Abstract
Background
We aimed to compare the outcomes of mitral valve repair with flexible band (FB) versus complete semirigid ring (SR) in degenerative mitral valve disease patients.
Methods
From September 2011 to 2014, 171 patients were randomized and underwent successful mitral valve repair using a SR (n = 85) or FB (n = 86). There were no significant between-group differences at baseline.
Results
There were no early mortalities. The mean follow up was 24.7 months. The 2-year survival was 96.0 ± 2.3% (95% confidence interval [CI], 88.6–98.7%) and 94.3 ± 2.8% (95% CI, 85.5–97.9%) in the SR and FB groups, respectively (p = 0.899). The left ventricle remodeling was similar between the groups. Higher transmitral peak (8.5 [3.9–17] vs. 6 [2.1–18] mmHg, p < 0.001), mean pressure gradients (3.7 [1.3–8] vs. 2.8 [0.6–6.8] mmHg, p = 0.001), and systolic pulmonary artery pressure (34.5 [20–68] vs. 29.5 [8–48] mmHg, p < 0.001) was observed in the SR group. The 2-year freedom from recurrence of significant mitral regurgitation was significantly higher in the FB group than the SR group (p = 0.002). Residual mitral regurgitation was an independent prognostic factor of recurrence of mitral regurgitation. The 3-year freedom from reoperation was significantly higher in the FB group than the SR group (p = 0.044).
Conclusion
Patients with degenerative mitral valve disease may benefit from valve repair with FBs. Residual mitral regurgitation before discharge is an independent risk factor of late insufficiency recurrence.
Trial registration
ClinicalTrials.gov NCT03278574, retrospectively registered on 06.09.2017.
Electronic supplementary material
The online version of this article (10.1186/s13019-017-0679-0) contains supplementary material, which is available to authorized users.
Keywords: Degenerative mitral valve disease, Mitral regurgitation, Mitral valve repair
Background
In 1957, Lillehei et al. [1] proposed the mitral annuloplasty technique, a new concept in valve surgery. Remodeling annuloplasty, developed by Carpentier in 1983, increases leaflet coaptation, prevents future annular dilatation, and preserves leaflet mobility in patients with degenerative mitral valve (MV) disease [2, 3]. Currently, there are many commercially available mitral annuloplasty devices in the market, including complete or partial, rigid or flexible, and flat or saddle-shaped rings. However, no single annuloplasty device has been proven to have a clinical benefit above the others [4]. Theoretically, the flexible band does remodel mitral annulus providing reduction annuloplasty only. However, the rigid ring does not have enough flexibility for physiological annular motion during the cardiac cycle. A systematic review of clinical trials [5] showed comparable clinical outcomes between rigid and flexible rings. Currently, mitral ring selection is based on a surgeon’s preference rather than evidence [6]. Semirigid rings combine flexibility and stability; however, their clinical benefit has not been completely clarified. The present study aimed to compare the outcomes of MV repair with a flexible posterior annuloplasty band versus complete semirigid ring in patients with degenerative MV disease.
Methods
Study design
In this prospective, randomized study, 171 patients with degenerative MV disease who were scheduled for isolated MV repair in our Institute from September 2011 through September 2014 were enrolled (CONSORT flow diagram, Figure 1). Participants were randomly assigned following a simple randomization procedure to complete semirigid ring (SR group) or flexible posterior annuloplasty band (FB group), according to computerized random numbers on the day before surgery. Eligible participants were adults aged 18 years or more with degenerative MV disease [7] who met the indications for MV operation according to the American College of Cardiology/American Heart Association guidelines [8]. Exclusion criteria were previous open cardiac surgery, indication for concomitant aortic valve replacement, or left ventricle (LV) impairment (ejection fraction <40%). The Local Ethics Committee approved the study design, and all the patients provided informed consent. The present study was conducted in compliance with the Declaration of Helsinki.
Fig. 1.
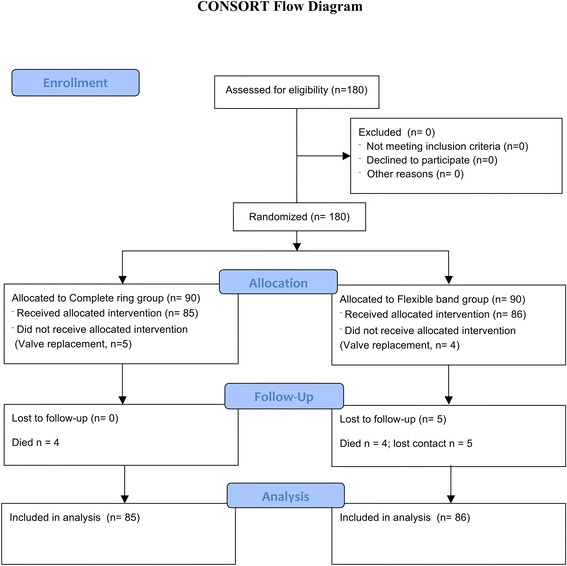
Consort flow diagram
Patients
Mean age of the participants in each group was 57 (23–75) and 54 (19–74) years, respectively. There were no differences in sex, age, preoperative New York Heart Association (NYHA) functional class, and comorbidities (Table 1).
Table 1.
