Abstract
Background:
In Lynch syndrome, inherited mismatch repair (MMR) defects predispose to colorectal cancer and to a wide spectrum of extra-colorectal tumours. Utilising a cohort study design, we aimed to determine the risk of extra-colorectal cancer and to identify yet unrecognised tumour types.
Methods:
Data from 1624 Lynch syndrome mutation carriers in the Danish hereditary non-polyposis colorectal cancer register were used to estimate the sex- and age-specific incidence rate ratios (IRRs) for 30 extra-colorectal malignancies with comparison to the general population.
Results:
Significantly increased IRRs were identified for 13 cancer types with differences related to gender, age and disease-predisposing gene. The different cancer types showed variable peak age incidence rates (IRs) with the highest IRs for ovarian cancer at age 30–49 years, for endometrial cancer, breast cancer, renal cell cancer and brain tumours at age 50–69 years, and for urothelial cancer, small bowel cancer, gastric cancer, pancreatic cancer and skin tumours after age 70.
Conclusions:
The broad spectrum of tumour types that develop at an increased incidence defines Lynch syndrome as a multi-tumour syndrome. The variable incidences in relation to age, gender and gene suggest a need for individualised surveillance.
Keywords: hereditary non-polyposis colorectal cancer, mismatch repair genes, pancreatic cancer, prostate cancer, breast cancer, colorectal cancer
Lynch syndrome is caused by mutations in the mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2, and represents one of the most common hereditary cancer syndromes (de la Chapelle, 2005). The MMR deficiency genotype is associated with a 58–75% life-time risk of cancer with frequent observations of synchronous/metachronous tumour development (Giardiello et al, 2014; Moller et al, 2015). Colorectal cancer and endometrial cancer predominate with estimated cumulative risks of 10–74% and 15–71%, respectively (Lu and Daniels, 2013; Vasen et al, 2013; Giardiello et al, 2014; Moller et al, 2015). Cancer of the ovaries, stomach, hepatobiliary tract, small bowel, brain and skin have been shown to develop at an increased incidence in Lynch syndrome, whereas the role of, for example, breast cancer, prostate cancer, pancreatic cancer and sarcoma remains uncertain with inconclusive risk estimates (Barrow et al, 2009; Kastrinos et al, 2009; Jensen et al, 2010; Urso et al, 2012; Lu and Daniels, 2013; Harkness et al, 2015; Dominguez-Valentin et al, 2016).
Genotype–phenotype correlations have been described in Lynch syndrome with a higher risk for urothelial cancer, skin tumours and brain tumours in MSH2 mutation carriers, an increased risk for gynaecological cancer in MSH6 mutation carriers, a higher risk for breast cancer in MLH1 mutation carriers and a lower risk for colorectal cancer in individuals with mutations in MSH6 and in PMS2 (Plaschke et al, 2004; Barrow et al, 2009; Harkness et al, 2015; Joost et al, 2015; Moller et al, 2015; Therkildsen et al, 2015). Increasing evidence suggests that cancer risk and tumour spectrum are influenced also by sex, genetic modifiers, obesity and life-style factors such as physical activity, smoking and diet (Watson et al, 2008a; Barrow et al, 2009; van Duijnhoven et al, 2013; Movahedi et al, 2015).
With the aim to determine the risk of extra-colorectal cancer and to identify yet unrecognised tumour types in Lynch syndrome, we determined the incidence rate ratios (IRRs) for 30 cancer types in a cohort study design, including 1624 eligible mutation carriers from the national Lynch syndrome cohort with comparison to a population-based cohort from Denmark.
Materials and methods
The Danish hereditary non-polyposis colorectal cancer (HNPCC) register contains more than 6000 families with suspected or verified hereditary colorectal cancer reported to the register by genetic counsellors, surgeons, pathologists and genetic diagnostic laboratories’ report. Families have been included based on family history of colorectal or endometrial cancer, or the demonstration of MMR defects based on routine reflex MMR testing of colorectal cancer. The Danish HNPCC register back-tracks and identifies all family members regardless of cancer history and subclassifies families according to genotypic and phenotypic features (Lindberg et al, 2016). Irrespective of family history, individuals/families with disease-predisposing MMR gene (MLH1, MSH2, MSH6, PMS2 or EPCAM) mutations (classes 4–5 according to IARC system) are defined as Lynch syndrome (Thompson et al, 2014). All family members, also non-carriers, are contained in the register, which is allowed to contact at-risk individuals by mail to ensure information and offer genetic diagnostics. The study was granted acceptance from the Danish Data Protection Agency. According Danish regulations, anonymised registry studies are not subject to ethical review.
Patient selection and data processing
All proven or obligate MMR gene mutation carriers from the HNPCC register were eligible for the study and are referred to as the Lynch syndrome cohort. Family data and genetic test results were collected from the HNPCC register. Data on primary extra-colorectal cancer diagnoses were obtained from the population-based Danish Cancer Registry, which is based on mandatory reporting from pathologists and clinicians throughout the country. All diagnoses were verified by pathological reports or medical reports. Benign tumours, carcinoma in situ/dysplasia, basal cell carcinomas of the skin and metastases from other primary tumours were excluded. Person years at risk were determined as the period from date of birth to date of diagnosis of any kind of cancer or date of death, whichever came first. Patients with a primary and a secondary cancer in different organs were, unless clinically regarded as metastases, classified as metachronous cancers and were allowed to contribute to the respective incidence estimates. Vital status was extracted from the Danish Civil Registration System.
The population-based cohort included data from the Nordcan database on age-specific cancer events and person years at risk in the Danish background population with data completeness in the time period 1 January 1978 to 31 December 2013 (Engel et al, 2016). These data were stratified according to year, sex, age and disease. Double registrations were not allowed and cancer events and person years at risk in the Lynch syndrome cohort were subtracted from the population-based data in the Nordcan database. Lynch syndrome-associated cancers were matched to categories from the Nordcan database. Of the 36 cancer groups specified in the Nordcan database, Lynch syndrome cancer diagnoses were stratified into 30 distinct extra-colorectal cancer subgroups. Motivated by the focus on extra-colorectal cancer, data on colon cancer and rectal cancer (including anal cancer that could not be distinguished from rectal cancer in the Nordcan database) were excluded. Furthermore, unspecified cancer types and rare diagnoses that were pooled in the Nordcan database were excluded. For sarcoma, the analyses were restricted to 7 cases as the Nordcan database classified 17 sarcomas from the Lynch syndrome cohort according to organ rather than histotype. Patient selection for the Lynch syndrome cohort is described in Supplementary Figure 1.
