a putative receptor associated with the angiotensin receptor AT1 (7). The apelin/APJ system has multiple effects on cardiovascular physiology and pathophysiology (8). Apelin reduced blood pressure in a mouse model of atherosclerosis and acutely in patients with heart failure (9–11). Furthermore, apelin promotes cardiac contractility (12) and vessel formation (13) and prevents aortic inflammation by decreasing the mRNA level of interleukin 6 and tumor necrosis factor α (14). Prepro-apelin mRNA is ubiqutous in human tissues, with increased levels in the placenta (15), which suggests a potential placental production of apelin during pregnancy. Apelin levels were reduced in serum and placental chorionic villi of patients with preeclampsia (16–18). The expression of APJ was also reduced in placentas of patients with preeclampsia in association with poor fetal growth (19). However, several other studies reported increased levels of apelin in the serum and placenta of patients with preeclampsia (20,21). Despite these controversial results, a potential association between apelin and preeclampsia is indicated.
Given the pleiotropic effect of apelin on angiogenesis, vasodilation, inhibition of inflammation and oxidative stress, it was hypothesized that apelin ameliorated the pathogenesis of preeclampsia. A rat model of preeclampsia induced by reduced uterine perfusion pressure (RUPP) was used to verify the effect of apelin on the development of preeclampsia.
Materials and methods
Animals
A total of 28 female Sprague-Dawley (SD) rats (age, 3 months; weight, 160–200 g) were obtained from the Animal Center of Hebei Medical University (Shijiazhuang, China) and were housed under standard conditions (temperature, 20±8°C; humidity, 60±10%; lights on from 6:00 to 18:00) with standard rodent chow and water provided ad libitum. All animal procedures complied with the Animal Management Regulations of the Ministry of Health, P.R. China (document no. 55, 2001) and the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH; publication no. 85–23, revised 1996), and were approved by the Animal Care Committee of Hebei Medical University (Shijiazhuang, China).
Experimental procedure
Gestational day (GD) 0 of pregnancy was identified by the existence of sperm in a vaginal smear after an overnight breeding with a male rat. Pregnant rats were then randomly divided into four groups for treatment: Control (N group; n=6), apelin (APLN group; n=7), preeclampsia (PE group; n=7) and preeclampsia plus apelin (PE+APLN group; n=8). The APLN and PE+ALPN rats were administered apelin-13 (Bachem, Bubendorf, Switzerland) subcutaneously (6×10−8 mol/kg, twice a day) from GD 11, and the other rats were injected with an equal volume of saline. On GD 14, preeclampsia was induced by RUPP (see procedure below). On GD 19, blood pressure was measured in all rats and the 24-h urine was collected using metabolism cages and urine protein was measured, and blood and placenta were collected. Blood was assembled for separation of serum, and fetal and placental weights were evaluated in each rat. Placentas of rats were split, weighed, part of them were immediately emerged into 4% paraformaldehyde for 48 h at 20°C and embedded in paraffin. serial paraffin sections (5-µm thick) were prepared and stained with hematoxylin and eosin (HE) for 3 min and 2 min, respectively at 20°C. On each HE section, four high-power fields (magnification, ×200) were selected to detect placental tissue morphology used light microscope.
CRUPP model in rats
The RUPP model in SD rats is an established preeclampsia model (22–25). In the PE and PE+ALPN group, rats were anesthetized by inhalation of isoflurane (4% for induction and 1–3% for maintenance; Pharmaceutical Partners of Canada; Fresenius Kabi, Toronto, ON, Canada), the abdominal cavity was opened by a midline incision and the abdominal aorta was uncovered above the iliac bifurcation. A silver clip [inner diameter (ID), 0.203 mm] was placed around the aorta. To block compensatory flow through ovarian arteries, two more silver clips (ID, 0.100 mm) were placed around the left and right ovarian arteries between the ovary and uterine horn. For rats in the N and APLN groups, arteries were similarly manipulated and silver clips were placed on intra-abdominal fat.
