Bcl-2 is the founding member of a large family of proteins that regulate cell survival and cell death. Since its discovery in B-cell lymphomas over 30 years ago, research on the biological functions and mechanisms of action of Bcl-2 family proteins in controlling cell life and death decisions has revealed important roles in multiple diseases, paving the way for at least one novel therapeutic agent.1, 2 In this issue of Cell Death and Differentiation, several of the world’s leaders in research on Bcl-2 family proteins provide complementary reviews covering multiple facets of this intriguing class of proteins, ranging from cellular and molecular mechanistic insights about how these proteins work and addressing their roles in evolution of programmed cell death mechanisms, mammalian development, cancer genetics, cancer biology and applications towards novel cancer therapies.3, 4, 5, 6, 7, 8, 9 With the recent approval by FDA of the orally bioavailable and highly selective Bcl-2 inhibitor, venetoclax, for some subtypes of chronic lymphocytic leukemia (CLL), Bcl-2 family proteins are now validated as promising drug targets, with the potential to provide significant advances in the standard of care for patients suffering from oncological maladies and possibly for certain non-oncological diseases as well.
The story of Bcl-2 began with discovery of the gene’s involvement in chromosomal translocations found in non-Hodgkin’s lymphomas (NHLs) by the Croce group. Most low-grade NHLs contain a t(14;18) chromosomal translocation that places the BCL-2 gene on chromosome 18 into juxtaposition with the immunoglobulin heavy-chain locus on chromosome 14, thus driving high levels of BCL-2 gene expression in B cells. These NHLs are termed ‘low grade’ because the B cells accumulate without having a high proliferative rate, which was an early clue that BCL-2 operated in a manner distinct from previously discovered oncogenes (MYC, RAS, etc.) by prolonging cell lifespan rather than by driving cell division. Croce and co-workers3 review the journey that led to their discovery of BCL-2. They also explain how they uncovered the mystery of why CLL B cells express very high levels of Bcl-2 mRNA and protein without genetic changes in the BCL-2 gene structure or sequence.10 For CLL, they homed in on 13q14 chromosomal deletions found in the majority of CLLs, leading them to discover the first example of genetic loss of microRNA-encoding genes that serve as tumor suppressors. Croce and co-workers describe how deletion and mutation of the gene cluster encoding miR15 and miR16 relieves Bcl-2 mRNA from silencing and explains at least in part the high levels of Bcl-2 expression seen in most CLLs. Interestingly, the Croce group’s investigations knocking out homologs of miR15/16 in mice suggest that these microRNAs oppose neoplasia by doing more than suppressing Bcl-2 expression, presumably via effects on additional target genes. Moreover, they discovered a homologous additional microRNA gene cluster (mir15b/mir16.2) that when knocked out in mice also causes accumulation of CD5+ B-lymphocytes, but has little effect on Bcl-2 expression, implicating other target genes.
Adams and Cory4 review the seminal work from their laboratories that firmly established the antiapoptotic function of the Bcl-2 protein, thus revealing the world’s first example of an anti-apoptotic oncogene. They emphasize how enforced Bcl-2 expression overcomes the proapoptotic influence of oncogenic stress, caused by dysregulation of oncogenes such as C-MYC and CYCLIN-D1 (aka, BCL-1) that drive cellular proliferation. Consequently, malignant cells are often dependent on (addicted to) Bcl-2 or other antiapoptotic proteins to protect them from the apoptotic drive of oncogenic stress, thus illustrating why malignant cells should be selectively vulnerable to agents that inhibit Bcl-2 and thereby conferring a reasonable therapeutic index. They cite a recent example of Bcl-2 addiction in the case of acute myelogenous leukemias that harbor IDH1 (isocitrate dehydrogenase-1) gene mutations.
