Summary
Objective
Evaluate the effects of an online commercial weight management program, with and without provision of a ‘smart’ scale with instructions to weigh daily and weekly tailored feedback, on weight loss and the frequency of body‐weight self‐monitoring.
Methods
Participants (N = 92; body mass index 27–40 kg/m2) were randomized to 6 months of no‐cost access to the Weight Watchers Online (WWO) platform alone, or enhanced with a cellular‐connected ‘smart’ scale, instructions to weigh daily and weekly pre‐scripted email feedback (Weight Watchers Online Enhanced [WWO‐E]). The number of days that weight was self‐monitored (via ‘smart’ scale in WWO‐E and manually in WWO) was recorded automatically across the 6‐month trial. Objective weight was measured at baseline, 3 and 6 months.
Results
While both groups achieved statistically significant weight loss, mean ± standard error weight loss did not differ between WWO‐E and WWO at 3 months (5.1 ± 0.6 kg vs. 4.0 ± 0.7 kg, respectively; p = 0.257) or 6 months (5.3 ± 0.6 kg vs. 3.9 ± 0.7 kg, respectively; p = 0.116). However, a greater proportion of WWO‐E lost ≥5% of initial body weight at 3 months (52.2% vs. 28.3%; p = 0.033), but not 6 months (43.5% vs. 30.4%; p = 0.280), compared with WWO. Mean ± standard deviation days with self‐monitored weight was higher in WWO‐E (80.5 ± 5.6; 44.7% of days) than WWO (12.0 ± 1.0; 6.7% of days; p < 0.001) across the 6‐month study period.
Conclusions
This is the first study to show that provision of a ‘smart’ scale with weekly tailored feedback substantially increased the frequency of self‐weighing and the proportion of participants achieving an initial clinically significant ≥5% weight loss (52% vs. 28%) in an online commercial weight management program. Both WWO and WWO‐E produced significant weight loss over 6 months. While mean weight losses were slightly greater in the enhanced group, the difference was not statistically significant in this small sample. This study provides support for the clinical utility of online commercial weight management programs and the potential for supporting technology such as ‘smart’ scales to improve adherence to body‐weight self‐monitoring and clinical outcomes.
Keywords: diet, eHealth, obesity, physical activity, weight loss
Introduction
Given the popularity of online weight management programs and the potential for digital tools and programs to reach large numbers of individuals for relatively low cost 1, it is important to evaluate their performance and identify strategies to maximize their efficacy. Most of the prior research in this area has focused on programs developed by researchers 2, 3, 4, 5. Few studies have evaluated the weight losses produced by commercial providers of online weight management programs or explored strategies for improving their outcomes.
Systematic reviews and meta‐analyses have shown that regular self‐monitoring of body weight is one of the most important and effective behavioural strategies for weight loss 6, 7, 8, 9, 10. It has been suggested that self‐monitoring of body weight should occur at least weekly in order to have a beneficial effect on weight loss and that daily self‐monitoring may produce greater benefits 6, 8, 9, 10. Providing feedback enhances the value of self‐monitoring and has been shown to facilitate weight loss in both commercial 11 and research‐based online weight loss programs 12.
Historically, self‐monitoring of body weight occurred by stepping on a home scale and recording the entry in a paper diary; feedback was delivered in a verbal and/or written format in structured weight loss programs 6. Advances in technology have made it possible to automate both self‐monitoring (e.g. via ‘smart’ scales with online connectivity for self‐monitoring of weight) and feedback (e.g. via use of algorithms and libraries of pre‐scripted feedback messages) 13. This allows for more efficient self‐monitoring and feedback that requires less effort both from the individuals losing weight and providers of weight loss programs. The potential of such an approach was demonstrated in a study by Steinberg et al. that randomized 91 individuals with overweight or obesity to a 6‐month delayed control condition or a weight loss intervention consisting of a cellular‐connected ‘smart’ scale for daily weighing, web‐based weight loss graph and weekly emails with tailored feedback and lessons 14. This very low intensity intervention produced a mean weight loss of 4.4% of initial body weight at 3 months and 6.6% at 6 months; 42.6% achieved a weight loss of at least 5% of initial body weight at 6 months 14.