Arterial blood pressure measurement
On GD 19, rats were anesthetized again with isoflurane (inhalation administration), and a carotid arterial catheter (Scientific Commodities Inc., Lake Havasu City, AZ, USA) filled with heparin saline (50 U/ml) was then inserted for blood pressure measurement. Arterial pressure was monitored with a pressure transducer (Chengdu Instrument Factory, Chengdu, China) and continuously recorded for 30 min after a 15-min stabilization period on a recorder (Chengdu Instrument Factory). Systolic blood pressure (SBP), diastolic blood pressure, mean arterial pressure (MAP) and heart rate (HR) were measured.
Semi-quantitative polymerase chain reaction (qPCR) analysis
The mRNA expression of endothelial nitric oxide synthase (eNOS) was detected by qPCR. Placentas of rats were split, weighed, immediately snap-frozen in liquid nitrogen and stored at −80°C. After the tissue was crushed in liquid nitrogen by using a mortar and pestle, total RNA was extracted by using the RNeasy Protect Mini kit (cat. no. 74106; Qiagen, Hilden, Germany). The isolation procedure followed the manufacturer's instructions. Complementary DNA (cDNA) was synthesized from 1 µg mRNA by using the Bio-Rad iScript cDNA reverse transcription kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR amplification was then performed using premix Taq Version (cat. no. R004A; Takara Biotechnology Co., Ltd., Dalian, China), cDNA and primers (Sangong Biotech, Shanghai, China) with the following sequences: eNOS sense, 5′-CCCAGGAGAGATCCACCTCA-3′ and anti-sense, 5′-CGGAAGGGTGCAATACCAGT-3′; GAPDH sense, 5′-GCAACTCCCATTCTTCCACC-3′ and anti-sense, 5′-TGGTATTCGAGAGAAGGGAGGG-3′. The condition of PCR was as follows: 30 cycles at 95°C for 10 sec; 55°C for 30 sec; and 72°C for 1 min. Subsequently, 5 µl PCR products was subjected to 1.5% agarose gel electrophoresis (150 V for 30 min) and the results were analyzed using a UV Gel imaging analysis system.
Western blot analysis
Placenta samples (100 mg) were frozen with liquid nitrogen, homogenized in 1 ml RIPA buffer (high) (cat. no. R0010, Beijing Solarbio Science Technology Co., Ltd., Beijing, China) and then centrifuged at 12,000 × g for 10 min at 4°C, and the protein concentration was determined using NanoDrop-1000 spectrophotometer (Gene Co., Ltd., Beijing, China). Protein extracts were re-suspended in a 5X sample buffer and boiled in water for 15 min at 100°C. Total protein (100 µg) was subjected to 10% SDS-PAGE and transferred to nitrocellulose membranes (cat. no. 1620150; Bio-Rad Laboratories, Inc., Hercules, CA, USA), followed by incubation with the primary antibodies anti-eNOS (cat. no. sc-654; 1:1,000 dilution) and anti-GAPDH (cat. no. sc-25778; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Subsequently, membranes were incubated with the IRDye 800CW goat anti-rabbit immunoglobulin G (H+L) (Rockland Immunochemicals Inc., Pottstown, PA, USA) for 1 h at room temperature. Blots were visualized by enhanced chemiluminescence (Beijing Applygen Technologies, Beijing, China), and bands were analyzed by using ImageJ software (vision 1.37; NIH, Bethesda, MD, USA). The protein contents were normalized to that of GADPH.
Measurement of NO and eNOS in serum
Contents of NO and activity of eNOS in serum were measured by using commercial kits (cat. no. 20150205; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Measurement of total anti-oxidant capacity (T-AOC) and malondialdehyde (MDA) levels in serum
To detect the level of oxidative stress, T-AOC and MDA levels were measured by using commercial kits (cat. no. 20150525; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions.
Statistical analysis
Values are expressed as the mean ± standard deviation and analyzed with SAS 9.1 software (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance was used to compare more than two groups, and Tukey's honest significant difference test was used to assess between-group differences. P<0.05 was considered to indicate a statistically significant difference.