Baseline
| SR Group, n = 85 |
FB Group, n = 86 |
Р value | |
|---|---|---|---|
| FED, n (%) | 65 (76.5) | 56 (65.1) | 0.103 |
| Forme fruste, n (%) | 12 (14.1) | 18 (20.9) | 0.241 |
| Barlow, n (%) | 8 (9.4) | 12 (14.0) | 0.355 |
| Male, n (%) | 59 (69.4) | 58 (67.4) | 0.782 |
| Age, years | 57 (23–75) | 54 (19–74) | 0.092 |
| Height, cm | 173 (148–192) | 175 (151;193) | 0.079 |
| Weight, kg | 79.1 ± 14.6 | 77.1 ± 16.4 | 0.388 |
| BMI, kg/m2 | 26.9 ± 4.3 | 25.4 ± 4.6 | 0.522 |
| BSA, m2 | 1.94 ± 0.21 | 1.93 ± 0.24 | 0.801 |
| NYHA I, n (%) | 7 (8.2) | 12 (14.0) | 0.234 |
| NYHA II, n (%) | 22 (25.9) | 29 (33.7) | 0.263 |
| NYHA III, n (%) | 54 (63.5) | 43 (50.0) | 0.742 |
| NYHA IV, n (%) | 2 (2.4) | 2 (2.3) | 0.991 |
| AF, n (%) | 22 (25.9) | 16 (18.6) | 0.252 |
| Paroxysmal, n (%) | 2 (2.4) | 2 (2.3) | 0.991 |
| Persistent, n (%) | 5 (5.9) | 3 (3.5) | 0.459 |
| Long-standing, n (%) | 11 (12.9) | 9 (10.5) | 0.614 |
| Permanent, n (%) | 4 (4.7) | 2 (2.3) | 0.398 |
| CAD, n (%) | 10 (11.8) | 4 (4.7) | 0.090 |
| Arterial hypertension, n (%) | 46 (54.1) | 42 (48.8) | 0.490 |
| Diabetes mellitus, n (%) | 4 (4.7) | 5 (5.8) | 0.746 |
| Moderate renal impairment, n (%) | 1 (1.2) | 1 (1.2) | 0.993 |
| Peripheral vascular disease, n (%) | 5 (5.9) | 3 (3.5) | 0.459 |
| Cerebrovascular disease, n (%) | 5 (5.9) | 4 (4.7) | 0.719 |
| LV EF, % | 65.5 (49–80) | 67.0 (51–89) | 0.212 |
| PA pressure, mm Hg | 48.5 (29–80) | 45.0 (29–94) | 0.053 |
SR semirigid ring, FB flexible band, BMI body mass index, BSA body surface area, NYHA New York Heart Association functional class, AF atrial fibrillation, CAD coronary artery disease, LV EF left ventricle ejection fraction, PA pulmonary artery
Outcome measures
The primary endpoint was freedom from moderate or severe mitral regurgitation (MR) recurrence. Secondary endpoints included survival, freedom from reoperations, and freedom from severe MR recurrence. The severity of MR was evaluated and defined in accordance with the recommendations [9]. Valve-related complications were evaluated and defined in accordance with the guidelines [10].
Surgical techniques
Real-time two-dimensional/three-dimensional transesophageal echocardiography (TEE, Phillips iE33, Philips Ultrasound Inc., PA, USA) was performed after the induction of anesthesia for MV lesion estimation. Cold crystalloid cardioplegic solution (Custodiol® HTK Solution, Dr. Franz Köhler Chemie, Alsbach-Hahnlein, Germany) was used for myocardial protection with antegrade root flow.
The two most experienced surgeons in MV repair in our Institute performed the operations in the study. Surgeons were evenly split between the treatment groups. The surgical technique for MV repair was chosen according to the MV lesion (Table 2). The most common techniques were quadrangular (“sliding maneuver”) or triangular resection for posterior MV prolapse, and artificial chordal replacement (separate or loop technique) for anterior leaflet pathology. The minimally invasive approach through the right fourth intercostal space with femoral–femoral cannulation for arterial and venous lines was used in 29.4% (n = 25) and 31.4% (n = 27) of patients in the SR and FB groups, respectively.
Table 2.
Intraoperative data
| SR Group, n = 85 |
FB Group, n = 86 |
P value | |
|---|---|---|---|
| Approach: | |||
| Conventional, n (%) | 60 (70.6) | 59 (65.1) | 0.778 |
| Minimally invasive, n (%) | 25 (29.4) | 27 (31.4) | 0.778 |
| CPB time, min | 140 (110;179.5) | 160 (122;206) | 0.091 |
| Cross clamp time, min: | |||
| Conventional | 92 (73;117) | 101 (79;125) | 0.230 |
| Minimally invasive | 116 (101;129) | 123 (106;156) | 0.171 |
| Mitral valve analyze and intervention in certain patients | |||
| А1 prolapse, n (%) | 9 (10.6) | 11 (12.8) | 0.654 |
| А2 prolapse, n (%) | 23 (27.1) | 33 (38.4) | 0.115 |
| А3 prolapse, n (%) | 13 (15.3) | 19 (22.1) | 0.254 |
| P1 prolapse, n (%) | 6 (7.1) | 12 (13.9) | 0.142 |
| P2 prolapse, n (%) | 59 (69.4) | 68 (79.1) | 0.119 |
| P3 prolapse, n (%) | 17 (20.0) | 27 (31.4) | 0.088 |
| AMVL prolapse, n (%) | 31 (36.5) | 40 (46.5) | 0.183 |
| PMVL prolapse, n (%) | 73 (85.9) | 74 (86.1) | 0.975 |
| DMVL prolapse, n (%) | 19 (22.4) | 28 (32.5) | 0.135 |
| Chordae rupture, n (%) | 64 (75.3) | 53 (61.6) | 0.055 |
| AMVL resection, n (%) | 1 (1.2) | 4 (4.7) | 0.178 |
| PMVL resection, n (%) | 37 (43.5) | 49 (56.9) | 0.079 |
| AMVL neochordae, n (%) | 22 (25.9) | 29 (33.7) | 0.263 |
| PMVL neochordae, n (%) | 30 (35.3) | 27 (31.4) | 0.589 |
| Ring size, mm | 32 (30;32) | 34 (30;34) | 0.456 |
| Concomitant procedure | |||
| Maze IV procedure, n (%) | 18 (21.2) | 14 (16.3) | 0.412 |
| CABG, n (%) | 10 (11.8) | 4 (4.7) | 0.090 |
| TV repair, n (%) | 32 (37.6) | 24 (27.9) | 0.175 |
| SAM-syndrome, n (%) | 0 | 1 (1.2) | 0.319 |
| Intraoperative TEE | |||
| Depth of coaptation, mm | 9 (7;11) | 6 (5;8) | 0.006 |
| Peak MV gradient, mm Hg | 8.0 (6.7;10.9) | 6.4 (4;9) | <0.001 |
| Mean MV gradient, mm Hg | 3 (2.4;4) | 2 (2;3.9) | <0.001 |
SR semirigid ring, FB flexile band, AMVL anterior mitral valve leaflet, PMVL posterior mitral valve leaflet, DMVL prolapse dual (anterior and posterior) mitral valve leaflet prolapse, CABG coronary artery bypass grafting, TV tricuspid valve, CPB cardiopulmonary bypass, TEE transesophageal echocardiography
All mitral valve repairs were performed with flexible bands (“C Flex”, CardiaMed, Penza, Russia) or complete rings (“D Ring”, CardiaMed, Penza, Russia), which routinely use since 2005. The ring size was selected according to the size of the anterior leaflet and the intertrigonal distance. In Barlow’s cases, 36–40 mm rings were used. Flexible bands were implanted along the posterior mitral semicircle with an extension to both commissures for 3–5 mm. Valve competence was assessed intraoperatively by saline test and “ink-test” (symmetrical closure line, coaptation depth ≥ 5 mm, absence of leaflet prolapse and regurgitation jet).