The anti-apoptotic role of Bcl-2 has also been linked to chemoresistance, making it more difficult to kill malignant cells with cytotoxic anticancer drugs.11 Montero and Letai8 review their work on dynamic BH3 (Bcl-2 homology 3) profiling as a means of ascertaining how ‘primed’ cancer cells are to undergo apoptosis, which correlates with clinical chemoresistance. The concept is that endogenous antagonists of the antiapoptotic Bcl-2 family proteins (such as Bad, Bim, Bid, Puma, Noxa, Bcl-G, etc.) bind via an amphipathic α-helical domain called BH3 and thus synthetic BH3 peptides titrated into cancer cells can reveal how close or how far cells are from being driven into apoptosis. Dynamic BH3 profiling has shown correlations with clinical responses (tumor shrinkage) for leukemias and some types of solid tumors but the predictivity of this method for longer-term patient outcomes (disease-free or overall survival) merits further investigation. Also, because in vitro analysis of isolated leukemia and cancer cells fails to recapitulate the complexity of the in vivo tumor microenvironment, the dynamic BH3 profiling method may be challenging to extend to solid tumors.
The complex network of BH3-mediated interactions among anti- and pro-apoptotic Bcl-2 family proteins is covered by several of the review articles, as well as current understanding of the molecular mechanisms that couple Bcl-2 family proteins to mitochondria-initiated pathways for cell death.4, 5, 6, 7, 8, 9 The canonical view, shared largely by the authors, envisions that Bax and Bak (proapoptotic proteins that share overall sequence and structural similarity to Bcl-2) oligomerize in the mitochondrial outermembrane to form chains whose ends eventually connect to create lipidic pores that release apoptogenic proteins into the cytosol (cytochrome c, SMAC, Htra2, etc.), thereby triggering a downstream cascade of cell death protease (caspase) activity. Bcl-2 and its antiapoptotic cousins binding to Bax and Bak prevent the oligomerization to preserve mitochondrial integrity, unless displaced by BH3-containing pro-apoptotic proteins. Structural and biophysical information about Bcl-2 family proteins in solution and in membranes is reviewed by Andrews and co-workers,5 highlighting several of the unresolved questions. Green and Kalkavan6 also cover the question of diversity of mechanisms for promoting Bax/Bak oligomerization in mitochondrial membranes, referencing their work on lipid mediators derived from the sphingomyelin pathway – in addition to the well-defined role for BH3-containing agonists of Bax/Bak (such as Bid and Bim) that operate apparently through a hit-and-run mechanism to trigger Bax and Bak oligomerization in membranes.
Green and Kalkavan also described the role of Bok,6 a protein with sequence and structural similarity to Bax and Bak, that appears to constitutively oligomerize in mitochondrial membranes if allowed to accumulate to sufficient levels, which has been documented to occur in the context of overwhelming accumulation of unfolded proteins that exhaust the capacity of the ERAD pathway. Interestingly, anti-apoptotic Bcl-2 family proteins appear to be incapable of interfering with Bok, suggesting perhaps opportunities to bypass Bcl-2-mediated blockade of apoptosis in cancers or to restore apoptosis in cancers that have impaired expression or activity of Bax and Bak – which is well documented in human malignancies. The ERAD-based mechanism of Bok ‘activation’ conceivably could also contribute to the promising clinical activity observed for proteasome inhibitors combined with venetoclax for myeloma treatment.1
While Bcl-2 family protein regulation of mitochondrial outer membrane permeability represents their paramount function, unresolved questions about non-mitochondrial roles for Bcl-2 family proteins are also lightly touched upon in reviews offered in this issue by Andrews and co-workers.5 Several of the Bcl-2 family proteins can associate with proteins within membranes of the endoplasmic reticulum, impacting intracellular Ca2+ regulation, which can have impact on cell proliferation (note that Bim-knockout mice have a defect in lymphocyte proliferation5), mitochondrial bioenergetics through endoplasmic reticulum/mitochondrial Ca2+ coupling, and cell survival. Scarcely touched upon by the reviews is the well-established role for Bcl-2 and several anti-apoptotic Bcl-2 family members in suppression of autophagy, a cellular mechanism potentially important in cancer cell survival in nutrient depleted microenvironments. Overall, how relevant these non-mitochondrial functions are to the biology of Bcl-2 family proteins is debated but conceivably could vary depending on cellular context (e.g. postmitotic neurons versus tumor cells). Interestingly, in their review about roles of Bcl-2 family proteins from an evolutionary perspective,8 Strasser and Vaux7 point out that in the nematode (Caenorhabditis elegans), the Bcl-2 homolog Ced-9 does not regulate mitochondrial outer membrane permeability. Thus, alternative roles for Bcl-2 family proteins are relevant in some species.