The purpose of this study was to evaluate the effects of the Weight Watchers Online (WWO) program, with and without provision of a ‘smart’ scale with instructions to weigh daily and weekly tailored feedback, on weight loss and the frequency of body‐weight self‐monitoring. To our knowledge, this is the first study to evaluate the effect of ‘smart’ scales and feedback in a randomized controlled trial in which all participants receive an active weight loss treatment. It is also one of the only studies to evaluate weight losses produced by an online commercial program.
Methods
Participants
English‐speaking men and women age 18–70 years with a body mass index (BMI) of 27–40 kg/m2 and access to an Internet‐connected computer were eligible. Individuals with a medical contraindication for weight loss (e.g. some types of cancer) or unsupervised exercise (e.g. chest pain during exercise), weight loss of ≥5% of initial body weight within the previous 6 months, weight loss surgery, unstable severe psychiatric illness or current use of weight loss medication were excluded. Patients with type 2 diabetes mellitus were admitted to the trial on the condition that they had physician consent to participate.
Study design
This 6‐month randomized controlled trial was conducted simultaneously at Brown University/The Miriam Hospital and the University of Tennessee Knoxville. Ads in local print media were used for recruitment May–June 2015. A phone screen was used to establish eligibility. Informed consent and baseline assessments were completed at an initial in‐person session at the research centres. During the following week, participants were required to visit a study website twice to demonstrate Internet access. Participants then attended a randomization visit at the research centres where they received printed instructions for beginning treatment.
All participants received 6 months of no‐cost access to WWO, accessible via website and mobile app. The dietary component focused on the PointsPlus plan, which assigns a PointsPlus value to each food and beverage based on its fat, fibre, carbohydrate and protein content. The fitness component focused on activity PointsPlus, which assigned each activity an activity PointsPlus value based on its type, duration, intensity and the participant's body weight. Upon sign‐up, an individualized PointsPlus budget consisting of a daily PointsPlus target and weekly PointsPlus allowance was calculated based on height, weight, age and gender. Participants were instructed to track their daily food intake and physical activity via the WWO website or mobile app, for the calculation of PointsPlus and activity PointsPlus values for foods and activities, respectively. Participants assigned to the WWO condition were instructed to self‐monitor their body weight no more than once per day and no less than once per week by manually recording the weight obtained from their home scale in their WWO account, either via the WWO website or mobile app. Summaries of the tracked self‐monitoring data (dietary intake, activity and weight) were provided to the participant via graphs and charts within the member WWO account.
The WWO program included video lessons delivered via links in weekly emails for the first 9 weeks. Each video described a validated behavioural weight loss skill (e.g. goal setting, portion size estimation and social support) 7 along with guidance and encouragement for implementation. The WWO program also provided participants with support from trained Weight Watchers staff via one‐on‐one, real‐time, online chatting available 24 h 7 d per week and from other Weight Watchers members via the online community. Lastly, participants could access menu and recipe ideas, informational articles and tip sheets online.
Half of the participants were randomized to an ‘Enhanced’ program (WWO‐E) that included provision of a cellular‐connected ‘smart’ scale with instructions to weigh daily and tailored weekly email feedback on weighing frequency and weight change. ‘Smart’ scales automatically transmitted body weights to participants' WWO accounts and the research team via the secure cellular network used for mobile phones and pager devices. Participants were instructed to disallow others from using the ‘smart’ scale, and a software filter was used to prevent body weights of others in the household from being recorded in the WWO account. A pre‐scripted email feedback message was sent weekly for 24 weeks. The feedback message was tailored based on frequency of body‐weight self‐monitoring (≥5 d per week vs. less often) and weight loss (average weight loss of ≥1 lb per week vs. less during weeks 1–12; total weight loss of ≥10 lbs during weeks 13–24). The messages were supportive in tone, encouraged continued body‐weight self‐monitoring, praised self‐monitoring and weight loss and recommended behavioural strategies (e.g. putting the scale where you can see it) to help increase body‐weight self‐monitoring. The feedback messages were incorporated into the standard WWO video lesson emails for the first 9 weeks, and study‐specific emails (consisting of only recommended behavioural strategies and feedback messages, without links to videos) were created for weeks 10–24.