After bypass weaning, patients were followed-up with TEE. In cases with residual MR ≥ grade 2, cardiopulmonary bypass was re-established, and the valve was re-repaired or replaced; residual MR grade 1 (mild) was left alone. Intraoperative data and concomitant procedures are shown in Table 2.
Patient follow-up
All patients underwent TTE evaluation before discharge. In total, 170 patients were discharged and followed-up periodically by cardiologists and surgeons. After discharge, examinations were scheduled annually. When annual clinic visits were unavailable, follow-up was performed by contact with the referring cardiologist, the patient or their family. Anticoagulation therapy with an international normalized ratio (INR) target in the range of 2.5–3.0 was prescribed for all patients after surgery. The decision to stop anticoagulation therapy with Coumadin was based on echocardiography (normal left ventricle function, presence of atrial contractility) and Holter data (absence of atrial fibrillation, flutter, tachycardia) after 3 months. Echocardiograms obtained from outside physicians were re-analyzed at our Institute by the most experienced echocardiographers. Clinical follow-up was completed in 165 patients (97.1%), and five patients were lost to follow-up. Follow-up echocardiography was performed 6–12 months after the operation and every year thereafter. Seventy-nine (94.0%) of the 84 discharged patients in the SR group and 78 (90.7%) of the 86 patients in the FB group underwent follow-up TTE. The mean duration from the operation to echocardiographic follow-up was 20.2 (95% confidence interval [CI], 18.6–21.8) months. The mean clinical follow-up period was 24.7 (95% CI, 23.5–27.0) months.
Statistical analysis
Assuming 5% significance (two sided), 80% power, event rates of 20 and 5% in the complete ring and flexible band groups, respectively, and a hazard ratio for significant MR recurrence of 0.23 and expected 5% withdrawal rate, the sample size (total n = 170 in both groups) was calculated using log-rank test (Freedman method) of freedom from MR recurrences in two groups. Data are presented as mean ± SD or median with range. The variables of the two groups were compared using the unpaired t test for continuous variables with normal distribution or the Mann-Whitney U test for other distributions. To analyze the risk factors of early postoperative complications, we reviewed preoperative and intraoperative variables (Tables 1, 2, 3); a multivariate logistic regression model was used to calculate odds ratios (ORs). Estimates of survival and event-free survival for reoperation and the recurrence of significant MR were calculated using the Kaplan-Meier method and are reported with 95% CIs. Patients were censored at the time of reoperation or at the time of death. Estimates are reported with their standard errors. Comparison of the curves was established by using the log-rank test for mid-term results. To analyze risk factors of late mortality, reoperation, and MR recurrence, we reviewed preoperative and intraoperative variables (Tables 1, 2, 3); multivariable Cox proportional hazard regression models were used to calculate HRs. HRs and 95% CIs were calculated. The inclusion criterion for the multivariable model was P value ≤0.2) in the univariable analysis. The significance level in the final “multivariable” model assessed as the 0.05. Stata/MP for Windows v. 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.) was used for the statistical analysis.
Table 3.
Echocardiography data
| SR Group, n = 85 | FB Group, n = 86 | Group comparison, P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | At discharge | Р value at discharge | Follow up, n = 79 | P value | Baseline | At discharge | P value at discharge | Follow up, n = 78 | P value | Baseline | At discharge | Follow up | |
| RA, cm | 5.4 (4.1–8.9) | 4.9 (4.0–7.9) | <0.001 | 4.8 (3.6–7.8) | 0.499 | 5.3 (3.3–8.5) | 4.8 (3.7–6.8) | <0.001 | 4.8 (3.0–7.5) | 0.805 | 0.155 | 0.187 | 0.204 |
| LA, cm | 6.1 (4.3–9.6) | 5.2 (4.4–8.2) | <0.001 | 5.1 (3.5–7.9) | 0.460 | 5.7 (4.4–9.5) | 5.1 (4.0–7.2) | <0.001 | 4.9 (3.2–7.2) | 0.070 | 0.070 | 0.067 | 0.291 |
| S МО (Doppler) cm2 | 3.8 (2.6–6.9) | 3.3 (2.4;7.6) | <0.001 | 3.2 (1.9–5.7) | 0.806 | 4.1 (2.8–9.8) | 3.3 (2.6–4.9) | <0.001 | 3.3 (1.8–4.8) | 0.054 | 0.062 | 0.202 | 0.699 |
| Peak MV pressure gradient, mm Hg | 8.7 (3–24) | 8.8(2.7–17.0) | 0.684 | 8.5 (3.9–17) | 0.206 | 7.9(3.0–22.0) | 7.0 (2.1–15.2) | 0.027 | 6 (2.1–18.0) | 0.212 | 0.680 | <0.001 | <0.001 |
| Mean MV pressure gradient, mm Hg | 2.8 (1–8) | 3.0 (1.0–8.0) | 0.236 | 3.7 (1.3–8.0) | 0.288 | 2.6 (1.0–7.0) | 2.7 (0.9–5.7) | 0.872 | 2.8 (0.6–6.8) | 0.232 | 0.985 | 0.002 | 0.001 |
| Severe MR, n (%) | 85 (100) | 0 | <0.001 | 7 (8.9) | 0.023 | 86 (100) | 0 | <0.001 | 1 (1.3) | 1.0 | 1.0 | 1.0 | 0.031 |
| Total moderate or severe MR, n (%) | 85 (100) | 5 (5.9) | <0.001 | 12 (15.2) | 0.023 | 86 (100) | 4 (4.6) | <0.001 | 7 (9.0) | 0.248 | 1.0 | 0.719 | 0.343 |
| LV EDD, cm | 5.78 ± 0.59 | 5.03 ± 0.53 | <0.001 | 4.82 ± 0.67 | 0.135 | 5.78 ± 0.74 | 5.04 ± 0.52 | <0.001 | 4.83 ± 0.47 | 0.017 | 0.962 | 0.874 | 0.908 |
| LV ESD, cm | 3.49 ± 0.55 | 3.36 ± 0.49 | 0.055 | 3.3 ± 0.7 | 0.731 | 3.51 ± 0.71 | 3.35 ± 0.58 | 0.783 | 3.15 ± 0.46 | <0.001 | 0.815 | 0.891 | 0.263 |
| LV EDV, ml | 172.5 ± 42.0 | 120.9 ± 32.6 | <0.001 | 111.2 ± 42.7 | 0.487 | 169.0 ± 50.0 | 123.4 ± 32.3 | <0.001 | 105 ± 23.1 | 0.001 | 0.624 | 0.618 | 0.375 |