The study was designed to minimise ascertainment bias through use of data from the national Danish Cancer register. In addition, reflex testing for MMR defective colorectal cancer independent of family history was on a national basis introduced more than 10 years ago. Further, we performed a subgroup analysis of cancers diagnosed after the initiation of surveillance in the family, which thus excluded diagnoses and corresponding person years at risk before this date (Supplementary Figure 1).
Statistical analyses
Person years at risk and cancer events in the Lynch syndrome cohort and in the population-based Nordcan cohort were stratified by age, sex and year of diagnosis and aggregated into four age groups (0–29, 30–49, 50–69 and 70 years or above) using the %STRATIFY SAS macro, which removes individuals from the at-risk group if cancer is diagnosed within the study period, and SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) (Rostgaard, 2008). For the entire study cohort, time at risk was determined as the time from study entry (1 January 1978) or date of birth, whichever occurred latest to study exit (31 December 2013) or date of death, whichever occurred first. For the subgroup in surveillance, study entry dates were defined as the date of first colonoscopic surveillance in the family. Relatedness was handled as conditioned independent given the MMR mutation, age, year of diagnosis and sex.
Stratified and aggregated data were transferred into R i386 3.1.0 (R: A Language and Environment for Statistical Computing, 2011). Incidence rates (IRs) were calculated as the number of events divided by person years at risk in each age group. Incidence rate ratios were calculated as the ratio between the IRs in Lynch syndrome and in the background population. Exact conditional 95% confidence intervals (CIs) and P-values were calculated using the exact Poisson test. Separate analyses stratified by age group and cancer type (and MMR gene mutation and sex for cancer types with more than 15 events) were performed to assess IRs in relation to genotype and phenotype. Significance levels were corrected for multiple testing using Bonferroni correction with the null hypothesis that Lynch syndrome mutation carriers do not have an increased risk of each specific cancer subtype (with significance set at P<0.0125). All P-values were two-sided.
Pooled IRs for extra-colorectal cancer were based on summarised IRs for all cancer types within each age group and asymptotic 95% CIs were calculated following the central limit theorem of Poisson distributions.
Results
Extra-colorectal cancers
The total Lynch syndrome cohort comprised 1624 mutation carriers from 365 Lynch syndrome families that contributed with 51 237 person years at risk. The cohort included families with pathogenic variants in MLH1 (n=94), MSH2 (n=155, including one family with an EPCAM mutation), MSH6 (n=98) and PMS2 (n=18; Table 1). During the study period, 762 mutation carriers developed 1095 malignancies that included 535 colorectal cancers and 560 extra-colorectal cancers. The extra-colorectal malignancies represented 30 distinct cancer types with the most common being endometrial cancer (n=163), urothelial cancer (n=75), breast cancer (n=47), non-melanoma skin tumours (n=39) and ovarian cancer (n=30; Supplementary Table 1).
Table 1. MMR gene distribution in the entire Lynch syndrome cohort and the subgroup in surveillance.
|
Entire Lynch syndrome cohort |
Lynch syndrome cohort in surveillance |
|||
|---|---|---|---|---|
| Individuals | Extra-colonic cancers | Individuals | Extra-colonic cancers | |
| n (%) | n (%) | n (%) | n (%) | |
| Cohort size | 1624 | 560 | 1336 | 222 |
| Unaffected | 862 (53.1) | — | 1028 (76.9) | — |
| MMR gene mutation | ||||
| MLH1 | 443 (27.3) | 128 (22.9) | 370 (27.7) | 60 (27.0) |
| MSH2/EPCAM | 701 (43.2) | 289 (51.6) | 616 (46.1) | 129 (58.1) |
| MSH6 | 419 (25.8) | 140 (25.0) | 317 (23.7) | 33 (14.9) |
| PMS2 | 61 (3.8) | 3 (0.5) | 33 (2.5) | 0 (0) |
| Sex, men | 754 (46.4) | 178 (31.8) | 631 (47.2) | 91 (41.0) |
Abbreviation: MMR=mismatch repair.
Overall IRRs for extra-colorectal cancer
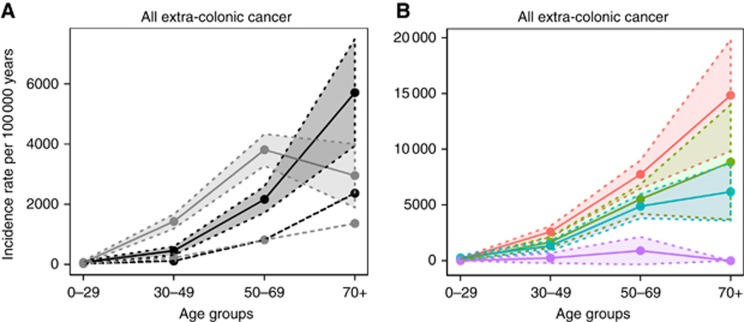
The IRs of any type of extra-colorectal cancer were separately estimated in men and women from the Lynch syndrome cohort compared to population-based cohorts. Significantly increased IRRs were identified in the Lynch syndrome cohort for any extra-colorectal malignancy for all age groups and both sexes compared to the background population (Figure 1A). Within the Lynch syndrome cohort, women had significantly higher IRRs than men with an IRR of 3.1 at 30–49 years and an IRR of 1.8 at 50–69 years (Figure 1A).
Figure 1.
Incidence rates for all extra-colorectal cancer in Lynch syndrome. Age-dependent incidence rates and 95% confidence intervals for all extra-colorectal cancers were estimated in Lynch syndrome mutation carriers in relation to (A) gender or (B) mutated MMR gene. (A) Solid lines, Lynch syndrome; dotted lines, population-based cohort; black lines, men; grey lines, women; shaded areas=95% confidence intervals. (B) Blue, MLH1; red, MSH2; green, MSH6; purple=PMS2; shaded areas=95% confidence intervals.