Results
Apelin ameliorates hypertension and proteinuria in RUPP rats
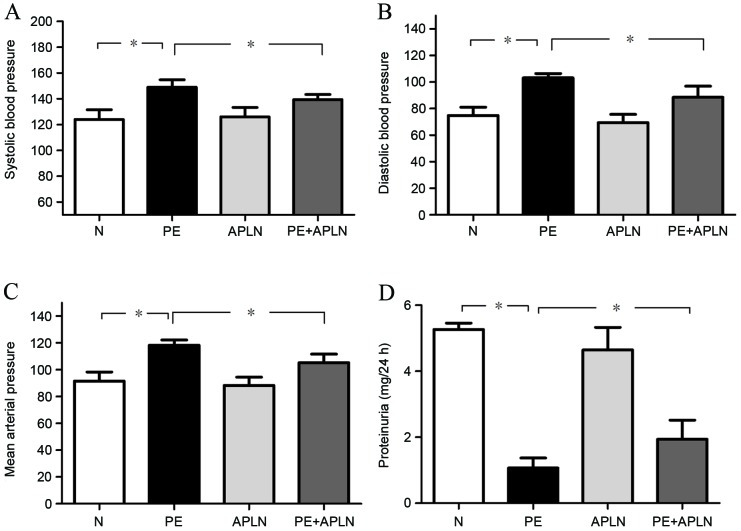
Compared with N rats, PE rats had significantly increased SBP, DBP and MAP (P<0.05; Fig. 1A-C). Apelin administration in PE rats (PE+APLN) significantly inhibited the elevation of SBP, DBP and MBP (P<0.05), while no difference was observed between N and APLN rats (P>0.05).
Figure 1.
Effect of apelin on (A-C) blood pressure and (D) proteinuria in rats subjected to reduced uterine perfusion pressure. Values are expressed as the mean ± standard deviation. *P<0.05. Groups: N, control (n=6); APLN, apelin (n=7); PE, preeclampsia (n=7); PE+APLN, preeclampsia plus apelin (n=8).
Compared with N rats, PE rats had significantly decreased proteinuria (P<0.05; Fig. 1D). Apelin administration reversed the PE-associated decreases in proteinuria (P<0.05). Proteinuria did not differ between N and APLN rats (P>0.05).
Apelin improves pregnancy outcomes in PE rats
Compared with N rats, PE rats exhibited a significantly decreased total fetal and total placental weight as well as embryo survival rate (P<0.05; Fig. 2). Apelin administration reversed the decreased total fetal weight and total placental weight, while further increasing the embryo survival rate (P<0.05). N and APLN rats did not exhibit any differences regarding these features (P>0.05).
Figure 2.
Effect of apelin on pregnancy outcomes in rats. Values are expressed as the mean ± standard deviation. (A) The total fetal weight in different groups; (B) the total placental weight in different groups; (C) the embryo survival rate in different groups. *P<0.05. N, control; APLN, apelin; PE, preeclampsia.
Effect of apelin on placental tissue morphology
Villi exhibited greater differences than other parts of the rat placenta among the groups on HE staining (Fig. 3). Compared with the placentas of N rats, those of PE rats had villous edema, irregularly arranged cells of various sizes and hyperchromatic nuclei, whereas PE+APLN rats had resolved edema of the placental villi and cells were regularly arranged with less hyperchromatic areas.
Figure 3.
