Abstract
Background
The current US Environmental Protection Agency (EPA) maximum contaminant level (MCL) for arsenic in public water systems (10 µg/L) took effect in 2006. Arsenic is not federally regulated in private wells. The impact of the 2006 MCL on arsenic exposure in the US, as confirmed through biomarkers, is presently unknown. We evaluated national trends in water arsenic exposure in the US, hypothesizing that urinary arsenic levels would decrease over time among participants using public water systems but not among those using well water. We further estimated the expected number of avoided lung, bladder, and skin cancer cases.
Methods
We evaluated 14,127 participants in the National Health and Nutrition Examination Survey (NHANES) 2003–2014 with urinary dimethylarsinate (DMA) and total arsenic available. To isolate water exposure, we expanded a residual-based method to remove tobacco and dietary contributions of arsenic. We applied EPA risk assessment approaches to estimate the expected annual number of avoided cancer cases comparing arsenic exposure in 2013–2014 vs. 2003–2004.
Findings
Among public water users, fully adjusted geometric means (GMs) of DMA decreased from 3.01 µg/L in 2003–2004 to 2.49 µg/L in 2013–2014 (17% reduction; 95% confidence interval 10%, 24%; p-trend<0.01); no change was observed among well water users (p-trend= 0.35). Assuming these estimated exposure reductions will remain similar across a lifetime, we estimate a reduction of 200 to 900 lung and bladder cancer cases per year depending on the approach used.
Interpretation
The decline in urinary arsenic among public water but not private well users in NHANES 2003–2014 indicates that the implementation of the current MCL has reduced arsenic exposure in the US population. Our study supports prior work showing that well water users are inadequately protected against drinking water arsenic, and confirms the critical role of federal drinking water regulations in reducing toxic exposures and protecting human health.
Funding
This work was supported by the National Institute of Environmental Health Sciences (1R01ES025216, R01ES021367, 5P30ES009089 and P42ES010349). A. E. Nigra was supported by 5T32ES007322.
Introduction
Arsenic is an established carcinogen naturally occurring in drinking water across the United States.1 For decades, the US Environmental Protection Agency (EPA) maximum contaminant level (MCL) in public water systems was 50 µg arsenic/L. The current arsenic MCL of 10 µg/L took effect in January 2006. However, the compliance determination process allowed additional time to test and address noncompliance for public water systems with mean annual arsenic levels exceeding 10 µg/L, based on quarterly samples. Private wells, the main source of drinking water for approximately 45.5 million Americans, however, are not enforced under the arsenic MCL.2 In 2000, EPA estimated that the excess population risk of lung and bladder cancer at water arsenic concentrations of 50 µg/L were between 1 in 100 and 1 in 300.3 The impact of the 2006 change in the MCL on individual arsenic exposure in the United States is unknown.
In our primary analysis, we used data from the National Health and Nutrition Examination Survey (NHANES) to evaluate national trends in water arsenic exposure. We hypothesized that urinary arsenic levels would decrease over time among NHANES participants on public water systems but not among those on well water. The public NHANES database does not allow public access to geographical information of the participants. Mexican-Americans in the United States, however, are more likely to live in the Southwest.4 In the Southwest, many cities’ public water supplies are based upon water sources with naturally occurring arsenic above the MCL (e.g. Los Angeles, Albuquerque, Scottsdale, and Tucson) and the enactment of the current MCL has resulted in infrastructure investments to ensure water arsenic below 10 µg/L.5 We therefore hypothesized that the decline in urinary arsenic levels would be more pronounced among Mexican-American NHANES participants. Additionally, we estimated the expected number of avoided skin, lung, and bladder cancer cases assuming exposure reductions persisted across the lifetime.