Measures
The primary outcome, objective body weight, was measured at the research centres at baseline, 3 and 6 months using a calibrated (Tanita® BWB‐800 scale, Tanita, Arlington Heights, IL, USA). Weight loss from baseline was calculated in kg and % of initial body weight.
Each time that body weight was self‐monitored, either manually in WWO or via the ‘smart’ scale in WWO‐E, the date was recorded automatically by the online system. The number of days of body‐weight self‐monitoring was calculated for each participant for each week of the 6‐month program; a sum was also calculated to represent the total number of days that body weight was self‐monitored over the 6‐month study period. The proportion of participants who self‐monitored body weight at least once during the week was also calculated for each week of the program, and a sum was calculated to represent the number of weeks out of 24 that each participant self‐monitored their body weight at least once during the week.
Participants rated their overall satisfaction with the weight loss program that they received on a Likert scale of 1 (Very Dissatisfied) to 7 (Very Satisfied) at 3 and 6 months.
Data were collected by blinded research assistants, and the investigators were blinded to study outcomes until the completion of the trial.
Statistical analysis
All analysis was performed using ibm spss statistics® for Windows, Version 20.0 (IBM Corp., Armonk, New York). Participants' baseline characteristics and the frequency of body‐weight self‐monitoring were summarized and compared between‐groups using chi‐squared and t‐test as appropriate and with correction for unequal variance between‐groups as needed. Linear mixed‐effects models controlling for baseline BMI, age, gender, race/ethnicity (non‐Hispanic White vs. all others) and level of education (at least college or university education vs. all others) were used to evaluate changes in weight (kg) at 3 and 6 months. Intercepts and the slope of time (represented as months since baseline) were treated as random effects. Treatment condition was represented as a fixed effect and was allowed to interact with the effect of time. This approach allowed all participants to contribute to the analysis. A similar approach was used to evaluate change in the proportion of participants weighing at least weekly over the 24‐week study period. Chi‐squared was used to compare rates of retention between‐groups and the proportion of participants achieving a clinically significant weight loss of ≥5% of initial body weight (Dropouts were assumed not to have met the threshold.). Bivariate correlations were used to test for associations between frequency of body‐weight self‐monitoring and weight loss in WWO and WWO‐E, separately. The Fisher r‐to‐z transformation was used to compare the strength of the correlation between the two conditions. All statistical tests were two‐tailed, with α = 0.05. There were no significant differences in baseline demographics between the two study sites, and including site in the outcomes analysis had no effect on the pattern of results and did not significantly improve model fit. Therefore, the results reported are for models without the effect of site. This pilot study was designed to obtain preliminary estimates of intervention effects on weight and frequency of body‐weight self‐monitoring. With a total sample size of at least 90 participants split evenly between conditions, statistical power was expected to be at least 0.80 to detect a between‐groups difference of at least 3.1 kg in weight loss at 3 and 6 months and a between‐groups difference of at least 24 d (about 1 d per week) in the frequency of body‐weight self‐monitoring.
Results
See Figure 1 for the CONSORT diagram illustrating participant flow though the trial. Of 230 individuals screened for eligibility, 37 refused participation and 101 were excluded, most often because of a BMI outside of the eligible range or a medical contraindication for weight loss or unsupervised exercise. The remaining 92 participants who were enrolled and randomized to WWO (n = 46) or WWO‐E (n = 46) were predominantly women (83.7%), non‐Hispanic White (91.3%), college or university educated (58.7%) and had a mean ± standard deviation (SD) age of 55.6 ± 11.0 and a BMI of 34.0 ± 3.7 kg/m2 (Table 1). There were no significant differences between‐groups at baseline. Retention was 96% (n = 88) at 3 months and 95% (n = 87) at 6 months with no differences between‐groups (ps > 0.117).
Figure 1.