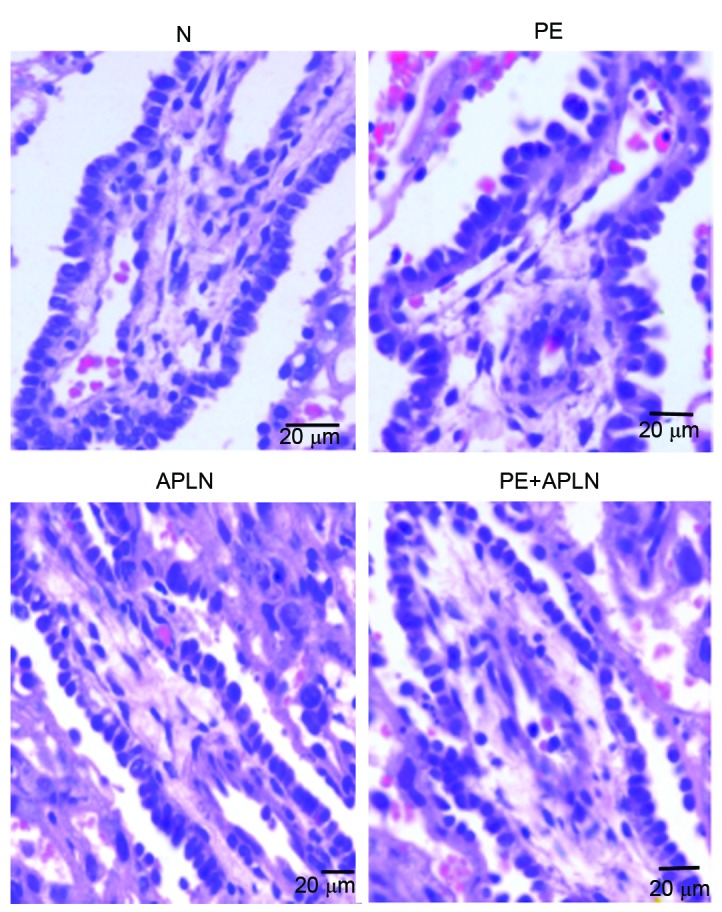
Histology of placental tissue from rats (hematoxylin and eosin staining; scale bar, 20 µm). Compared with the placentas of N rats, those of PE rats had villous edema, irregularly arranged cells of various sizes and hyperchromatic nuclei, whereas PE+APLN rats had resolved edema of the placental villi and cells were regularly arranged with less hyperchromatic areas. N, control; APLN, apelin; PE, preeclampsia.
Apelin restores eNOS/NO signaling in RUPP rats
Compared with N rats, PE rats had significantly downregulated protein and mRNA levels of eNOS in the placenta and decreased activity of eNOS in the serum (P<0.05). Apelin administration to PE rats led to an upregulation of the protein and mRNA levels of eNOS (P<0.05). N and APLN rats did not exhibit any differences in eNOS levels (P>0.05; Fig. 4).
Figure 4.
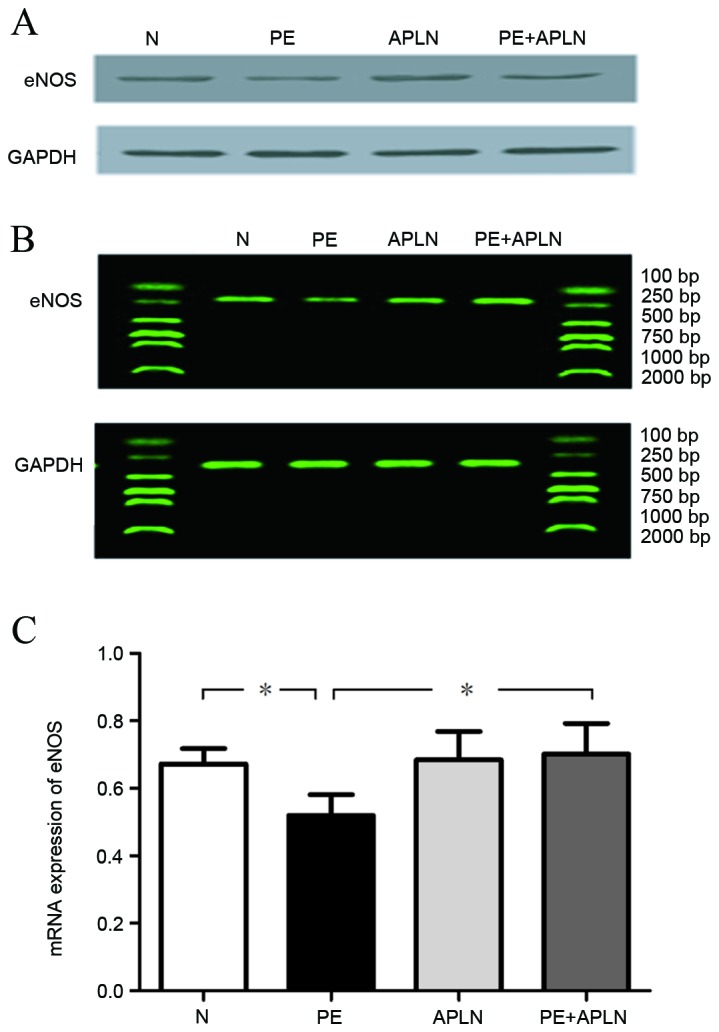
Effect of apelin on (A) protein and (B and C) mRNA expression of eNOS in the placenta of rats. Values are expressed as the mean ± standard deviation. *P<0.05. eNOS, endothelial nitric oxide synthase; N, control; APLN, apelin; PE, preeclampsia.