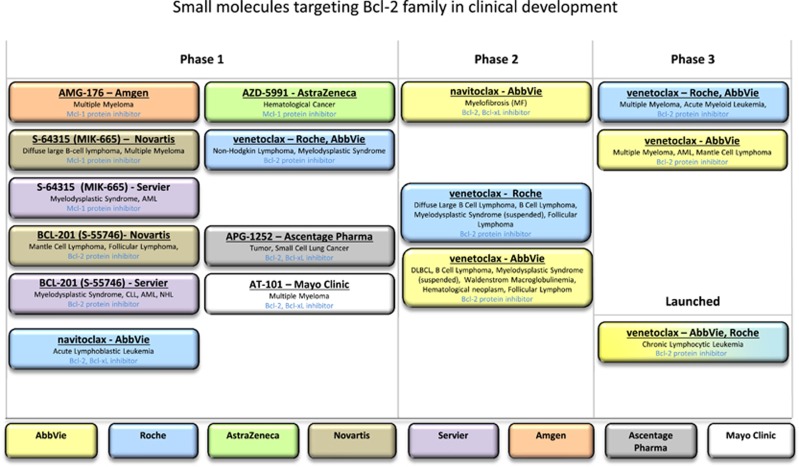
Several of the articles in this special issue of Cell Death and Differentiation review to greater or lesser extent the efforts to create synthetic chemical mimics of BH3 peptide as therapeutic agents for cancer. These efforts have culminated thus far in one FDA-approved drug, venetoclax, an oral selective small-molecule inhibitor of Bcl-2 that lacks significant activity against other anti-apoptotic members of the human Bcl-2 family (Bcl-XL, Mcl-1, Bfl-1, Bcl-W, Bcl-B), with several additional molecules in development (Figure 1). Unfortunately, no contribution appears in this special issue of Cell Death and Differentiation from members of the industry-led teams whose research and development led to the discovery of venetoclax, the world’s first Bcl-2-inhibiting drug.13 That journey represents a milestone in drug discovery science, in that it entailed the first successful application of the so-called SAR by NMR (structure–activity relation by nuclear magnetic resonance) technology to tackle a very challenging protein–protein interaction target, an accomplishment recently recognized with the 2017 Prix Galien.14 In addition to the countless scientific, technical, medical and other professionals who contributed to that drug, Abbvie management also deserves praise for allowing protracted investment in the Bcl-2 inhibitor program over a time frame that few companies would have courage to continue.
Figure 1.
Molecules in clinical development that target Bcl-2 family proteins. Based on data available at www.clinictrials.gov, the figure indicates molecules targeting a Bcl-2 family protein that are currently in clinical development according to their phase of development. The sponsors of the molecules are indicated by color, as shown at the bottom
Montero and Letai8 reviewed some of the efforts to go beyond Bcl-2 to discover and develop small-molecule inhibitors of other Bcl-2 family proteins, particularly Mcl-1. Bcl-2 inhibitors show monotherapy activity only in some types of malignancy and preclinical studies have clearly demonstrated a role for Mcl-1 in resistance to Bcl-2-targeting compounds. Small molecules targeting Mcl-1 are currently in early clinical development (Figure 1). In their review on the role of Bcl-2 family proteins in mammalian development, Opferman and Kothari9 point out that mcl-1 gene ablation in mice results in compromised survival of hematopoietic stem cells and neutrophils, unlike bcl-2 gene inactivation. This raises the question of whether Mcl-1-targeting compounds will provide a reasonable therapeutic index. Challenges with therapeutic index were previously encountered with compounds that target Bcl-XL (navitoclax), due to Bcl-XL’s requirement for platelet stability. While preclinical assessment of Mcl-1-inhibiting compounds has provided hope, the structural differences in the BH3-binding pockets of rodent versus human Mcl-1 proteins may underestimate the impact on hematopoiesis. In the case of Bcl-2, mouse gene knockout studies faithfully predicted the lymphopenia seen in the clinic with molecules such as venetoclax, but not the neutropenia that has been observed. Mice lacking Bcl-2 suffer from postnatal loss of spinal motor neurons, sensory neurons (DRGs) and sympathetic neurons, but venetoclax does not appreciably cross the blood–brain barrier, and neurotoxicity has heretofore not been an issue.