The incidence of extra-colorectal cancer was also studied in relation to disease-predisposing MMR gene. In PMS2 mutation carriers, no increased IR for extra-colorectal cancer was demonstrated and as only three extra-colorectal cancers (one endometrial cancer, one gastric cancer and one prostate cancer) developed, PMS2 was omitted from further analyses. The median ages at onset for any extra-colorectal cancer were 53, 53 and 55 years for MLH1, MSH2 and MSH6 mutation carriers, respectively. Higher IRRs were found for MSH2 mutations compared to MSH6 mutations with significant differences from age 30 (IRR=2.0 at age 30–49 years, 1.6 at age 50–69 years and 2.4 at age 70 and above; Figure 1B).
Incidence rates for specific cancer types
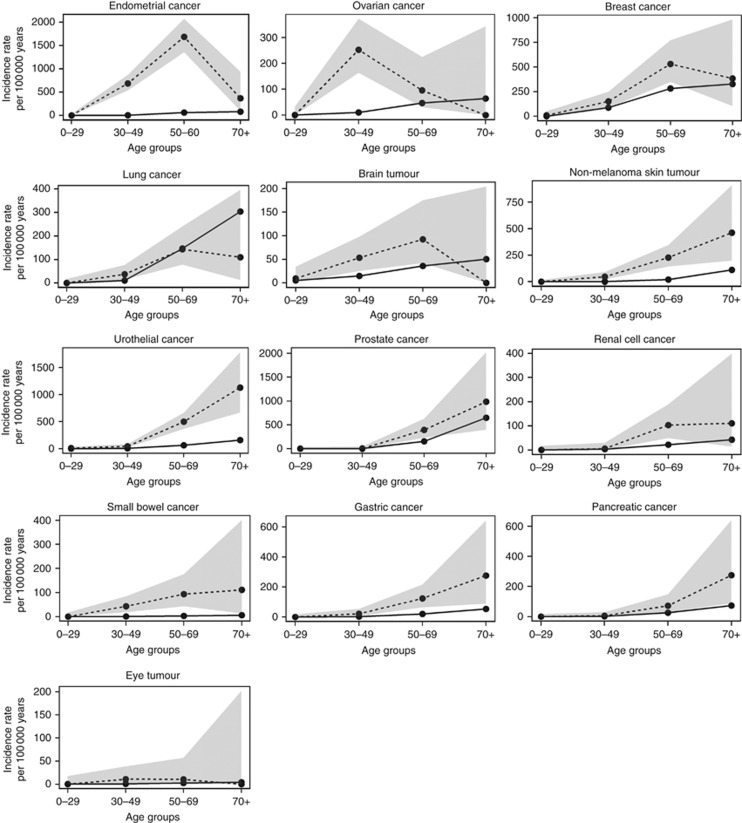
Of the 30 cancer types represented in the Lynch syndrome cohort, significantly increased IRRs in at least one age group were demonstrated for 13 diagnoses. Of these, endometrial cancer, ovarian cancer, urothelial cancer, gastric cancer, brain tumours, non-melanoma skin tumours and cancer of the small bowel have been linked to Lynch syndrome, whereas the role of breast cancer, prostate cancer, lung cancer, kidney cancer, pancreatic cancer and eye tumours in Lynch syndrome is uncertain (Figure 2; Table 2; Supplementary Table 2).
Figure 2.
Cancer types with significantly increased incidence rate ratios in the Lynch syndrome cohort. Age-dependent incidence rates and 95% confidence intervals are showed for 13 specific cancer types with at least one time point being significantly increased in the Lynch syndrome cohort compared to the population-based cohort. Solid lines, Lynch syndrome; dotted lines, the population-based cohort; shaded areas, 95% confidence intervals. Note the scale is changing on the y-axis between cancer types.
Table 2. Age-dependent incidence rate ratios for extra-colonic cancer in Lynch syndrome mutation carriers.
|
Lynch syndrome
vs
population-based cohort |
|||||
|---|---|---|---|---|---|
| Cancer | Age groups | IRR | 95% CI lower | 95% CI upper | P-values |
| Endometrial cancer (n=163) | 0–29 | 0 | 0 | 278.7 | 1 |
| 30–49 | 133.0 | 96.2 | 178.9 | <0.0001 | |
| 50–69 | 27.4 | 20.7 | 35.5 | <0.0001 | |
| 70+ | 4.6 | 0.8 | 14.1 | 0.01218 | |
| Urothelial cancer (n=75) | 0–29 | 34.2 | 2.0 | 154.7 | 0.00165 |
| 30–49 | 8.3 | 2.8 | 18.9 | <0.0001 | |
| 50–69 | 8.2 | 5.5 | 11.7 | <0.0001 | |
| 70+ | 7.1 | 3.6 | 12.5 | <0.0001 | |
| Breast cancer (n=47) | 0–29 | 6.7 | 0.0 | 48.2 | 0.13853 |
| 30–49 | 1.7 | 0.8 | 3.1 | 0.05996 | |
| 50–69 | 1.9 | 1.1 | 3.0 | 0.00224 | |
| 70+ | 1.2 | 0.2 | 3.6 | 0.59032 | |
| Non-melanoma skin tumours (n=39) | 0–29 | 0 | 0 | 89.2 | 1 |
| 30–49 | 17.8 | 6.4 | 38.9 | <0.0001 | |
| 50–69 | 11.0 | 6.0 | 18.3 | <0.0001 | |
| 70+ | 4.2 | 1.4 | 9.5 | 0.00086 | |
| Ovarian cancer (n=30) | 0–29 | 0 | 0 | 41.9 | 1 |
| 30–49 | 24.4 | 13.9 | 39.6 | <0.0001 | |
| 50–69 | 2.0 | 0.5 | 5.6 | 0.10343 | |
| 70+ | 0 | 0 | 7.4 | 1 | |
| Prostate cancer (n=24) | 0–29 | 0 | 0 | 14 505.7 | 1 |
| 30–49 | 0 | 0 | 37.2 | 1 | |
| 50–69 | 2.5 | 1.3 | 4.5 | 0.00059 | |
| 70+ | 1.5 | 0.5 | 3.7 | 0.23773 | |
| Small bowel cancer (n=19) | 0–29 | 0 | 0 | 737.4 | 1 |
| 30–49 | 107.3 | 35.7 | 245.7 | <0.0001 | |
| 50–69 | 32.3 | 11.6 | 70.4 | <0.0001 | |
| 70+ | 18.1 | 1.1 | 81.6 | 0.00567 | |
| Lung cancer (n=23) | 0–29 | 0 | 0 | 136.8 | 1 |
| 30–49 | 3.5 | 1.1 | 8.3 | 0.00471 | |
| 50–69 | 1.0 | 0.4 | 1.8 | 1 | |
| 70+ | 0.4 | 0.0 | 1.6 | 0.19510 | |
| Gastric cancer (n=21) | 0–29 | 0 | 0 | 226.0 | 1 |
| 30–49 | 8.0 | 1.4 | 24.7 | 0.00172 | |
| 50–69 | 6.1 | 2.6 | 12.1 | <0.0001 | |