Accordingly, the serum content of NO in PE rats was lower than that in N rats and the activity of serum eNOS was decreased (P<0.05; Fig. 5). Apelin treatment reversed the PE-induced reduction of the serum NO content and eNOS activity (P<0.05). The serum content of NO and the activity of eNOS did not differ between N and APLN rats (P>0.05).
Figure 5.
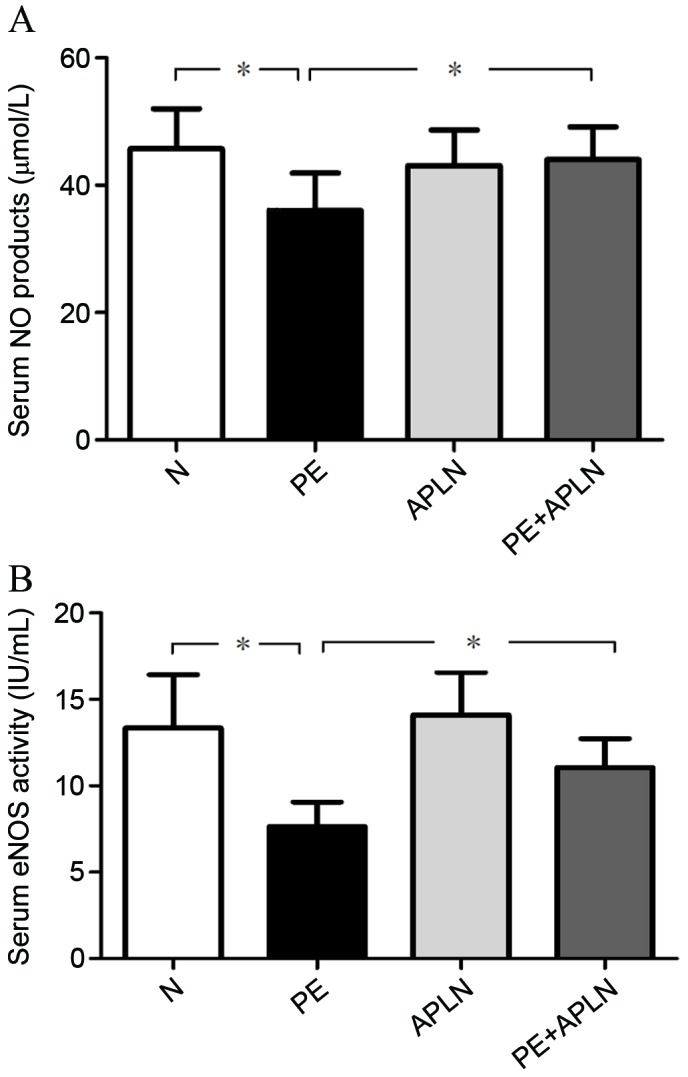
(A) Serum content of NO and (B) serum activity of eNOS in rats. Values are expressed as the mean ± standard deviation. *P<0.05. eNOS, endothelial nitric oxide synthase; N, control; APLN, apelin; PE, preeclampsia.
Apelin ameliorates oxidative stress in RUPP rats
The serum T-AOC was 8.39±1.04, 7.20±0.88, 8.63±1.27 and 8.78±1.00 U/ml and serum MDA levels were 0.41±0.05, 0.55±0.09, 0.28±0.03 and 0.44±0.08 µmol/l in N, PE, APLN and PE+APLN rats, respectively. Compared with N rats, PE rats exhibited significantly increased serum MDA levels and decreased serum T-AOC levels (P<0.05; Fig. 6). Apelin administration reversed these effects (P<0.05). The N and APLN rats did not differ in these features (P>0.05).
Figure 6.