| LV ESV, ml | 53.0 (19–145) | 44.5 (22–135) | <0.001 | 35 (18–102) | 0.130 | 50.5 (16–146) | 45 (20–140) | 0.165 | 38 (18–154) | <0.001 | 0.127 | 0.360 | 0.808 |
| LV EF, % | 65.5 (49–80) | 61 (35–76) | <0.001 | 61 (35–87) | 0.518 | 67.0 (51–89) | 59 (35–78) | <0.001 | 65 (36–78) | <0.001 | 0.212 | 0.247 | 0.043 |
| PA systolic pressure, mm Hg | 48.5 (42;56) | 36 (29–46) | 0.003 | 34.5 (20–68) | 0.080 | 45 (39;54.5) | 36.5 (25–49) | <0.001 | 29.5(8–48) | 0.833 | 0.0n3 | 0.416 | <0.001 |
SR semirigid ring, FB flexible band, RA size of right atrium, LA size of left atrium, S MO mitral orifice area, MV mitral valve, MR mitral regurgitation, LV left ventricle, EDD end diastolic diameter, ESD end systolic diameter, EDV end diastolic volume, ESV end systolic volume, EF ejection fraction, PA pulmonary artery
Availability of data and materials
The data that support the findings of this study are available from Meshalkin National Medical Research Center but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publically available. Data are however available from the authors upon reasonable request and with permission of Meshalkin National Medical Research Center.
Results
Overall repair rate was 95%. The mean (median) cross clamp time did not differ between the SR and FB groups. The subgroup analysis of variances by the surgical approach revealed no differences in cross clamp time between the minimally invasive (25 and 27 patients, respectively, p = 0.171) and median sternotomy subgroups (60 and 59 patients, respectively, p = 0.230).
The incidence of systolic anterior motion (SAM) syndrome was observed in 1 patient in the FB group. Conservative management was not effective and SAM was successfully treated using posterior MV leaflet folding technique. Two patients from SR group were re-repaired due to residual moderate MR revealed by intraoperative TEE.
Intraoperative TEE control revealed that MV coaptation depth was significantly higher in the SR group than the FB group (9 [7–11] vs. 6 [5–8] mm; p = 0.006); however, the FB group had a lower rate of peak and mean transmitral pressure gradients (p < 0.001; Table 2).
There were no early (at 30/60/90 days) deaths. One 65 years old man with p3 prolapse and coronary artery disease who was underwent triangular posterior leaflet resection and concomitant CABG in the SR group died after 6 months of hospital stay because of severe multiple organ failure. Two patients (one from each group) with severe LV systolic dysfunction in early postoperative period required extracorporeal life support with complete recovering after 7 days.
The mean intensive care unit stay was 2 days in both groups (p = 0.453). Ventilation and inotropic support time also did not differ between the groups (Table 4). There were no significant differences in terms of heart failure, prolonged ventilation or requirement of extracorporeal life support. Electrical cardioversion for atrial fibrillation paroxysm was required in 7 (8.2%) and 3 (3.5%) cases in the SR and FB groups, respectively (p = 0.186). Five patients (SR group, 4 vs. FB group, 1; p = 0.169) were re-explored for bleeding on the first postoperative day. Pacemaker implantation rates were 5.9% (due to sinus node dysfunction, 3 patients; complete AV-conductance disturbances, 2 patients) and 4.7% (due to sinus node dysfunction, 3 patients; complete AV-conductance disturbances, 1 patient) in the SR and FB groups, respectively (p = 0.719).
Table 4.
Early (30-days) results
| SR Group, n = 85 |
FB Group, n = 86 |
Р value | |
|---|---|---|---|
| Hospital mortalitya, n (%) | 1 (1.2) | 0 | 0.313 |
| ICU stay, days | 2 (1–14) | 2 (1–14) | 0.453 |
| Ventilation time, h | 7 (0–49) | 5 (1–41) | 0.068 |
| Inotropic support, h | 10 (0–151) | 14 (0–105) | 0.591 |
| Heart failure, n (%) | 23 (27.1) | 19 (22.1) | 0.451 |
| Prolonged ventilation, n (%) | 14 (16.5) | 15 (17.4) | 0.866 |
| ECLS, n (%) | 1 (1.2) | 1 (1.2) | 0.993 |
| AF Early paroxysm, n (%), | 39 (45.9) | 30 (34.9) | 0.143 |
| Electrical cardioversion, n (%) | 7 (8.2) | 3 (3.5) | 0.186 |
| Valve-related complications, n (%): | |||
| MI, n (%) | 5 (5.9) | 3 (3.5) | 0.459 |
| TIA, n (%) | 4 (4.7) | 0 | 0.042 |
| Stroke, n (%) | 0 | 1 (1.2) | 0.319 |
| AKF, n (%) | 5 (5.9) | 4 (4.7) | 0.719 |
| IE, n (%) | 1 (1.2) | 1 (1.2) | 0.993 |
| Ring dehiscence, n (%) | 1 (1.2) | 0 | 0.313 |
| Leak, n (%) | 0 | 0 | – |
| Thrombosis, n (%) | 0 | 0 | – |
| Other embolic events, n (%) | 0 | 0 | – |
| Structural dysfunction, n (%) | 0 | 0 | – |
| Bleeding, n (%); | 9 (10.6) | 6 (7.0) | 0.404 |
| Re-exploration for bleeding, n (%) | 4 (4.7) | 1 (1.2) | 0.169 |
| Lymphorrhea, n (%) | 0 | 1 (1.2) | 0.319 |
| Pacemaker implantation, n (%) | 5 (5.9) | 4 (4.7) | 0.719 |
| Pleural effusion, n (%) | 14 (16.5) | 20 (23.2) | 0.266 |
| Deep sternal infection, n (%) | 0 | 0 | – |
| Superficial infection, n (%) | 1 (1.2) | 1 (1.2) | 0.993 |
| Hospital stay, days | 17 (9–178) | 17 (7–36) | 0.455 |
SR semirigid ring, FB flexible band, ICU intensive care unit, ECLS extracorporeal life support, AF atrial fibrillation, MI myocardial infarction, TIA transient ischemic attack, AKF acute kidney failure, IE infective endocarditis
ahospital death occurred 6 months after surgery
There were no significant between-group differences in major valve-related complications. There were no cases of thromboembolic events, leakage, or structural dysfunction. There were 4 cases of transient ischemic attack with reversible neurologic deficits in the SR group versus none in the FB group (p = 0.042).