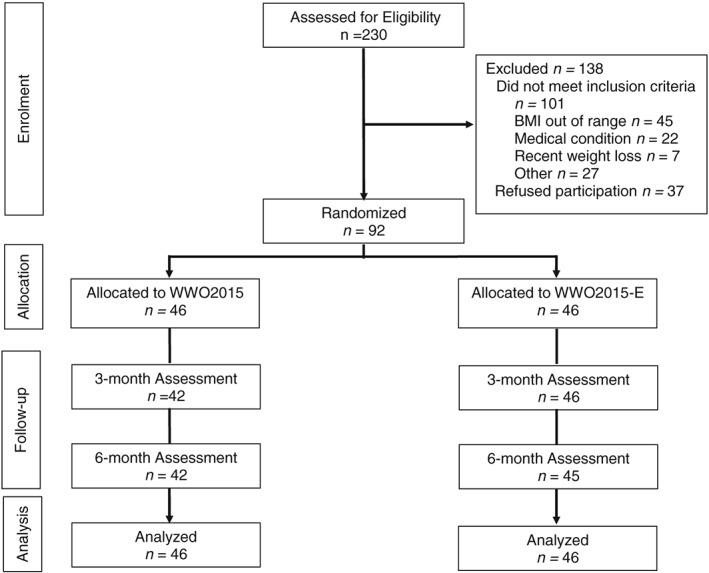
The CONSORT flow diagram includes data on assessment of eligibility, patient enrolment, allocation to condition, follow‐up and primary analysis.
Table 1.
Participant characteristics at baseline
| WWO (n = 46) | WWO‐E (n = 46) | Total (n = 92) | |
|---|---|---|---|
| Gender, no. (%) | |||
| Men | 7 (15.2) | 8 (17.4) | 15 (16.3) |
| Women | 39 (84.8) | 38 (82.6) | 77 (83.7) |
| Age, mean (SD), years | 54.9 (10.2) | 56.4 (11.8) | 55.6 (11.0) |
| Race, no. (%) | |||
| American Indian | 0 (0.0) | 2 (4.3) | 2 (2.2) |
| Black | 5 (10.9) | 1 (2.2) | 6 (6.5) |
| White | 41 (89.1) | 43 (93.5) | 84 (91.3) |
| Ethnicity, no. (%) | |||
| Not Hispanic or Latino | 46 (100) | 46 (100) | 92 (100) |
| Education, no. (%) | |||
| High school or less | 4 (8.7) | 2 (4.3) | 6 (6.5) |
| Some college | 14 (30.4) | 18 (39.1) | 32 (34.8) |
| College or university degree | 12 (26.1) | 12 (26.1) | 24 (26.1) |
| Graduate degree | 16 (34.8) | 14 (30.4) | 30 (32.6) |
| Weight, mean (SD), kg | 95.1 (15.5) | 93.1 (14.7) | 94.1 (15.1) |
| Body mass index, mean (SD), kg m−2 | 34.1 (3.5) | 33.9 (3.9) | 34.0 (3.7) |
SD, standard deviation; WWO, Weight Watchers Online; WWO‐E, Weight Watchers Online Enhanced.
As shown in Table 2, both conditions achieved statistically significant weight loss (p < 0.001), but the mean ± standard error weight losses did not differ between‐groups at 3 months (5.1 ± 0.6 kg in WWO‐E vs. 4.0 ± 0.7 kg in WWO, p = 0.257) or 6 months (5.3 ± 0.6 kg in WWO‐E vs. 3.9 ± 0.7 kg in WWO, p = 0.116). However, at 3 months, a significantly greater proportion of participants assigned to WWO‐E (52.2%) achieved a ≥5% weight loss compared with WWO (28.3%; p = 0.033). This difference did not persist at 6 months (43.5% in WWO‐E vs. 30.4% in WWO, p = 0.280).
Table 2.
Weight loss outcomes
| WWO (n = 46) | WWO‐E (n = 46) | |
|---|---|---|
| Weight Loss, mean (SE), kg | ||
| 3 months | 4.0 (0.7) | 5.1 (0.6) |
| 6 months | 3.9 (0.7) | 5.3 (0.6) |
| Weight Loss, mean (SE), % | ||
| 3 months | 4.1 (0.7) | 5.4 (0.7) |
| 6 months | 4.0 (0.7) | 5.8 (0.7) |
| Proportion achieving weight loss of ≥5% of initial weight, no. (%) | ||
| 3 months | 13 (28.3) | 24 (52.2) |
| 6 months | 14 (30.4) | 20 (43.5) |
Statistically significant weight loss was observed in both conditions at 3 and 6 months (p < 0.001). Bolded values indicate a significant difference between groups (p < 0.05).