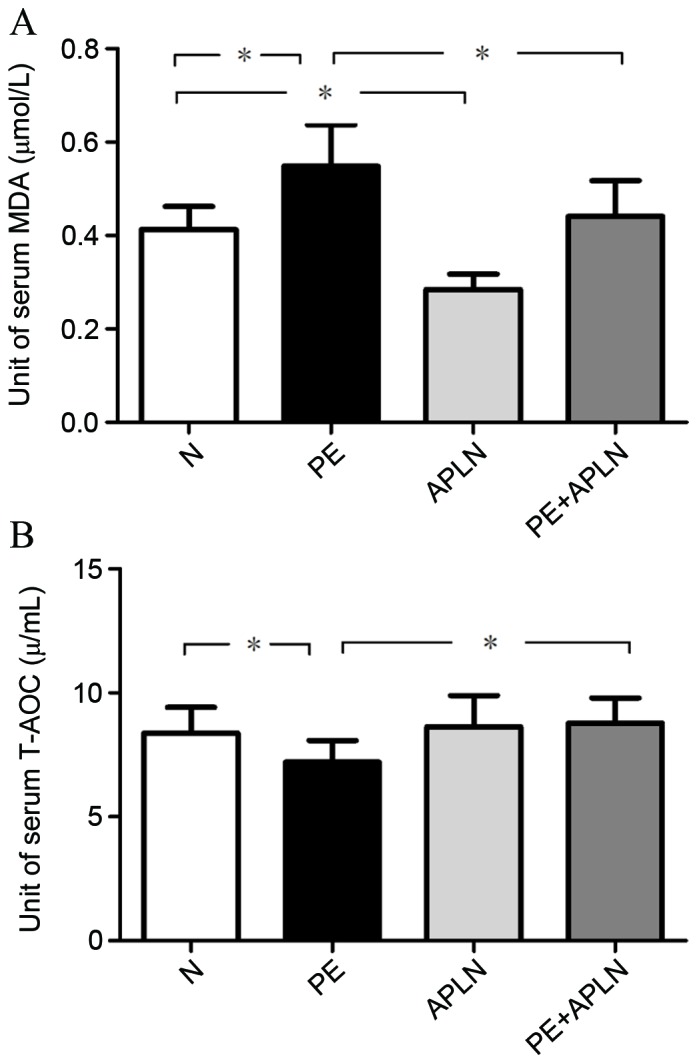
Effect of apelin on (A) the content of MDA and (B) T-AOC in the serum in rats. Values are expressed as the mean± standard deviation. *P<0.05. N, control; APLN, apelin; PE, preeclampsia; T-AOC, total anti-oxidant capacity; MDA, malondialdehyde.
Discussion
In the present study, a rat preeclampsia model was successfully established by RUPP, as demonstrated by hypertension and poor outcomes of pregnancy. Apelin treatment significantly ameliorated the symptoms of preeclampsia in RUPP rats. Furthermore, it improved the impaired eNOS/NO signaling pathway and prevented activation of oxidative stress in RUPP rats.
The RUPP animal model of placental ischemia is well established, and in pregnant animals, this procedure invokes numerous symptoms of preeclampsia representative of those in humans, such as hypertension and intrauterine growth restriction (22–25). The present study observed that blood pressure parameters in PE rats, including SBP, DBP and MAP, were greater than those in N rats and total fetal weight as well as the embryo survival rate were lower. These results demonstrated the successful establishment of the preeclampsia model in the rats of the present study. Of note, apelin treatment reversed the PE-associated elevation in blood pressure, increased the total fetal weight and further increasing the embryo survival rate. These results demonstrated the beneficial effects of apelin on preeclampsia.
Endothelial dysfunction is considered to have a key role in the progression of preeclampsia. Placental hypoperfusion from a lack of spiral artery remodeling in initial placental development releases circulating factors into the maternal vasculature. Analysis of plasma from women with preeclampsia indicated impaired endothelium-dependent vasodilation in healthy resistance vessels (26,27). One critical mechanism regulating vascular function during pregnancy is the NO vasodilation pathway. NO, a powerful vasodilator, is produced from L-arginine by NOS and has three isoforms: eNOS (also referred to as NOS-3), inducible NOS (also referred to as NOS-2) and neuronal NOS (also referred to as NOS-1). In the endothelium, NO production is primarily associated with eNOS (28,29). NO is also a potent vasodilator in the uterine artery vasculature and contributes to the elevated blood flow and fetal growth during pregnancy (30). Animal models with production of NO inhibited by genetic modification or pharmacological blockade with L-NAME demonstrated structural and functional impairments in terms of uterine artery adaptation and decreased uterine artery blood flow (31). Given that the etiology of preeclampsia is considered to be abnormal placentation and that endothelial dysfunction is a key feature of the disease, enhancement of NO bioavailability is an attractive strategy for preventing and treating preeclampsia (6). One randomized controlled trial of NO supplementation with L-arginine combined with anti-oxidant vitamins revealed a 40% decrease in the risk of preeclampsia in women with a personal or close family history of preeclampsia (32). Apelin has a direct activating effect on the L-arginine/eNOS/NO pathway (33,34). The present study found that apelin treatment significantly improved the expression of eNOS in the placenta and the levels of NO and eNOS in the serum, which were all decreased in PE rats. These results suggested that restoration of the eNOS/NO pathway may be involved in the ameliorative effects of apelin on preeclampsia.