| 70+ | 5.2 | 1.2 | 14.4 | 0.00304 | |
| Brain tumours (n=21) | 0–29 | 1.8 | 0.1 | 8.0 | 0.30849 |
| 30–49 | 3.6 | 1.4 | 7.6 | 0.00061 | |
| 50–69 | 2.6 | 0.9 | 5.6 | 0.00984 | |
| 70+ | 0 | 0 | 5.6 | 1 | |
| Kidney cancer (n=13) | 0–29 | 0 | 0 | 58.9 | 1 |
| 30–49 | 1.7 | 0.0 | 11.9 | 0.45410 | |
| 50–69 | 4.7 | 1.8 | 9.9 | 0.00007 | |
| 70+ | 2.6 | 0.2 | 11.8 | 0.17865 | |
| Pancreatic cancer (n=13) | 0–29 | 0 | 0 | 573.5 | 1 |
| 30–49 | 2.3 | 0.0 | 16.7 | 0.34888 | |
| 50–69 | 2.8 | 0.8 | 6.6 | 0.01541 | |
| 70+ | 3.7 | 0.9 | 10.4 | 0.01182 | |
| Eye tumours (n=3) | 0–29 | 0 | 0 | 85.5 | 1 |
| 30–49 | 14.5 | 0.8 | 65.4 | 0.00869 | |
| 50–69 | 4.0 | 0.0 | 28.9 | 0.21992 | |
| 70+ | 0 | 0 | 68.0 | 1 | |
Abbreviations: CI=confidence intervals; IRR=incidence rate ratio.
Incidence rates and 95% CI for the Lynch syndrome cohort and the population-based cohort are shown in Supplementary Table 2. Bold P values indicate significance following Bonferroni correction.
Strikingly different peak incidence ages were identified for the different tumour types (Figure 2). Ovarian cancer and eye tumours had the highest IRs in the age group 30–49 years with an IR of 252.7 (95% CI=163.5–373.0) for ovarian cancer and an IR of 10.7 (95% CI=1.3–38.6) for eye tumours (Supplementary Table 2). Though the absolute numbers were low, Lynch syndrome mutation carriers showed a 14.5-fold increased IR for eye tumours, including uveal malignant melanoma, compared to the population-based cohort. Endometrial cancer, breast cancer and brain tumours showed peak IRs in the age group 50–69 years; endometrial cancer had an IR of 1686.1 (95% CI=1355.8–2072.5), breast cancer an IR of 530.3 (95% CI=349.5–771.5) and brain tumours an IR of 92.5 (95% CI=42.3–175.7; Supplementary Table 2). Compared to the general population, the overall highest IRRs applied to endometrial cancer in the age group 30–49 years with a 133-fold increased IR (95% CI=103.3–168.6, P<0.0001; Table 2). For other tumour types, that is, non-melanoma skin tumours, urothelial cancer, small bowel cancer, gastric cancer and pancreatic cancer the IR increased with age with the highest risk above age 70 (Figure 2; Table 2). Urothelial cancer showed significantly increased IRRs in all age groups, while non-melanoma skin tumours, small bowel cancer and gastric cancer developed at increased IRRs from age 30 compared to the population-based cohort. Pancreatic cancer showed significantly increased IRR from age 70 (IRR=3.7, 95% CI=1.2–8.7, P=0.01182).
An early age at onset was observed for lung cancer, kidney cancer and prostate cancer with significantly increased IRRs in the age groups 30–49 and 50–69 years, respectively (Figure 2; Table 2). Compared to the population-based cohort, Lynch syndrome mutation carriers had a 4.7-fold increased IR (95% CI=2.3–8.7, P<0.0001) of kidney cancer at the age 50–69 years and a 3.5-fold increased IR (95% CI=1.4–7.2, P=0.00471) for early-onset lung cancer at the age 30–49 years. The 23 lung cancers developed at median 53 years and included 11 non-small-cell lung cancers, 8 small-cell lung cancers, 1 neuroendocrine tumour and 3 with unclassified subtype. The IR of prostate cancer was 2.5-fold increased (95% CI=1.4–4.1) in male MMR mutation carriers at the age of 50–69 years compared to the population-based cohort. Though 17 sarcomas developed at a median age of 48.5 years, separate sarcoma analyses could only be performed based on 1 bone sarcoma and 6 connective tissue sarcomas. The latter showed a nonsignificant IRR of 6.3 (95% CI=1.3–18.4, P=0.01261) at age 50–69 years compared to the population-based cohort.