The median length of hospital stay after MV repair in the SR and FB groups was 17 (9–178) and 17 (7–36) days, respectively (p = 0.455, Table 4). The preoperative independent predictor of heart failure was left ventricular ejection fraction (LVEF) (OR, 0.94; 95% CI, 0.9–0.98); of myocardial infarction – cardiopulmonary bypass time with 1-min step (OR, 1.03; 95% CI, 1.01–1.1); and prolonged ventilation – preoperative NYHA functional class (OR, 2.6; 95% CI, 1.2–5.7).
Echocardiographic results
All echocardiographic parameters significantly changed from the preoperative to immediate postoperative period at discharge, except transmitral pressure gradients and LV end-systolic diameter (LVESD) (Table 3). There were no significant between-group differences in changes in LV function and left and right atria remodeling; however, transmitral peak and mean pressure gradients were significantly lower in the FB group. The mitral orifice area did not differ between the groups. The TTE before discharge revealed that 5 (5.9%) and 4 (4.7%) patients in the SR and FB groups, respectively, had grade 2 residual MR (p = 0.719). Follow-up echocardiography data at 24 months are shown in Table 3. The repeated-measures analysis of variance in the FB group revealed significant changes in LV end-diastolic diameter (LVEDD), LV end-diastolic volume (LVEDV), and LV end-systolic volume (LVESV) at discharge; moreover, these parameters and LVESD were also decreased at 24 months of follow up. Meanwhile, the LVEF significantly decreased at discharge; however, it significantly increased during the follow-up. In the SR group, there were no significant differences in LV function changes at serial examinations. We also revealed higher transmitral pressure gradients in the SR group, including significant differences in systolic pulmonary artery pressure among patients with sinus rhythm, with favorable results in the FB group.
Survival analysis
At the latest follow-up, 158 patients were alive. There were 3 late deaths among the 84 patients in the SR group (mortality 3.6%), and 4 late deaths among the 81 patients in the FB group (mortality 4.9%). The causes of death in the SR group were endocarditis (1 case), malignancy (1 case), and unknown cause (1 case); two of them were regarded as cardiac-related deaths. The causes of death in the FB group were ischemic stroke (2 cases), acute myocardial infarction (1 case), and pulmonary edema (1 case), all of which were cardiac-related. The Kaplan-Meier survival rates at 2 years were 96.0 ± 2.3% (95% CI, 88.6–98.7%) and 94.3 ± 2.8% (95% CI, 85.5–97.9%) in the SR and FB groups, respectively (Figure 2) (log-rank, p = 0.899).
Fig. 2.
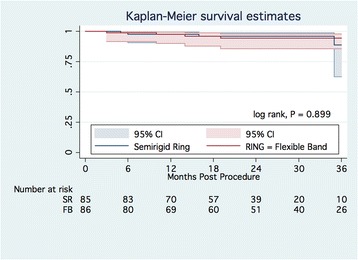
Kaplan-Meier survival estimates. Abbreviation: SR – semirigid ring; CI, confidence interval
The Kaplan-Meier freedom from cardiac-related death at 2 years was 97.7 ± 2.0% (95% CI, 89.1–99.3%) and 94.3 ± 2.8% (95% CI, 85.5–97.9%) in the SR and FB groups, respectively (Additional file 1: Figure S1) (log-rank, p = 0.411). The multivariable Cox regression hazard model did not identify independent risk factors of late death.
Clinical status
Before surgery, 65.9% and 52.3% of patients in the SR and FB groups, respectively, had an NYHA functional classification of III and IV. Most patients demonstrated significant improvement of functional capacity at the last follow-up (43.2% and 34.6% of 81 SR patients and 44.1% and 40.3% of 77 FB patients had an NYHA functional classification of I and II, respectively). A total of 57 (75%) and 61 (84.7%) patients presented with sinus rhythm in the SR and FB groups, respectively (p = 0.201).
Thromboembolism and anticoagulation-related hemorrhage
There was no major bleeding case during follow-up. Two patients form each group had transient ischemic attack. In the FB group, 2 out of 3 patients who had stroke died. Two patients had atrial fibrillation and were kept on anticoagulation therapy, and another one was in sinus rhythm at the last follow up and was not anticoagulated. Central retina occlusion occurred in 1 case in each group. The overall freedom from thromboembolic events at 3 years after MV repair in the SR and FB groups was 94.7 ± 3.6% (95% CI, 80.6–98.7%) and 90.8 ± 3.7% (95% CI, 80.4–95.9%), respectively (log-rank, p = 0.416).
Infective endocarditis
Three patients had infective endocarditis. Two of them were in the SR group, and one of them died because of pacemaker lead endocarditis and heart failure. The 2 patients who survived were treated with antibiotics alone. Freedom from infective endocarditis at 3 years was 97.3 ± 1.9% (95% CI, 89.4–99.3%) and 98.5 ± 1.5% (95% CI, 89.9–99.8%) in the SR and FB groups, respectively (log-rank, p = 0.573).