Oxidative stress has a key role in the progression of preeclampsia. It may trigger apoptosis of syncytium and subsequent secretion of pro-inflammatory cytokines and anti-angiogenic factors, which may ultimately induce endothelial dysfunction and cause symptoms of preeclampsia (4). Accumulating evidence has demonstrated that preeclampsia is associated with high levels of oxidative stress markers, including lipid peroxidation products and decreased anti-oxidant capacity (35). Given that excessive oxidative stress is associated with the pathology of preeclampsia, reducing oxidative stress may be one method to prevent and treat preeclampsia (36). Accordingly, the salutary effect of apelin on suppressing oxidative stress has been demonstrated in numerous cell and tissue types (37,38). In line with this, the present study also demonstrated that apelin attenuated the increased oxidative stress in RUPP rats. These results suggested that inhibition of oxidative stress may be involved in the ameliorative effects of apelin on preeclampsia.
Of note, the present study had several limitations. It investigated the therapeutic effect of apelin on preeclampsia in rats with PE only, and further study of the effect in other animal models of preeclampsia will be performed in the future. Due to the transient half-life of apelin, long-acting analogs of apelin are required. In addition, with the multiple effects of apelin, other mechanisms by which it ameliorates progression of preeclampsia should be thoroughly investigated.
In conclusion, the results of the present study suggested that apelin ameliorates the pathologies of preeclampsia. Restoring the eNOS/NO pathway and inhibiting oxidative stress may be involved in the ameliorative effect of apelin on preeclampsia. Apelin is a potential drug for preventing and treating preeclampsia.
Acknowledgements
The present study was funded by the Key Research Program of Hebei Provincial Health and Family Planning Commission (grant no. 20150233).
References
- 1.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: The boyd collection revisited. Am J Obstet Gyneco. 1999;181:718–724. doi: 10.1016/S0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 2.Walker JJ. Pre-eclampsia. Lancet. 2000;356:1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 3.Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 5.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in africa, asia, latin america and the caribbean. Br J Obstet Gynaecol. 1992;99:547–553. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 6.Oyston CJ, Stanley JL, Baker PN. Potential targets for the treatment of preeclampsia. Expert Opin Ther Targets. 2015;19:1517–1530. doi: 10.1517/14728222.2015.1088004. [DOI] [PubMed] [Google Scholar]
- 7.O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13–R35. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 8.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O'Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 10.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, et al. Acute cardiovascular cffects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 12.Szokodi I, Tavi P, Földes G, Voutilainen-Myllylä S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysä J, Toth M, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–440. doi: 10.1161/01.RES.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 13.Kidoya H, Takakura N. Biology of the apelin-APJ axis in vascular formation. J Biochem. 2012;152:125–131. doi: 10.1093/jb/mvs071. [DOI] [PubMed] [Google Scholar]
- 14.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 16.Bortoff KD, Qiu C, Runyon S, Williams MA, Maitra R. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy. 2012;31:398–404. doi: 10.3109/10641955.2012.690054. [DOI] [PubMed] [Google Scholar]
- 17.Inuzuka H, Nishizawa H, Inagaki A, Suzuki M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H, Udagawa Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy. 2013;32:410–421. doi: 10.3109/10641955.2013.813535. [DOI] [PubMed] [Google Scholar]
- 18.Yamaleyeva LM, Chappell MC, Brosnihan KB, Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor RN, et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2015;309:E852–E860. doi: 10.1152/ajpendo.00272.2015. [DOI] [PubMed] [Google Scholar]