Gene- and sex-specific IRs
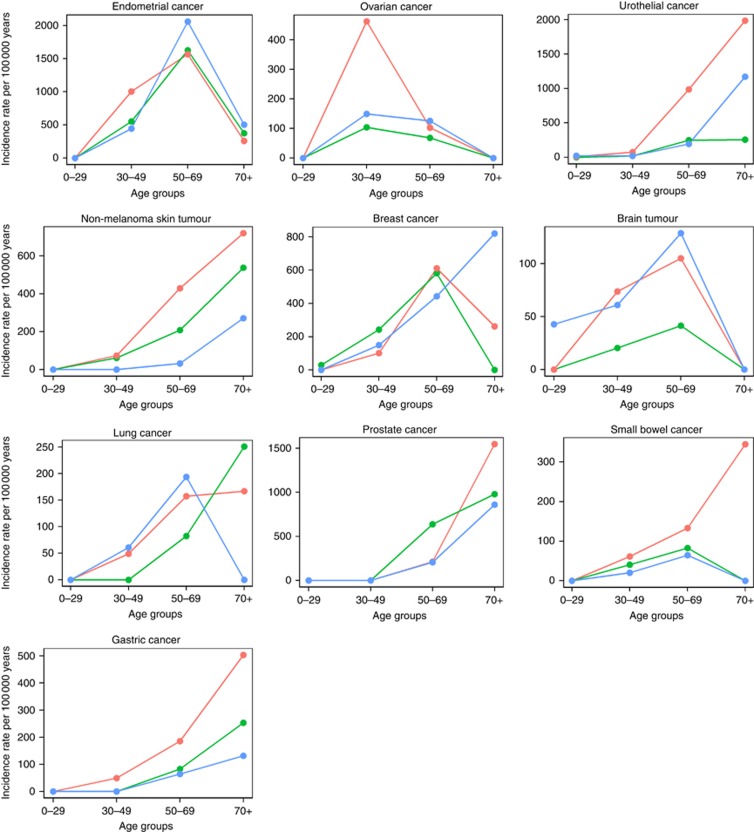
Cancer type-specific IRs were in subsets with minimum 15 events estimated according to the disease-predisposing MMR genes. Significantly increased IRRs were identified in MSH2 mutation carriers compared to MLH1 and MSH6 for four cancer types, that is, endometrial cancer, urothelial cancer, non-melanoma skin tumours and ovarian cancer, with particular increased incidence in the younger age groups (Figure 3). In the age group 30–49 years, the IRR for endometrial cancer was 2.3 (95% CI=1.2–4.7, P=0.0107) for MSH2 compared to MSH6, and the IRR for ovarian cancer was 4.5 (95% CI=1.3–23.6, P=0.0077) for MSH2 compared to MLH1. In the age group 50–69 years, the IRR for urothelial cancer was 4.0 (95% CI=1.7–11.5, P=0.0006) for MSH2 compared to MLH1 and 5.1 (95% CI=2.1–14.7, P=0.00003) compared to MSH6, and for non-melanoma skin cancer the IRR was 13.3 (95% CI=2.1–558.3, P=0.00084) for MSH2 compared to MSH6. No significant difference was found in relation to the mutated MMR gene for gastric cancer, small bowel cancer, breast cancer, lung cancer, prostate cancer and brain tumours.
Figure 3.
Incidence rates for the 10 most frequent cancer types in Lynch syndrome. Age-dependent incidence rates were calculated separately by the mutated MMR genes MLH1 (blue), MSH2 (red) and MSH6 (green) for cancer types with more than 15 events.
No difference in relation to sex was observed for urothelial cancer, gastric cancer, small bowel cancer, lung cancer or brain tumours, whereas non-melanoma skin tumours showed a higher IRR for male compared to female MMR mutation carriers above age 70 with an IRR of 11.6 (95% CI=1.5–524.5, P=0.0056).
Incidence rates for ascertained tumour types
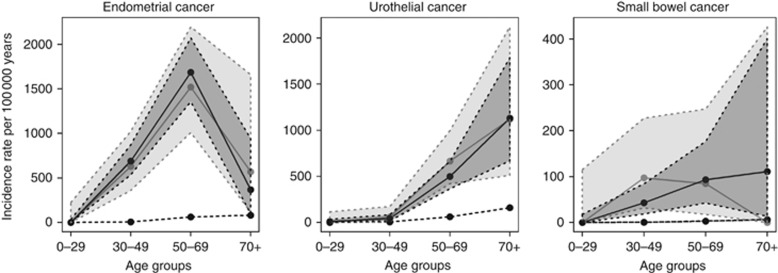
To correct for potential ascertainment bias, we performed separate subgroup analysis in 1336 mutation carriers from 309 families (including 12 773 person years at risk) with time at risk restricted to the time after initiation of surveillance in the family (Supplementary Figure 1). The analysis encompassed 222 extra-colorectal cancers, including 47 endometrial cancers, 35 urothelial cancers, 23 ovarian cancer, 13 gastric cancer, 10 brain tumours and 8 cancers of the small bowel (Table 1; Supplementary Table 1). A limited number of individuals in the oldest age group restricted power above age 70, whereas robust risk estimates were obtained in the age groups 30–49 and 50–69 years. In here, endometrial cancer showed IRRs of 121.5 (95% CI=69.4–197.5, P<0.0001) and 24.7 (95% CI=16.4–35.7, P<0.0001), urothelial cancer had IRRs of 11.3 (95% CI=2.3–33.1, P=0.00254) and 10.9 (95% CI=6.9–16.4, P<0.0001) and cancer of the small bowel had IRRs of 224.6 (95% CI=79.2–573.1, P<0.0001) and 29.4 (95% CI=6.1–85.8, P=0.00017; Figure 4; Supplementary Table 3). Significantly increased IRRs were also identified for breast cancer, non-melanoma skin tumours, ovarian cancer, gastric cancer, brain tumours and pancreatic cancer (Supplementary Table 3).
Figure 4.
Incidence rates for ascertained tumour types in the entire Lynch syndrome cohort and a subgroup of the cohort excluding cancer events before the first colonoscopic surveillance in the family. Age-dependent incidence rates and 95% confidence intervals are shown for the entire Lynch syndrome cohort (black line) and the subgroup herein (grey line) compared to the general population (dotted line) for endometrial cancer, urothelial cancer and small bowel cancer. Shaded areas, 95% confidence intervals.