SE, standard error.
Figure 2 depicts patterns of body‐weight self‐monitoring in both conditions throughout the 6‐month trial. The mean ± SD number of days per week that body weight was self‐monitored ranged from 2.5 ± 2.7 to 3.8 ± 2.5 in WWO‐E over 24 weeks; much higher than the 0.3 ± 0.4 to 1.4 ± 0.6 observed in WWO. This led to a significant difference between WWO‐E and WWO in the total number of days that body weight was self‐monitored (80.5 ± 54.5 vs. 12.0 ± 9.6, respectively, p < 0.001). The emphasis on daily self‐weighing in WWO‐E also increased the mean ± SD number of weeks that participants self‐monitored their body weight at least once during the week (19.8 ± 6.2 in WWO‐E vs. 11.1 ± 8.6 kg in WWO, p < 0.001), a commonly accepted threshold of clinical significance.
Figure 2.
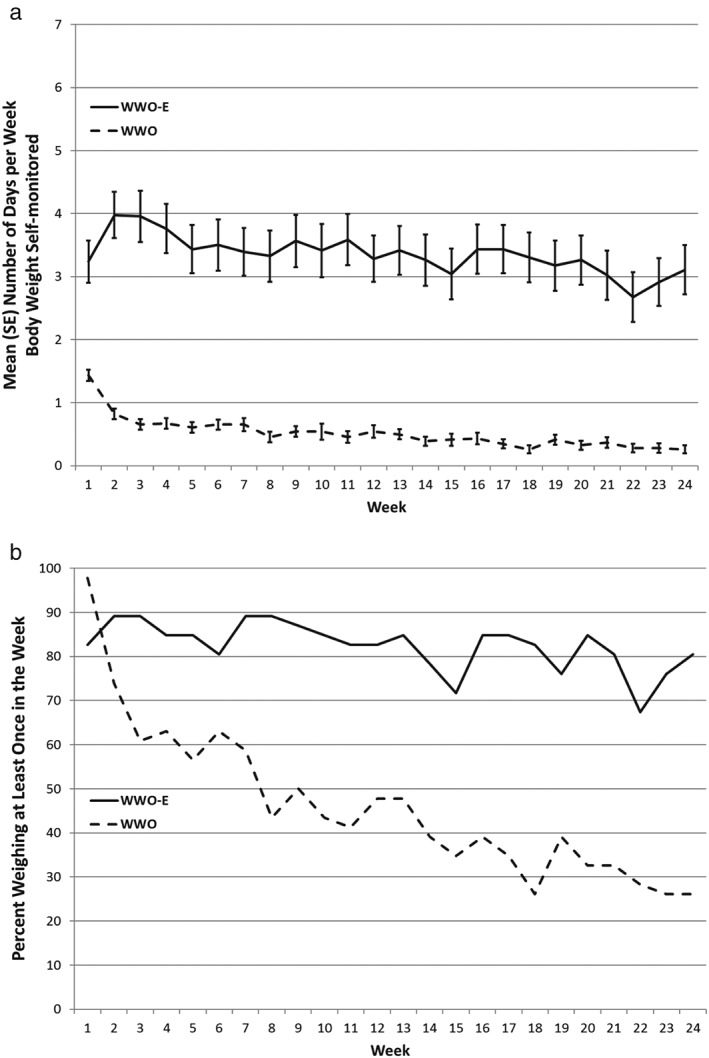
(a) Mean (standard error) number of days and (b) proportion (%) of participants weighing at least once each week in the Weight Watchers Online (WWO) and Weight Watchers Online Enhanced (WWO‐E) conditions.