- 19.Furuya M, Okuda M, Usui H, Takenouchi T, Kami D, Nozawa A, Shozu M, Umezawa A, Takahashi T, Aoki I. Expression of angiotensin II receptor-like 1 in the placentas of pregnancy-induced hypertension. Int J Gynecol Pathol. 2012;31:227–235. doi: 10.1097/PGP.0b013e31823b6e71. [DOI] [PubMed] [Google Scholar]
- 20.Cobellis L, De Falco M, Mastrogiacomo A, Giraldi D, Dattilo D, Scaffa C, Colacurci N, De Luca A. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22:1–8. doi: 10.14670/HH-22.1. [DOI] [PubMed] [Google Scholar]
- 21.Kucur M, Tuten A, Oncul M, Acikgoz AS, Yuksel MA, Imamoglu M, Balci Ekmekci O, Yilmaz N, Madazli R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy. 2014;33:467–475. doi: 10.3109/10641955.2014.944709. [DOI] [PubMed] [Google Scholar]
- 22.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 23.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 25.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA, Granger JP. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension. 2016;67:740–747. doi: 10.1161/HYPERTENSIONAHA.115.06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashworth JR, Warren AY, Johnson IR, Baker PN. Plasma from pre-eclamptic women and functional change in myometrial resistance arteries. Br J Obstet Gynaecol. 1998;105:459–461. doi: 10.1111/j.1471-0528.1998.tb10134.x. [DOI] [PubMed] [Google Scholar]
- 27.Hayman R, Warren A, Johnson I, Baker P. Inducible change in the behavior of resistance arteries from circulating factor in preeclampsia: An effect specific to myometrial vessels from pregnant women. Am J Obstet. Gynecol. 2001;184:420–426. doi: 10.1067/mob.2001.109733. [DOI] [PubMed] [Google Scholar]
- 28.Lekontseva O, Jiang Y, Schleppe C, Davidge ST. Altered neuronal nitric oxide synthase in the aging vascular system: Implications for estrogens therapy. Endocrinology. 2012;153:3940–3948. doi: 10.1210/en.2012-1071. [DOI] [PubMed] [Google Scholar]
- 29.Amaral LM, Pinheiro LC, Guimaraes DA, Palei AC, Sertório JT, Portella RL, Tanus-Santos JE. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med. 2013;17:1300–1307. doi: 10.1111/jcmm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckman DM, Gupta R, Rosenfeld CR, Morgan TM, Charles SM, Mertz H, Moore LG. Pregnancy increases myometrial artery myogenic tone via NOS- or COX-independent mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;303:R368–R375. doi: 10.1152/ajpregu.00490.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61:259–266. doi: 10.1161/HYPERTENSIONAHA.112.201996. [DOI] [PubMed] [Google Scholar]
- 32.Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S, Macones G, Strauss JF. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: Randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, Yu F, Duan XH, Tang CS, Qi YF. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides. 2007;28:2023–2029. doi: 10.1016/j.peptides.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Busch R, Strohbach A, Pennewitz M, Lorenz F, Bahls M, Busch MC, Felix SB. Regulation of the endothelial apelin/APJ system by hemodynamic fluid flow. Cell Signal. 2015;27:1286–1296. doi: 10.1016/j.cellsig.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui IA, Jaleel A, Tamimi W, Al Kadri HM. Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet. 2010;282:469–474. doi: 10.1007/s00404-010-1538-6. [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Tian FJ, Lin Y. Oxidative stress in placenta: Health and diseases. Biomed Res Int. 2015;2015:293271. doi: 10.1155/2015/293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Than A, Zhang X, Leow MK, Poh CL, Chong SK, Chen P. Apelin attenuates oxidative stress in human adipocytes. J Biol Chem. 2014;289:3763–3774. doi: 10.1074/jbc.M113.526210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pisarenko O, Shulzhenko V, Studneva I, Pelogeykina Y, Timoshin A, Anesia R, Valet P, Parini A, Kunduzova O. Structural apelin analogues: Mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury. Br J Pharmacol. 2015;172:2933–2945. doi: 10.1111/bph.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]