Discussion
In Lynch syndrome, surveillance and preventive measures effectively reduce morbidity and mortality from colorectal cancer and prolong life expectancy, which in turn increase the time at risk for other syndrome-associated tumour types (Jarvinen et al, 2009; Lu and Daniels, 2013). Indeed, 61% of cancer-related deaths in Lynch syndrome mutation carriers have been estimated to be caused by cancer types other than colorectal and endometrial cancer (Pylvanainen et al, 2012). In the Danish Lynch syndrome cohort, cancer has been diagnosed in 47% of the mutation carriers with 51% being extra-colorectal cancers. We demonstrate significantly increased IRs for 13 cancer types and thereby further define Lynch syndrome as a multi-tumour syndrome. Increased IRs applied from age 30 (Figure 1). In the age group 30–69 years, female mutation carriers had a higher IR than male mutation carriers due to early-onset endometrial cancer, ovarian cancer and breast cancer (Figure 2). The overall IRR of extra-colorectal cancer was highest in MSH2 mutation carriers, which was largely explained by urothelial cancer, endometrial cancer, ovarian cancer and skin tumours (Figures 1 and 2).
An increased risk of gynaecologic cancer, that is, endometrial cancer and ovarian cancer, has been defined in Lynch syndrome (Lu and Daniels, 2013; Moller et al, 2015). Our findings are in accordance with previous studies in Lynch syndrome and provide additional age-specific IRRs (Engel et al, 2012; Win et al, 2012). Endometrial cancer showed the highest IRR relative to the general population at age 30–49 (IRR=133), whereas the highest IR applied to the age group 50–69 (IR=1686.1). The increased IR for endometrial cancer applied to all MMR genes though the IRR for MSH2 at age 30–49 years was significantly higher compared to MSH6 and MLH1, which is in agreement with studies showing increased risk in MSH2 mutation carriers (Moller et al, 2015). The results remained stable in the subgroup analysis with a 122-fold increased IR in the age group 30–49 years. The high risk for endometrial cancer is generally accepted to motivate surveillance though the benefit is uncertain, partly because these cancers present with gynaecologic bleedings in early stages (Lu and Daniels, 2013; Syngal et al, 2015; Moller et al, 2015). Ovarian cancer showed an IRR of 24.4 with a distinct peak at age 30–49. The early onset and the narrow age span of Lynch syndrome-associated ovarian cancer challenges recommendations for surveillance and/or prophylactic surgery (Helder-Woolderink et al, 2016). Though the absolute risk of ovarian cancer has been estimated to be 1–4% at age 40 by Moller et al, 2015, the relative risk compared to the background population still remain high especially in the young age groups.
The increased IRRs identified for urothelial cancer, cancer of the small bowel and gastric cancer are comparable with reports from Win et al (2012) and Engel et al (2012), and were not influenced by sex. The estimates remained stable in the subset analyses with significantly increased IRRs between ages 30 and 69 (Supplementary Table 3). The incidence of urothelial cancer in MSH2 compared to MLH1 and MSH6 in the age group 50–69 years strengthens the links between MSH2 and urothelial cancer and suggests that urinary surveillance is particularly relevant in MSH2 mutation carriers (Watson et al, 2008b; Aarnio et al, 2012). Gastric cancer and cancer of the small bowel have lifetime risks <10% and gastrointestinal endoscopic surveillance is therefore not broadly recommended, though Helicobacter pylori screening and potential eradication is relevant (Win et al, 2012; Vasen et al, 2013).
The role of breast cancer in Lynch syndrome is controversial (Watson et al, 2008b; da Silva et al, 2010; Harkness et al, 2015). The IRR for breast cancer of 1.9 identified in our series is comparable to the standardised IRRs (SIR) estimated in recent reports and did not show any impact in relation to MMR gene (Engel et al, 2012; Win et al, 2012; Harkness et al, 2015). As the risk increase is at most doubled and most countries offer population screening for breast cancer, current data do not call for additional preventive measures.
We identified an increased IRR of non-melanoma skin tumours from age 30 with significantly higher incidence in MSH2 mutation carriers compared to MSH6 mutation carriers. Muir–Torre syndrome refers to a variant of Lynch syndrome with development of multiple skin tumours, particularly sebaceous adenomas and carcinomas, and a link to MSH2 mutations (South et al, 2008). Though the overall life-time risk of skin tumours in Lynch syndrome families would not call for intensive surveillance programmes, these results argue for increased awareness of skin cancer especially in MSH2 mutation carriers.
Increased IRRs were identified for a number of tumour types that have in case reports and smaller studies been suggested to develop as part of Lynch syndrome, that is, kidney cancer, lung cancer, pancreatic cancer and prostate cancer (Figure 2; Table 2) (Nolan et al, 2009; Kastrinos et al, 2009; Ryan et al, 2014; Therkildsen et al, 2016). Though MMR deficiency and microsatellite instability are rarely observed in kidney cancers a link between kidney cancer and Lynch syndrome has been suggested (Aarnio et al, 1999; Therkildsen et al, 2016). Pancreatic cancer developed at an increased IRR of 3.7 from age 70, which is somewhat lower than a relative risk of 8.6 reported by Kastrinos et al, 2009. Several studies have suggested an increased risk of prostate cancer in Lynch syndrome and our identification of an IRR of 2.5 is within the range of previously published SIR of 2.1–2.5 (Engel et al, 2012; Win et al, 2012; Raymond et al, 2013; Ryan et al, 2014). Data on lung cancer in Lynch syndrome are scarce, and our study is the first to suggest a significantly increased IRR of 3.5 at an early age (30–49 years) (Pande et al, 2012; Warth et al, 2016). Eye tumours have not previously been described in Lynch syndrome mutation carriers and notably two of three eye tumours from this study were malignant melanomas. Though the IRRs of these rare tumour types may be increased at specific age groups, the absolute risk is limited (<5%) and does not motivate surveillance.
Extra-colorectal tumours in Lynch syndrome showed distinct peak incidence patterns (Figure 2). Ovarian cancer and eye tumours showed the highest risk at young age (30–49 years). Endometrial cancer, breast cancer, brain tumours and lung cancer developed with the highest incidence in the age group 50–69 years. Urothelial cancer, small bowel cancer, gastric cancer, kidney cancer, prostate cancer, pancreatic cancer and non-melanoma skin tumours showed a continuously increasing IR with age. Early age at onset characterises hereditary tumours and the earlier onset of, for example, prostate cancer and lung cancer in our series may suggest that these tumour types may develop at an earlier age in individuals with Lynch syndrome (Hampel et al, 2005; Meyer et al, 2009; Joost et al, 2015; Helder-Woolderink et al, 2016).