Recurrence of mitral regurgitation
Moderate or severe MR was observed in 12 (15.2%) of 79 patients and 7 (9.0%) of 78 patients in the SR and FB groups, respectively (p = 0.343). Among them, 3 patients in the SR group underwent redo MV surgery. The Kaplan-Meier estimate for freedom from recurrence of moderate or severe MR at 2 years was 85.3 ± 5.0% (95% CI, 71.9–92.6%) and 95.3 ± 2.7% (95% CI, 85.9–98.5%) in the SR and FB groups, respectively, (log-rank test, p = 0.011) (Figure 3A). The multivariable Cox proportional hazard model identified residual MR before discharge as an independent risk factor of late recurrence of significant MR (HR, 4.1; 95% CI, 1.5–11.5) (Additional file 2: Table S1).
Fig. 3.
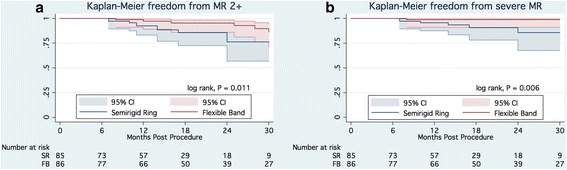
a. Kaplan-Meier freedom from moderate and severe mitral regurgitation. b. Kaplan-Meier freedom from severe mitral regurgitation. Abbreviation: MR, mitral regurgitation; SR – semirigid ring; CI, confidence interval
Among them, severe MR was observed in 7 (9.7%) and 1 (1.3%) patient(s) in the SR and FB groups, respectively. The Kaplan-Meier estimate for freedom from severe MR at 2 years was 84.8 ± 6.8% (95% CI, 65.1–93.8%) and 98.7 ± 1.3% (95% CI, 90.8–99.8%) in the SR and FB groups, respectively (log-rank test, p = 0.006) (Figure 3B). The multivariable Cox proportional hazard model identified residual MR before discharge as an independent risk factor of late recurrence of severe MR (HR, 8.4; 95% CI, 1.6–43.3).
Reoperations
Reoperations were required in 3 patients in the SR group for partial ring dehiscence in all cases. The MV was re-repaired in 2 patients and replaced in 1 case. The Kaplan-Meier estimate for freedom from reoperation at 2 years of follow-up was 97.0 ± 2.1% (95% CI, 88.4–99.3%) and 100% in the SR and FB groups, respectively (Figure 4) (log-rank, p = 0.044). The Cox regression hazard model did not identify any preoperative or intraoperative risk factor of late reoperations.
Fig. 4.
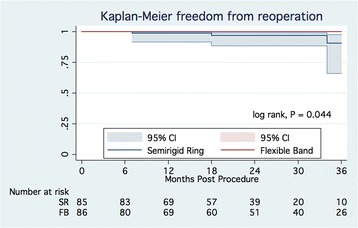
Kaplan-Meier freedom from reoperations. Abbreviation: SR – semirigid ring; CI, confidence interval
Discussion
In the late 1960’s, Carpentier developed annuloplasty rings, considered the “gold standard” for the surgical treatment of MR [11]. Subsequent studies showed that the MV annulus continually changes size and shape during the cardiac cycle [12–14]. This led to the development of a flexible ring that could conform to the physiologic changing annular shape [15, 16]. However, there is controversy regarding the optimal mechanical characteristics of annuloplasty rings and the use of rigid rings or flexible bands in degenerative MV disease [5, 17].
David et al. [18] conducted one of the first randomized studies comparing the rigid ring (13 patients) and flexible ring (12 patients). The results showed significantly better LV systolic function and stroke volume/LVEDV index in the flexible group and no significant reduction in LVESV and LVESD in the rigid ring group. However, the clinical outcomes of these 2 ring types have not been reported. In the present study, there were similar results in LV changes with no significant differences between the SR and FB groups intermediately and 2 years postsurgery. However, only the FB group showed a significant reduction in LVEDD, LVESD, LVEDV, and LVESV (when comparing postoperative values and those 2 years postsurgery).
Chang et al. conducted the largest randomized trial (363 patients) comparing the Carpentier-Edwards rigid ring and flexible Duran annuloplasty ring [19]. The mean duration from the operation to follow-up echocardiography was 26.7 ± 24.1 months. LVEF, LVESD, and LVEDD parameters changed significantly at serial examinations in both groups with no significant difference between the two ring types. There were no significant differences in survival, reoperation rates, and recurrences of significant MR at a mean follow-up of 46.6 ± 32.6 months. However, their study was limited by the heterogeneous MR etiology, the long recruitment period, and the fact that the echocardiography parameters were not presented at separate time points, which may have caused bias.
Shahin et al. [20] conducted a randomized study comparing the Carpentier-Edwards rigid Classic and semi-flexible Physio rings (mean follow up, 5.1 years). There were no between-groups differences in terms of mortality, morbidity, and LV function. However, an unexplained 16% difference in mortality was considered clinically important.
At least 3 randomized studies did not demonstrate the clinical benefits of flexible or rigid types of annuloplasty devices [18–20]. However, one of them [18] suggested better LV systolic function with flexible rings. Similar results were reported by Okada et al. [21], and concern that rigid rings could restrict LV wall motion was raised.
In a prospective echocardiographic study by Unger-Graeber et al. [22], no significant difference was shown between the Carpentier rigid ring and flexible Duran ring in terms of transmitral velocity and pressure gradient. In contrast, our study showed higher transmitral pressure gradients in the SR group.
After David et al. criticized the rigid ring for impaired LV function [18] and Kreindel et al. [23] reported the potential risk of SAM syndrome after rigid ring annuloplasty, Carpentier et al. introduced a new concept of mitral annuloplasty with the semirigid prosthetic ring, which combines remodeling and flexibility [24]. At mid-term follow-up between 6 and 18 months, 93.2% of 94 followed-up patients were free from MR recurrence with a transmitral pressure gradient at 3.55 ± 1.93 mmHg. Thereafter, excellent mid-term results with semirigid rings have been reported by other investigators [25, 26]. Since then, the concept of a semirigid ring has gained popularity, and the semirigid ring (instead of the rigid ring) has been successfully adopted in mitral annuloplasty for degenerative MV disease. Flexible posterior annuloplasty bands have also shown high effectiveness in preserving mitral annulus flexibility and provide good mid-term and long-term durability [4, 27, 28].