There was a statistically significant between‐groups difference (p < 0.001) in the change in the proportion of participants weighing at least weekly over the 24‐week study period. The proportion of WWO participants who self‐monitored their body weight decreased significantly from nearly 100% at the start of the trial to under 30% at 24 weeks (coefficient = −0.22, p < 0.001). In contrast, there was no significant change in the proportion of participants self‐monitoring body weight in WWO‐E (coefficient = −0.03, p = 0.334), which fluctuated from about 70–90% for the duration of the trial.
The total number of days of body‐weight self‐monitoring was correlated with weight loss at 6 months in WWO (r = 0.66, p < 0.001) but not WWO‐E (r = 0.27, p = 0.073); the Fisher r‐to‐z transformation confirmed that the correlation was significantly stronger in WWO than WWO‐E (z = 2.30, p = 0.021). Weight loss was not significantly greater among participants in WWO‐E who self‐monitored body weight at an average of at least 3, 4 or 5 times per week vs. less often (ps > 0.080); this comparison was not feasible in WWO because too few participants self‐monitored body weight at these high frequencies.
Mean ± SD satisfaction with the weight loss program was moderately high and did not differ significantly between‐groups at both 3 months (4.8 ± 1.6 in WWO‐E vs. 4.3 ± 1.2 in WWO, p = 0.090) and 6 months (5.2 ± 1.4 in WWO‐E vs. 4.8 ± 1.4 in WWO, p = 0.234).
Discussion
The weight losses produced by online commercial programs, and strategies for improving them, have rarely been studied. Regular self‐monitoring of body weight is frequently cited as one of the most important behavioural weight loss strategies 6, 7, 8, 9, 10. It is therefore a logical target to improve outcomes of online weight loss programs. Steinberg et al. recently showed that a weight loss intervention consisting of a cellular‐connected ‘smart’ scale for daily weighing, web‐based weight loss graph and weekly emails with tailored feedback and lessons resulted in a body‐weight self‐monitoring frequency of approximately 6 d per week and produced a weight loss of approximately 4 kg at 3 months and 6 kg at 6 months 14. The current study is the first to test a similar intervention as an adjunct to a popular commercial online weight loss program in a randomized controlled trial in which all participants received an active weight loss treatment.
Both versions of the online commercial program tested in this trial produced statistically significant reductions in weight. While the mean weight loss was estimated to be about 25% greater in WWO‐E (5 kg) compared with WWO (4 kg), the study was not powered to detect this magnitude of difference as statistically significant. However, it was possible to show that a greater proportion of WWO‐E (52.2%) compared with WWO (28.3%) achieved a clinically significant weight loss of ≥5% of initial body weight at 3 months; this effect did not persist at 6 months. The lack of statistical power to detect smaller but clinically significant between‐groups differences in weight loss is a clear limitation of this study, but it sets the stage for future research to replicate and confirm the beneficial effect of increased body‐weight self‐monitoring on weight loss in a commercial online weight management program.
The frequency of body‐weight self‐monitoring increased substantially from less than 1 d per week on average in the standard WWO programme to over 3 d per week on average in WWO‐E. The number of weeks that body weight was self‐monitored at least once during the week, an important metric for clinically significant body‐weight self‐monitoring, was also superior in WWO‐E. Despite these improvements, average body‐weight self‐monitoring frequency in WWO‐E was less than the daily recommendation and less than the average of 6.1 d per week observed by Steinberg et al. 14. In addition, while the ‘smart’ scale and associated resources increased the frequency of body‐weight self‐monitoring, they did not increase overall satisfaction with the program. Nevertheless, the average weight loss produced by WWO‐E (over 5 kg) was similar to that observed in Steinberg et al. 14. Reviews and meta‐analyses show that such mean weight losses of 5–6 kg are the most that are typically achieved via automated online programs developed by researchers and commercial providers 2, 3, 4, 5.
Most prior studies of the association have found that more frequent body‐weight self‐monitoring is associated with larger weight loss 6, 7, 8, 9, 10, but this was true only for WWO and not WWO‐E in the current study. One interpretation of this finding is that non‐automated body‐weight self‐monitoring has served as a proxy for overall adherence to a behavioural weight loss program. By reducing the burden of body‐weight self‐monitoring, but not other behavioural strategies necessary for successful weight loss, self‐monitoring may become a less powerful indicator of overall adherence to a program. The weaker association does not appear to suggest that partially automating body‐weight self‐monitoring reduced its effectiveness as a behavioural weight loss strategy given that WWO‐E produced weight loss outcomes that were at least as good as WWO. Additional research is thus needed to further develop our understanding of how best to capitalize on automation of self‐monitoring behaviours to maximize weight loss outcomes.