Methods
Study population and urine arsenic measurements
We analyzed data from the 2003–2014 cycles of NHANES, a nationally-representative sample of the general non-institutionalized US population.6 All NHANES protocols were approved by the NCHS institutional review board (IRB), and all participants gave written informed consent. Our study was exempt from IRB approval because we used de-identified, publicly available data. Arsenic was measured in spot urine samples collected during the examination in a one-third random subsample of participants ≥ 6 years of age (n=16,332). Total urinary arsenic concentrations were measured via inductively coupled plasma-mass spectrometry with dynamic reaction cell (ICP-DRC-MS), and speciated arsenic concentrations were determined via high performance liquid chromatography (HPLC) coupled to ICP-MS; these analyses have been described elsewhere in detail.7 We used dimethylarsinate (DMA), the main metabolite of inorganic arsenic in humans, and total urine arsenic to reflect water arsenic exposure. Inter-assay coefficients of variation varied from 2.2 to 6.0% for DMA and from 1.0 to 19.4% for total arsenic. The limit of detection (LOD) varied from 1.70 to 1.91 µg/L for DMA and from 0.26 to 0.74 µg/L for total arsenic. The percentage of samples below the LOD was 17.2% for DMA and 0% for total arsenic. Undetectable DMA was replaced by the LOD divided by the square root of two.8 Arsenite, arsenate, and monomethylarsonate were not evaluated as their LODs were relatively high and the levels for these species were mostly undetectable. Participants missing DMA, arsenobetaine, total arsenic, body mass index (BMI), education, urinary creatinine, dietary recall, or who were pregnant were excluded; these exclusions resulted in a final sample size of 14,127.
Statistical analysis
Urinary arsenic integrates multiple exposure sources, including water, diet and tobacco, which are contaminated with arsenic of anthropogenic and/or natural origins. Seafood contributes high levels of arsenobetaine and other largely non-toxic organic arsenicals to urine arsenic.9 To isolate exposure to water arsenic, we expanded a residual-based method previously validated to remove the contribution of seafood, to also remove tobacco and other dietary sources of urinary DMA and total arsenic.10 Estimated urinary DMA and total arsenic levels reflecting water arsenic were obtained by first regressing their original log-transformed levels (µg/L) on log-transformed arsenobetaine (µg/L), smoking status (never/former/current), and past 24-hour intake of rice, cereals, juice, wine, chicken, and turkey (log-transformed g/kg bodyweight). The conditional means of urinary DMA and total arsenic among non-smoking participants with undetectable arsenobetaine and no arsenic dietary sources (as listed above) were then added back to model residuals to estimate the amount of urinary DMA and total arsenic that likely represents water arsenic exposure in the US population. Food intake was derived from past 24-hour dietary recalls using recipe codes from the Food Commodity Index Database (FCID), averaging commodity weights across all recipe modification codes.11 Cereal intake was derived using USDA food codes only, as no FCID codes exist for cereal. Urinary cotinine is not yet available in the NHANES public database for the 2013–2014 cycle, preventing us from using cotinine in addition to self-reported smoking in our correction. Distributions of estimated urinary total arsenic and DMA likely due to drinking water are available in Supplemental Figure 1.
Geometric means (GMs) and geometric mean ratios (GMRs) of urinary DMA and total arsenic comparing each subsequent 2-year cycle to NHANES 2003–2004 were estimated separately for participants reporting a primary tap water source of “well or rain cistern” (categorized as well water users as rain cisterns are rare) and “community supply” (categorized as public water users) during the interview. Participants who reported not drinking tap water were excluded from this analysis. GMs and GMRs were adjusted for sex, age, race/ethnicity, BMI, and education. We conducted sensitivity analyses further adjusting for urinary creatinine with similar results (data not shown). All analyses were conducted in R using the ‘survey’ package to account for NHANES complex sampling design and weights.12,13
Risk analysis
While the change in the MCL from 50 to 10 µg/L was initiated in 2006, the compliance determination process under the drinking water arsenic rule allowed time to test and address an MCL exceedance. We thus examined the impact of the MCL on drinking water arsenic levels and subsequent exposures by comparing urinary arsenic measurements from NHANES participants in the 2003–2004 cycle (prior to the implementation of the MCL change) with those from the 2013–2014 cycle, assuming full compliance with the new MCL at that point in time.