The size of our cohort allowed for subgroup analysis to correct for ascertainment bias for the most frequently occurring cancer types, that is, endometrial cancer, urinary tract cancer and cancer of the small bowel (Figure 4). When the analyses were restricted to cancers that developed after the initiation of colonoscopic surveillance in the family, the results remained stable and thus demonstrate robust IRR estimates without evidence from ascertainment bias (Supplementary Table 3). Though the sample size in the oldest age group was limited due to high mortality rates, the peak age incidence pattern was consistent for endometrial cancer and urothelial cancer, whereas a shift was identified for cancer of the small bowel (Figure 4). Denmark has a long history of active diagnostics of Lynch syndrome with national registers for hereditary colorectal cancer, a national genetic network and reflex testing for MMR defects in colorectal cancer diagnostics since 2007. Though, at present 53% of the Lynch syndrome cohort remain without cancer, the estimated carrier frequency of 1:279 implies that a considerable fraction of the Lynch syndrome families still remain undiagnosed (Win et al, 2016). The impact from preventive interventions is estimated to be minimal based on use of historic data and lack of demonstrated efficacy from surveillance or chemoprevention for extra-colorectal cancer.
In summary, we identified increased IRRs for 13 extra-colorectal cancer types in the Danish Lynch syndrome cohort relative to the Danish background population. The very early-age peak incidences for ovarian cancer and brain tumours and the increased IRRs for urothelial cancer after age 50 and in MSH2 mutation carriers are relevant to consider in future surveillance strategies in Lynch syndrome. The broad tumour spectrum, the overall increased risk for MSH2 mutation carriers, the distinct and variable incidence patterns in relation to age, sex and cancer type are important to recognise for clinicians, geneticists and counsellors and provide a basis for targeted surveillance programmes.
Acknowledgments
This work was supported by grants from the Danish Cancer Research Fund (R90-A6150), the Swedish Cancer Research Fund (2014/442) and the ALF Funds at the Region Skåne.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Aarnio M, Saily M, Juhola M, Gylling A, Peltomaki P, Jarvinen HJ, Mecklin JP (2012) Uroepithelial and kidney carcinoma in Lynch syndrome. Fam Cancer 11: 395–401. [DOI] [PubMed] [Google Scholar]
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomaki P, Mecklin JP, Jarvinen HJ (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81: 214–218. [DOI] [PubMed] [Google Scholar]
- Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, Hill J, Evans DG (2009) Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 75: 141–149. [DOI] [PubMed] [Google Scholar]
- da Silva FC, de Oliveira LP, Santos EM, Nakagawa WT, Aguiar JS, Valentin MD, Rossi BM, de Oliveira FF (2010) Frequency of extracolonic tumors in Brazilian families with Lynch syndrome: analysis of a hereditary colorectal cancer institutional registry. Fam Cancer 9: 563–570. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A (2005) The incidence of Lynch syndrome. Fam Cancer 4: 233–237. [DOI] [PubMed] [Google Scholar]
- Dominguez-Valentin M, Joost P, Therkildsen C, Jonsson M, Rambech E, Nilbert M (2016) Frequent mismatch-repair defects link prostate cancer to Lynch syndrome. BMC Urol 16: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel C, Loeffler M, Steinke V, Rahner N, Holinski-Feder E, Dietmaier W, Schackert HK, Goergens H, von Knebel DM, Goecke TO, Schmiegel W, Buettner R, Moeslein G, Letteboer TG, Gomez GE, Hes FJ, Hoogerbrugge N, Menko FH, van Os TA, Sijmons RH, Wagner A, Kluijt I, Propping P, Vasen HF (2012) Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol 30: 4409–4415. [DOI] [PubMed] [Google Scholar]
- Engel G, Ferlay J, Cristensen N, Kejs AMT, Johannesen TB, Khan S, Milter MC, Ólafsdóttir E, Petersen T, Pukkala E, Stenz F, Storm HH (2016) NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.0 (17.12.2014). Available at: http://www.ancr.nu Association of the Nordic Cancer Registries. Danish Cancer Society (accessed on 14 April 2016).
- Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA, Kaltenbach T, Levin TR, Lieberman DA, Robertson DJ, Syngal S, Rex DK (2014) Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 109: 1159–1179. [DOI] [PubMed] [Google Scholar]
- Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, de la Chapelle A (2005) Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 129: 415–421. [DOI] [PubMed] [Google Scholar]
- Harkness EF, Barrow E, Newton K, Green K, Clancy T, Lalloo F, Hill J, Evans DG (2015) Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet 52: 553–556. [DOI] [PubMed] [Google Scholar]
- Helder-Woolderink JM, Blok EA, Vasen HF, Hollema H, Mourits MJ, De Bock GH (2016) Ovarian cancer in Lynch syndrome; a systematic review. Eur J Cancer 55: 65–73. [DOI] [PubMed] [Google Scholar]
- Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, Peltomaki P, Aaltonen LA, Mecklin JP (2009) Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 27: 4793–4797. [DOI] [PubMed] [Google Scholar]
- Jensen UB, Sunde L, Timshel S, Halvarsson B, Nissen A, Bernstein I, Nilbert M (2010) Mismatch repair defective breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 120: 777–782. [DOI] [PubMed] [Google Scholar]
- Joost P, Therkildsen C, Dominguez-Valentin M, Jonsson M, Nilbert M (2015) Urinary tract cancer in Lynch syndrome; increased risk in carriers of MSH2 mutations. Urology 86: 1212–1217. [DOI] [PubMed] [Google Scholar]
- Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, Bandipalliam P, Stoffel EM, Gruber SB, Syngal S (2009) Risk of pancreatic cancer in families with Lynch syndrome. JAMA 302: 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg LJ, Ladelund S, Frederiksen BL, Smith-Hansen L, Bernstein I (2016) Outcome of 24 years national surveillance in different hereditary colorectal cancer subgroups leading to more individualised surveillance. J Med Genet 54: 297–304. [DOI] [PubMed] [Google Scholar]
- Lu KH, Daniels M (2013) Endometrial and ovarian cancer in women with Lynch syndrome: update in screening and prevention. Fam Cancer 12: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LA, Broaddus RR, Lu KH (2009) Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 16: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rodland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Sampson JR, Capella G, Mecklin JP, Moslein G (2015) Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 66: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi M, Bishop DT, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard ML, Dunlop MG, Ho JW, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar RS, Side L, Scott RJ, Thomas HJ, Vasen HF, Burn J, Mathers JC (2015) Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 Study. J Clin Oncol 33: 3591–3597. [DOI] [PubMed] [Google Scholar]
- Nolan L, Eccles D, Cross E, Crawford G, Beck N, Bateman A, Ottensmeier C (2009) First case report of Muir-Torre syndrome associated with non-small cell lung cancer. Fam Cancer 8: 359–362. [DOI] [PubMed] [Google Scholar]
- Pande M, Wei C, Chen J, Amos CI, Lynch PM, Lu KH, Lucio LA, Boyd-Rogers SG, Bannon SA, Mork ME, Frazier ML (2012) Cancer spectrum in DNA mismatch repair gene mutation carriers: results from a hospital based Lynch syndrome registry. Fam Cancer 11: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke J, Engel C, Kruger S, Holinski-Feder E, Pagenstecher C, Mangold E, Moeslein G, Schulmann K, Gebert J, von Knebel DM, Ruschoff J, Loeffler M, Schackert HK (2004) Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol 22: 4486–4494. [DOI] [PubMed] [Google Scholar]
- Pylvanainen K, Lehtinen T, Kellokumpu I, Jarvinen H, Mecklin JP (2012) Causes of death of mutation carriers in Finnish Lynch syndrome families. Fam Cancer 11: 467–471. [DOI] [PubMed] [Google Scholar]
- R: A Language and Environment for Statistical Computing (2011) R Foundation for Statistical Computing: Vienna, Austria. Available at: http://www.R-project.org/.