The semirigid ring and flexible ring have not been sufficiently compared. An animal randomized study [29] showed that LV function was not altered with either flexible or semirigid ring annuloplasty. A Japanese, retrospective, propensity score matched study [30] evaluated intermediate echocardiography results only. The overall cohort’s LVEF decreased during the first week after surgery and then recovered gradually at 6 months and 1 year; LVEDD abruptly decreased and LVESD minimally decreased at the first week postoperatively, then gradually decreased at 6 months and later stabilized. There were no significant between-group differences in LVEF, LVEDD, and LVESD. They suggested that the semirigid ring might prevent LV impairment compared with the rigid ring. However, their study was limited by the retrospective design, low rate of followed-up patients, and difference in annuloplasty devices used in the flexible group.
Previously was shown that experience in mitral valve repair is an important determinant of operative efficiency and late survival [31], in our study both surgeons are well experienced and evenly split between groups. However, our results seem different to Castillo JG report [32]. We assume that limited experience in Barlow valves disease was influenced for our results. In this view complex mitral valve cases should be consolidated and addressed to one surgeon. It might be helpful to use scoring system [33] to allow stratification of complexity for degenerative mitral valve repair for improving results and develop local expertise.
We examined the immediate and mid-term results of patients with degenerative MV disease who underwent primary MV repair with a complete semirigid ring or flexible posterior annuloplasty band. Both groups had comparable early clinical results; however, the SR group had better coaptation depth, while the FB group demonstrated significantly lower transmitral pressure gradients. At serial echocardiographic examinations, only the FB group showed significant LV remodeling. Consistent with previous studies, there were no between-group differences in overall survival, freedom from cardiac-related death, and follow-up LV remodeling. Our study is the first to show the superiority of the flexible posterior band over the semirigid complete ring in terms of freedom from recurrences of significant and/or severe MR and risk of MV reoperation.
Conclusion
The present study is limited by the 2-year follow-up period and single-center design. Important limitation is low number of patients at late risk. Further study with a longer follow-up is warranted. In conclusion, patients with degenerative MV disease may benefit from valve repair with flexible bands. Residual MR is an independent risk factor of late insufficiency recurrence.
Acknowledgements
We would like to thank Editage for their editorial support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this study are available from Meshalkin National Medical Research Center but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publically available. Data are however available from the authors upon reasonable request and with permission of Meshalkin National Medical Research Center.
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- ITT
Intention-to-treat
- LV
Left ventricle
- LVEDD
Left ventricle end-diastolic diameter
- LVEDV
Left ventricle end-diastolic volume
- LVEF
Left ventricular ejection fraction
- LVESD
Left ventricle end-systolic diameter
- LVESV
Left ventricle end-systolic volume
- MR
Mitral regurgitation
- MV
Mitral valve
- NYHA
New York Heart Association
- OR
Odds ratio
- SAM
Systolic anterior motion
- TEE
Transesophageal echocardiography
Additional file
Kaplan-Meier freedom from cardiac-related death. Abbreviation: CI, confidence interval. (TIFF 1293 kb)
Cox proportional hazard model for recurrence of significant MR. (DOCX 15 kb)
Authors’ contributions
Please contact author for data requests. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Study was approved by local Ethical Committee at Aughust 1, 2011, approval number PA – 112.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13019-017-0679-0) contains supplementary material, which is available to authorized users.
Contributor Information
Alexandr V. Bogachev-Prokophiev, Email: bogachev.prokophiev@gmail.com
Alexandr V. Afanasyev, Phone: +7 (913) 754 4245, Email: av.afanasyev@icloud.com
Sergei I. Zheleznev, Email: jsi1962@mail.ru
Vladimir M. Nazarov, Email: vm_nazarov@mail.ru
Ravil M. Sharifulin, Email: ravil-sharifulin@rambler.ru
Alexandr M. Karaskov, Email: kam@meshalkin.ru
References
- 1.Lillehei CW, Gott VL, Dewall RA, Varco RL. Surgical correction of pure mitral insufficiency by annuloplasty under direct vision. J Lancet. 1957;77:446–449. [PubMed] [Google Scholar]
- 2.Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 3.Filsoufi F, Carpentier A. Principles of reconstructive surgery in degenerative mitral valve disease. Semin Thorac Cardiovasc Surg. 2007;19:103–110. doi: 10.1053/j.semtcvs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Brown ML, Schaff HV, Li Z, Suri RM, Daly RC, Orszulak TA. Results of mitral valve annuloplasty with a standard-sized posterior band: is measuring important? J Thorac Cardiovasc Surg. 2009;138:886–891. doi: 10.1016/j.jtcvs.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Zhao Q. Systematic evaluation of the flexible and rigid annuloplasty ring after mitral valve repair for mitral regurgitation. Eur J Cardiothorac Surg. 2011;40:480–487. doi: 10.1016/j.ejcts.2010.11.080. [DOI] [PubMed] [Google Scholar]
- 6.Wan S, Lee AP, Jin CN, Wong RH, Chan HH, Ng CS, et al. The choice of mitral annuloplastic ring-beyond “surgeon’s preference”. Ann Cardiothorac Surg. 2015;4:261–265. doi: 10.3978/j.issn.2225-319X.2015.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31:1958–1967. doi: 10.1093/eurheartj/ehq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the Management of Patients with Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 9.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2. Mitral and tricuspid regurgitation (native disease) Eur J Echocardiogr. 2010;11:307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 10.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grukemeier GL, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg. 2008;85(4):1490–1495. doi: 10.1016/j.athoracsur.2007.12.082. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A. Reconstructive valvuloplasty. A new technique of mitral valvuloplasty. Presse Med. 1969;77:251–253. [PubMed] [Google Scholar]