Alternatives to directly inhibiting Mcl-1 or other antiapoptotic Bcl-2 family proteins with small molecules are highlighted in the reviews by Adams and Cory4 and by Montero and Letai.8 For example, impressive phase 3 data were recently announced for relapsed/refractory CLL patients treated with anti-CD20 antibody rituximab in combination with venetoclax versus rituximab combined with the bendamustine (standard of care). In this regard, rituximab has been reported to downregulate Mcl-1 protein expression in circulating CLL cells in patients,15 which could enhance sensitivity to selective Bcl-2 inhibitors. Promising clinical responses have also been reported for venetoclax combined with other drugs that can downregulate Mcl-1 expression (as well as upregulate expression of proapoptotic BH3 proteins) – such as observed for DNA-hypomethylating agents combined with venetoclax in AML patients.
Although taking over 30 years from Bcl-2’s discovery to an approved therapeutic, it is early days for understanding how to optimally and fully exploit inhibitors of Bcl-2 (or other members of the Bcl-2 family) for treatment of human diseases. Venetoclax monotherapy and particularly certain venetoclax drug combinations have shown tremendous promise for improving the standard of care for hematological malignancies, but applications to solid tumors have been slower to materialize. Given the current interest in cancer immunotherapy, it will also be important to understand how Bcl-2 inhibitors impact the immune system – given the role for Bcl-2 in maintaining lymphocyte survival. Applications of Bcl-2 inhibitors to autoimmune diseases also merit investigation, given intriguing connections such as the autoimmune manifestations that have been described in Bcl-2 transgenic mice,7 the lupus-like syndrome observed in NZB/NZB mice, which harbor mutations in the miR15/16 gene cluster that suppresses Bcl-2 expression,3 and evidence of alternations in the expression of Bcl-2 family protein in lymphocytes of patients with various autoimmune disorders.16 Also, in humans, some anti-apoptotic Bcl-2 family members are predominantly or more highly expressed in immune cells (e.g., Bfl-1; Bcl-B) among all tissues,17 suggesting they may serve as future drug targets for autoimmunity. By analogy to the way that some anti-cancer drugs such as methotrexate have been repurposed for autoimmunity using lower dose schedules, one wonders whether low-dose (or short schedule) venetoclax could find a role in treatment of autoimmune disorders. The history with anti-CD20 monoclonal antibodies provides a possible roadmap how to extend applicability from lymphoma and leukemia into rheumatoid arthritis, multiple sclerosis, transplant and other non-oncological indications. Given the recent progress, the next 30 years of clinical research on Bcl-2 family inhibitors promises to be as exciting and rewarding as the prior 30 years of basic and translational research on these amazing proteins.
Footnotes
The author declares no conflict of interest.
References
- Ruefli-Brasse A, Reed JC. Therapeutics targeting Bcl-2 in haematological malignancies. Biochem J 2017474: 3643–3657. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A et al. Nat Rev Drug Discov 2017; 16: 273–284. [DOI] [PubMed]
- Pekarsky Y, Balatti V, Croce CM. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Kale J, Osterlund EJ, Andrews DW. Bcl-2 family proteins: changing partners in the dance towards death.. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Kalkavan H, Green DR. MOMP, cell suicide as a BCL2 family business. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Strasser A, Vaux DL. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ 2017. (in press). [DOI] [PMC free article] [PubMed]
- Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. BCL-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood 1993; 82: 1820–1828. [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res 1992; 52: 5407–5411. [PubMed] [Google Scholar]
- Reed JC. Priming cancer cells for death. Science 2011; 334: 1075–1076. [DOI] [PubMed] [Google Scholar]
- Souers AJ et al. Nat Med 2013; 19: 202–208. [DOI] [PubMed]
- Erlanson DA, Fesik SW, Hubbard RE, Jahnke W, Jhoti H. Twenty years on: the impact of fragments on drug discovery. Nat Rev Drug Discov 2016; 15: 605–619. [DOI] [PubMed] [Google Scholar]
- Byrd JC et al. The mechanism of tumor cell clearance by Rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 2002; 99: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Tischner D, Woess C., Ottina E, Villunger A. Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Differ 2010; 1: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Ha H et al. Expression of Bfl-1 in normal and tumor tissues: Bfl-1 overexpression in cancer is attributable to its preferential expression in infiltrating inflammatory cells. Hum Pathol 1998; 29: 723–728. [DOI] [PubMed] [Google Scholar]