- Raymond VM, Mukherjee B, Wang F, Huang SC, Stoffel EM, Kastrinos F, Syngal S, Cooney KA, Gruber SB (2013) Elevated risk of prostate cancer among men with Lynch syndrome. J Clin Oncol 31: 1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostgaard K (2008) Methods for stratification of person-time and events – a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, Jenkins MA, Win AK (2014) Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 23: 437–449. [DOI] [PubMed] [Google Scholar]
- South CD, Hampel H, Comeras I, Westman JA, Frankel WL, de la Chapelle A (2008) The frequency of Muir-Torre syndrome among Lynch syndrome families. J Natl Cancer Inst 100: 277–281. [DOI] [PubMed] [Google Scholar]
- Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW (2015) ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110: 223–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therkildsen C, Joost P, Lindberg LJ, Ladelund S, Smith-Hansen L, Nilbert M (2016) Renal cell cancer linked to Lynch syndrome: Increased incidence and loss of mismatch repair protein expression. Int J Urol 23: 528–529. [DOI] [PubMed] [Google Scholar]
- Therkildsen C, Ladelund S, Rambech E, Persson A, Petersen A, Nilbert M (2015) Glioblastomas, astrocytomas and oligodendrogliomas linked to Lynch syndrome. Eur J Neurol 22: 717–724. [DOI] [PubMed] [Google Scholar]
- Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, Bapat B, Bernstein I, Capellá G, den Dunnen JT, du SD, Fabre A, Farrell MP, Farrington SM, Frayling IM, Frebourg T, Goldgar DE, Heinen CD, Holinski-Feder E, Kohonen-Corish M, Robinson KL, Leung SY, Martins A, Moller P, Morak M, Nystrom M, Peltomaki P, Pineda M, Qi M, Ramesar R, Rasmussen LJ, Royer-Pokora B, Scott RJ, Sijmons R, Tavtigian SV, Tops CM, Weber T, Wijnen J, Woods MO, Macrae F, Genuardi M (2014) Application of a five-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants lodged on the InSiGHT locus-specific database. Nat Genet 46: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso E, Agostini M, Pucciarelli S, Bedin C, D'angelo E, Mescoli C, Viel A, Maretto I, Mammi I, Nitti D (2012) Soft tissue sarcoma and the hereditary non-polyposis colorectal cancer (HNPCC) syndrome: formulation of an hypothesis. Mol Biol Rep 39: 9307–9310. [DOI] [PubMed] [Google Scholar]
- van Duijnhoven FJ, Botma A, Winkels R, Nagengast FM, Vasen HF, Kampman E (2013) Do lifestyle factors influence colorectal cancer risk in Lynch syndrome? Fam Cancer 12: 285–293. [DOI] [PubMed] [Google Scholar]
- Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G, Colas C, Engel C, Frayling IM, Genuardi M, Heinimann K, Hes FJ, Hodgson SV, Karagiannis JA, Lalloo F, Lindblom A, Mecklin JP, Moller P, Myrhoj T, Nagengast FM, Parc Y, Ponz de LM, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Sijmons RH, Tejpar S, Thomas HJ, Rahner N, Wijnen JT, Jarvinen HJ, Moslein G (2013) Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 62: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A, Korner S, Penzel R, Muley T, Dienemann H, Schirmacher P, von Knebel-Doeberitz M, Weichert W, Kloor M (2016) Microsatellite instability in pulmonary adenocarcinomas: a comprehensive study of 480 cases. Virchows Arch 468: 313–319. [DOI] [PubMed] [Google Scholar]
- Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Jarvinen HJ, Myrhoj T, Sunde L, Wijnen JT, Lynch HT (2008. a) The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 123: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Jarvinen HJ, Myrhoj T, Sunde L, Wijnen JT, Lynch HT (2008. b) The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 123: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, Buchanan DD, Clendenning M, Rosty C, Ahnen DJ, Thibodeau SN, Casey G, Gallinger S, Marchand LL, Haile RW, Potter JD, Zheng Y, Lindor NM, Newcomb PA, Hopper JL, MacInnis RJ (2016) Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 26: 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, Parry S, Goldblatt J, Lipton L, Winship I, Leggett B, Tucker KM, Giles GG, Buchanan DD, Clendenning M, Rosty C, Arnold J, Levine AJ, Haile RW, Gallinger S, Le ML, Newcomb PA, Hopper JL, Jenkins MA (2012) Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 104: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.