- 12.Glasson JR, Komeda M, Daughters GT, Niczyporuk MA, Bolger AF, Ingels NB, et al. Three-dimensional regional dynamics of the normal mitral anulus during left ventricular ejection. J Thorac Cardiovasc Surg. 1996;111:574–585. doi: 10.1016/S0022-5223(96)70309-4. [DOI] [PubMed] [Google Scholar]
- 13.Grewal J, Suri R, Mankad S, Tanaka A, Mahoney DW, Schaff HV, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121:1423–1431. doi: 10.1161/CIRCULATIONAHA.109.901181. [DOI] [PubMed] [Google Scholar]
- 14.Lansac E, Lim KH, Shomura Y, Goetz WA, Lim HS, Rice NT, et al. Dynamic balance of the aortomitral junction. J Thorac Cardiovasc Surg. 2002;123:911–918. doi: 10.1067/mtc.2002.121286. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove DM, Arcidi JM, Rodriguez L, Stewart WJ, Powell K, Thomas JD. Initial experience with the Cosgrove-Edwards Annuloplasty system. Ann Thorac Surg. 1995;60:499–503. doi: 10.1016/0003-4975(95)00458-W. [DOI] [PubMed] [Google Scholar]
- 16.Dagum P, Timek T, Green GR, Daughters GT, Liang D, Ingels NB, Jr, et al. Three-dimensional geometric comparison of partial and complete flexible mitral annuloplasty rings. J Thorac Cardiovasc Surg. 2001;122:665–673. doi: 10.1067/mtc.2001.116313. [DOI] [PubMed] [Google Scholar]
- 17.Chee T, Haston R, Togo A, Raja SG. Is a flexible mitral annuloplasty ring superior to a semi-rigid or rigid ring in terms of improvement in symptoms and survival? Interact Cardiovasc Thorac Surg. 2008;7:477–484. doi: 10.1510/icvts.2007.174243. [DOI] [PubMed] [Google Scholar]
- 18.David TE, Komeda M, Pollick C, Burns RJ. Mitral valve annuloplasty: the effect of the type on left ventricular function. Ann Thorac Surg. 1989;47:524–527. doi: 10.1016/0003-4975(89)90426-8. [DOI] [PubMed] [Google Scholar]
- 19.Chang B, Youn Y, Ha J, Lim S, Hong Y, Chung N. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg. 2007;133(4):995–1003. [DOI] [PubMed]
- 20.Shahin GM, van der Heijden GJ, Bots ML, Cramer MJ, Jaarsma W, Gadellaa JC, et al. The Carpentier-Edwards classic and Physio mitral annuloplasty rings: a randomized trial. Heart Surg Forum. 2005;8:E389–E394. doi: 10.1532/hsf.419. [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Shomura T, Yamaura Y, Yoshikawa J. Comparison of the Carpentier and Duran prosthetic rings used in mitral reconstruction. Ann Thorac Surg. 1995;59:658–662. doi: 10.1016/0003-4975(94)01008-0. [DOI] [PubMed] [Google Scholar]
- 22.Unger-Graeber B, Lee RT, Sutton MS, Plappert M, Collins JJ, Cohn LH. Doppler echocardiographic comparison of the Carpentier and Duran anuloplasty rings versus no ring after mitral valve repair for mitral regurgitation. Am J Cardiol. 1991;67:517–519. doi: 10.1016/0002-9149(91)90014-C. [DOI] [PubMed] [Google Scholar]
- 23.Kreindel MS, Schiavone WA, Lever HM, Cosgrove D. Systolic anterior motion of the mitral valve after Carpentier ring valvuloplasty for mitral valve prolapse. Am J Cardiol. 1986;57:408–412. doi: 10.1016/0002-9149(86)90762-9. [DOI] [PubMed] [Google Scholar]
- 24.Carpentier AF, Lessana A, Relland JY, Belli E, Mihaileanu S, Berrebi AJ, et al. The “physio-ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg. 1995;60:1177–1185. doi: 10.1016/0003-4975(95)00753-8. [DOI] [PubMed] [Google Scholar]
- 25.Raffoul R, Uva MS, Rescigno G, Belli E, Scorsin M, Pouillart F, et al. Clinical evaluation of the Physio annuloplasty ring. Chest. 1998;113:1296–1301. doi: 10.1378/chest.113.5.1296. [DOI] [PubMed] [Google Scholar]
- 26.Accola KD, Scott ML, Thompson PA, Palmer GJ, Sand ME, Ebra G. Midterm outcomes using the physio ring in mitral valve reconstruction: experience in 492 patients. Ann Thorac Surg. 2005;79:1276–1283. doi: 10.1016/j.athoracsur.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 27.Gillinov AM, Cosgrove DM, 3rd, Shiota T, Qin J, Tsujino H, Stewart WJ, et al. Cosgrove-Edwards Annuloplasty system: midterm results. Ann Thorac Surg. 2000;69:717–721. doi: 10.1016/S0003-4975(99)01543-X. [DOI] [PubMed] [Google Scholar]
- 28.Chitwood WR, Rodriguez E, Chu MW, Hassan A, Ferguson TB, Vos PW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg. 2008;136:436–441. doi: 10.1016/j.jtcvs.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Green GR, Dagum P, Glasson JR, Daughters GT, Bolger AF, Foppiano LE, et al. Semirigid or flexible mitral annuloplasty rings do not affect global or basal regional left ventricular systolic function. Circulation. 1998;98:II128–II135. [PubMed] [Google Scholar]
- 30.Manabe S, Kasegawa H, Fukui T, Tabata M, Shinozaki T, Shimokawa T, et al. Do semi-rigid prosthetic rings affect left ventricular function after mitral valve repair? Circ J. 2013;77:2038–2042. doi: 10.1253/circj.CJ-12-1600. [DOI] [PubMed] [Google Scholar]
- 31.Burt BM, ElBardissi AW, Huckman RS, Cohn LH, Cevasco MW, Rawn JD, et al. Influence of experience and the surgical learning curve on long-term patient outcomes in cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:1061–68.e3. doi: 10.1016/j.jtcvs.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 32.Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–312. doi: 10.1016/j.jtcvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 33.Anyanwu AC, Itagaki S, Chikwe J, El-Eshmawi A, Adams DH. A complexity scoring system for degenerative mitral valve repair. J Thorac Cardiovasc Surg. 2016;151:1661–1670. doi: 10.1016/j.jtcvs.2016.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Meshalkin National Medical Research Center but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publically available. Data are however available from the authors upon reasonable request and with permission of Meshalkin National Medical Research Center.
The data that support the findings of this study are available from Meshalkin National Medical Research Center but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publically available. Data are however available from the authors upon reasonable request and with permission of Meshalkin National Medical Research Center.