Participants assigned to WWO‐E were encouraged to self‐monitor their weight daily, but feedback was provided only weekly, via email. Therefore, feedback was often delayed several days from the time of body‐weight self‐monitoring. Research shows that feedback is most effective when it occurs soon after the target behaviour occurs; ideally immediately after 15. Thus, it is possible that feedback delivered with a different frequency and/or format might improve effectiveness. For example, feedback could be delivered in the form of a notification delivered to a smartphone minutes after a weight is recorded. Shorter, more frequent messages may hold attention and be perceived as more responsive than a longer message delivered less frequently. However, the technology and message library needed to enable such an intervention is more complex and costly than what was used in this study.
The use of monitoring technology as a means of improving adherence to key weight‐related behaviours is receiving increased attention, particularly in online programs, which are especially suited to incorporating device data as part of the intervention. However, the few other studies published to date have reported limited benefits. For example, we recently conducted a study of WWO with and without a physical activity tracking device and associated online resources for physical activity goal setting and feedback 16. There was no indication that the tracking technology improved physical activity or weight loss outcomes. Furthermore, the WWO program on its own produced significantly larger weight losses than a control condition, whereas the weight losses of participants who also received the tracking technology were indistinguishable from control. A similar pattern of findings was observed by Jakicic et al., who randomized participants completing 6 months of group‐based behavioural weight loss treatment to a self‐monitoring website or a physical activity tracking device with associated online resources 17. Again, the tracking technology produced no significant improvements in psychical activity, and the trajectory of weight regain was stepper compared to participants who were not given a tracker. In contrast to these reports, the current study, and the study by Steinberg et al. 14 upon which it is based, found that the tracking technology produced significant improvements in the targeted behaviour (i.e. body‐weight self‐monitoring) and had beneficial effects on mean weight and/or the proportion of participants achieving an initial clinically significant weight loss. Taken together, these findings indicate potential for self‐monitoring technology to both enhance and undermine behavioural and clinical outcomes. Clearly, additional research to improve understanding of factors and circumstances that lead to technology having beneficial or detrimental effects is warranted.
This study has a number of important strengths: it was the first to study ‘smart’ scale technology to improve the frequency of body‐weight self‐monitoring in a popular internationally available online weight loss program in a randomized controlled trial in which all participants received an active weight loss treatment. It was also a multisite trial conducted at two geographically and culturally distinct research centres with excellent retention. Objectively measured frequency of body‐weight self‐monitoring was conducted automatically in both conditions by the electronic intervention platforms. It is one of the first studies to provide data on the effects of partially automating a key behavioural weight loss strategy. This study also has important limitations: the sample size was not adequate to detect smaller but clinically significant between‐groups differences in weight loss. The sample consisted primarily of non‐Hispanic White women. Finally, the effect of providing the ‘smart’ scale cannot be disentangled from the recommendation to weigh at least daily or the effect of providing feedback in WWO‐E.
Conclusions
Participants in a popular internationally available online weight loss program achieved clinically significant weight loss over 6 months. Provision of a cellular‐connected ‘smart’ scale with instructions to weigh daily as well as receiving weekly tailored feedback on weight change and weighing frequency delivered via email substantially increased the frequency of body‐weight self‐monitoring. The sample size was not sufficient to detect smaller but clinically significant effects on mean weight loss. However, the proportion of participants achieving a clinically significant weight loss of ≥5% of initial body weight was significantly improved with the ‘smart’ scale intervention but only early in treatment. The correlation between body‐weight self‐monitoring and weight loss was lower with the ‘smart’ scale intervention, possibly because the behaviour was partially automated, but no adverse effect on weight loss was observed. These findings highlight the clinical significance of widely available online weight management programs and the potential of digital health technology to improve the rate at which key weight management behaviours such as self‐monitoring are performed.