Water arsenic exposure was estimated from both urinary DMA and total arsenic measurements. For total arsenic, we assumed that water arsenic was present solely in the inorganic form and that the ratio of urinary arsenic to ingested water arsenic was 1:1 µg/L, based on earlier work in Taiwan and the United States.14,15 In the study population, the mean proportion of DMA in urinary total arsenic was 74%. We thus estimated water inorganic arsenic concentrations by multiplying urinary DMA levels by 1.36, assuming that water arsenic exposure is entirely inorganic arsenic.1
Body weight-adjusted lifetime average daily inorganic arsenic dose (LADD) was estimated by multiplying the drinking water arsenic concentration by the mean drinking water consumption rate for persons ≥ 21 years (only among those who reported consuming tap water) and dividing by the mean adult body weight (80 kg).16 Cancer risks were estimated by multiplying the resulting LADD by the EPA Integrated Risk Information System (IRIS) inorganic arsenic cancer slope factor. Risks were calculated separately using the current slope factor of 1.5 per mg (kgBW-day)−1, corresponding to skin cancer17, and the 2010 proposed slope factor of 25.7 per mg (kgBW-day)−1 for combined lung and bladder cancers.18
Expected 70-year cancer burdens were calculated by multiplying the estimated risks by the size of the population at risk. Burdens were calculated for the portion of the US population served by public water systems and separately for Mexican-Americans served by public water systems. The size of the US population served by public water systems was calculated by multiplying the 2014 US Census Bureau population estimate (318,563,456 persons) by the fraction of the overall NHANES sample served by public water systems (70.3%). The size of the Mexican-American population was calculated by multiplying the 2014 US Census Bureau population estimate by the Mexican-American fraction (9.4%), and then by the fraction of Mexican-Americans served by public water systems (61.5%). Seventy-year cancer burdens were divided by 70 to give an annual number of expected cancer cases due to the consumption of arsenic in drinking water. The number of cancer cases avoided as a result of the MCL was calculated by subtracting the expected post-MCL change 2013–2014 cancer burden from the pre-MCL change cancer burden.
For purposes of comparison, risks and burdens were also estimated using the dose-response methodology and metrics employed in the benefit-cost analysis supporting the establishment of the 10 µg/L MCL.18 Following the methods used by EPA,3 we calculated gender-specific unit cancer risk factors (Runit) for bladder and lung cancers by dividing 0.01 by the excess doses associated with 1% risk of bladder and lung cancers (ED01) and the lower bounds on those doses (LED01) from Model 1 as presented by Morales and colleagues. This model employs an exponential linear dose effect with a quadratic age effect and does not use a reference population. Using this approach, risk was estimated by multiplying gender-weighted, lung- and bladder-specific Runits (in cases/person per µg/L) by drinking water arsenic concentrations (estimated as described above). Lung and bladder cancer burdens and cases avoided were calculated from risk and population at risk estimates as described above. An example derivation for estimating the number of avoided lung and bladder cancer cases using total arsenic estimations appears in Supplemental Table 1. The funding source had no role in study design, collection, analysis, or interpretation of the data, writing of the report, or raw data collection. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Participant characteristics and urine arsenic concentrations