Funding
This project was funded by a research grant from Weight Watchers International, Inc. awarded to The Miriam Hospital and the University of Tennessee.
Conflict of Interest Statement
Dr Foster and Ms Wojtanowski are employed by Weight Watchers International, Inc. Ms Vander Veur was employed by Weight Watchers International, Inc. during the execution of the study and is now affiliated with the Drexel University Center for Weight, Eating and Lifestyle Science. Drs Thomas, Raynor, Bond and Wing received research support (i.e. partial salary paid through their academic institutions and funds to conduct the research) from Weight Watchers International, Inc. for this research study and others. Dr Tate serves on the Weight Watchers International, Inc. Scientific Advisory Board and has received consulting fees from Weight Watchers International, Inc. to provide guidance on program development, including those tested in this trial.
Acknowledgement
The authors thank the study participants for their contribution to the research.
Thomas, J. G. , Raynor, H. A. , Bond, D. S. , Luke, A. K. , Cardoso, C. C. , Wojtanowski, A. C. , Vander Veur, S. , Tate, D. , Wing, R. R. , and Foster, G. D. (2017) Weight loss and frequency of body‐weight self‐monitoring in an online commercial weight management program with and without a cellular‐connected ‘smart’ scale: a randomized pilot study. Obesity Science & Practice, 3: 365–372. doi: 10.1002/osp4.132.
Trial Registration
http://clinicaltrials.gov Identifier: NCT 02417220
References
- 1. Tate DF, Finkelstein EA, Khavjou O, Gustafson A. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med. 2009; 38: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arem H, Irwin M. A review of web‐based weight loss interventions in adults. Obes Rev. 2011; 12: e236–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mateo GF, Granado‐Font E, Ferré‐Grau C, Montaña‐Carreras X. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta‐analysis. J Med Internet Res. 2015; 17: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherrington A, Newham JJ, Bell R, Adamson A, McColl E, Araujo‐Soares V. Systematic review and meta‐analysis of internet‐delivered interventions providing personalized feedback for weight loss in overweight and obese adults. Obes Rev. 2016; 17: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013; 28: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke LE, Wang J, Sevick MA. Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011; 111: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self‐monitoring of weight: a key component of successful weight loss maintenance. Obesity. 2007; 15: 3091–3096. [DOI] [PubMed] [Google Scholar]
- 8. Madigan CD, Daley AJ, Lewis AL, Aveyard P, Jolly K. Is self‐weighing an effective tool for weight loss: a systematic literature review and meta‐analysis. Int J Behav Nutr Phys Act. 2015; 12: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanwormer JJ, French SA, Pereira MA, Welsh EM. The impact of regular self‐weighing on weight management: a systematic literature review. Int J Behav Nutr Phys Act. 2008; 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self‐weighing in weight management: a systematic literature review. Obesity. 2015; 23: 256–265. [DOI] [PubMed] [Google Scholar]
- 11. Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e‐mail counseling, computer‐automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006; 166: 1620–1625. [DOI] [PubMed] [Google Scholar]
- 12. Wing RR, Crane MM, Thomas JG, Kumar R, Weinberg B. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health. 2010; 100: 2513–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swan M. Sensor mania! the internet of things, wearable computing, objective metrics, and the quantified self 2.0. Journal of Sensor and Actuator Networks 2012; 1: 217. [Google Scholar]
- 14. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel‐Hodge C, Ward DS. The efficacy of a daily self‐weighing weight loss intervention using smart scales and e‐mail. 2013;21:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiClemente CC, Marinilli AS, Singh M, Bellino LE. The role of feedback in the process of health behavior change. Am J Health Behav. 2001; 25: 217–227. [DOI] [PubMed] [Google Scholar]
- 16. Thomas JG, Raynor HA, Bond DS, et al. Weight loss in Weight Watchers Online with and without an activity tracking device compared to control: a randomized trial. Obesity. 2017; 25: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 17. Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technlogy combined with a lifestyle intervention on long‐term weight loss: The IDEA randomized clinical trial. JAMA. 2016; 316: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]