Over the 10-year study period, the population in the each of the 2-year NHANES cycles became older, more racially/ethnically diverse, and the prevalence of never-smoking and wine intake increased (Table 1). The percentage of the population served by public water and private well systems remained similar. Before and after removing dietary and tobacco sources of arsenic, median (interquartile range, IQR) were, respectively, 3.46 (2.00, 5.82) and 2.63 (1.56, 4.22) µg/L for DMA, and 7.15 (3.68, 14.99) and 4.07 (2.69, 6.12) µg/L for total arsenic.
Table 1. Participant characteristics by NHANES survey cycle (N= 14,127).
| Overall | 2003/2004 (N= 2279) |
2005/2006 (N= 2285) |
2007/2008 (N= 2355) |
2009/2010 (N= 2679) |
2011/2012 (N= 2214) |
2013/2014 (N= 2315) |
|
|---|---|---|---|---|---|---|---|
| Age (yr) - mean (SE) | 39.9 (0.3) | 39.3 (0.6) | 39.6 (0.9) | 40.0 (0.6) | 40.1 (0.8) | 40.1 (1.0) | 40.4 (0.4) |
| Sex- % female (SE) | 50.6 (0.01) | 51.1 (1.3) | 50.2 (1.6) | 50.7 (1.3) | 50.9 (0.9) | 50.8 (0.9) | 50.0 (1.0) |
| Race/ethnicity - % (SE) | |||||||
| Non-Hispanic white | 67.4 (0.1) | 71.3 (4.2) | 70.7 (3.1) | 68.3 (3.6) | 65.3 (3.3) | 65.1 (3.8) | 64.1 (3.8) |
| Non-Hispanic black | 11.8 (0.01) | 11.7 (2.2) | 11.7 (1.9) | 12.3 (2.0) | 12.0 (1.0) | 12.1 (2.4) | 11.2 (1.8) |
| Mexican-American | 9.4 (0.01) | 8.6 (2.4) | 8.8 (1.2) | 9.1 (1.9) | 10.0 (2.1) | 9.1 (2.1) | 10.7 (2.2) |
| Other, including multiple | 11.4 (0.01) | 8.4 (1.4) | 8.8 (1.5) | 10.3 (1.8) | 12.7 (1.9) | 13.7 (1.4) | 14.0 (1.3) |
| Education - % (SE) | |||||||
| <High school (HS) | 18.0 (0.01) | 18.0 (1.7) | 17.9 (1.6) | 20.7 (2.1) | 18.8 (1.1) | 17.8 (2.1) | 15.0 (1.5) |
| HS or equivalent | 23.9 (0.01) | 28.7 (1.2) | 24.9 (1.3) | 25.1 (1.5) | 23.0 (1.3) | 20.1 (2.0) | 22.2 (1.5) |
| >HS | 58.1 (0.01) | 53.3 (1.8) | 57.2 (2.1) | 54.3 (2.4) | 58.2 (1.3) | 62.1 (3.3) | 62.8 (1.8) |
| Smoking - % (SE) | |||||||
| Never | 58.2 (0.01) | 53.8 (1.8) | 56.0 (1.5) | 56.7 (1.8) | 58.8 (2.0) | 59.9 (1.3) | 63.8 (1.8) |
| Former | 19.2 (0.01) | 19.0 (0.9) | 19.4 (1.4) | 19.3 (1.1) | 18.1 (1.3) | 18.7 (1.0) | 20.8 (1.4) |
| Current | 22.6 (0.01) | 27.3 (1.9) | 24.6 (1.3) | 24.0 (1.5) | 23.2 (1.2) | 21.3 (1.3) | 15.4 (1.0) |
| BMI - mean (SE) | 27.2 (0.10) | 27.0 (0.2) | 27.0 (0.3) | 26.8 (0.2) | 27.2 (0.2) | 27.3 (0.3) | 27.7 (0.3) |
| Poultry past 24-hr - % (SE)1 | 40.5 (0.01) | 43.4 (2.1) | 44.1 (1.5) | 39.8 (1.5) | 39.5 (1.3) | 39.4 (2.6) | 37.4 (1.6) |
| Rice past 24-hr - % (SE)1 | 19.9 (0.01) | 22.0 (1.8) | 22.6 (1.4) | 21.7 (1.7) | 24.4 (1.5) | 15.2 (1.2) | 13.8 (1.0) |
| Juice past 24-hr - % (SE)1 | 12.3 (0.01) | 12.8 (1.4) | 13.0 (1.0) | 13.1 (0.8) | 12.8 (0.6) | 13.2 (0.8) | 8.7 (0.7) |
| Wine past 24-hr - % (SE)1 | 6.9 (0.01) | 5.6 (0.9) | 7.3 (0.9) | 6.3 (1.1) | 6.3 (0.8) | 8.2 (1.5) | 7.4 (0.8) |
| Cereal past 24-hr - % (SE)1 | 25.3 (0.01) | 22.6 (1.3) | 27.3 (1.2) | 26.6 (1.5) | 27.1 (1.5) | 24.8 (1.3) | 23.4 (1.1) |
| Urine arsenobetaine (µg/L)2 | 0.84 (0.48, 4.98) | 1.02 (0.30, 5.10) | 1.54 (0.28, 6.79) | 0.70 (0.28, 4.18) | 0.94 (0.28, 6.18) | 0.84 (0.84, 4.39) | 0.82 (0.82, 3.58) |
| Public water - % (SE)3 | 70.3 (0.01) | 83.5 (0.04) | 64.5 (0.04) | 69.6 (0.02) | 68.2 (0.04) | 68.3 (0.03) | 72.6 (0.03) |
| Well water - % (SE)4 | 12.7 (0.01) | 12.8 (0.03) | 17.6 (0.04) | 12.3 (0.02) | 12.0 (0.03) | 12.1 (0.02) | 9.4 (0.02) |
| Urine DMA (µg/L)2 | 2.63 (1.56, 4.22) | 2.77 (1.69, 4.28) | 2.83 (1.82, 4.44) | 2.88 (1.71, 4.49) | 2.61 (1.53, 4.46) | 2.44 (3.99, 1.39) | 2.28 (1.39, 3.69) |
| Urine total arsenic (µg/L)2 | 4.07 (2.69, 6.12) | 4.51 (2.99, 6.56) | 4.39 (2.99, 6.77) | 4.67 (3.29, 7.19) | 4.64 (6.97, 3.23) | 3.31 (2.17, 4.80) | 3.16 (1.99, 4.66) |
SE = standard error of the mean. All percentages are weighted to account for NHANES complex sampling design and survey weights.
Consumers of poultry, rice, juice, wine, cereal, and seafood are defined as those consuming >0.4g/kgBW of that FCID commodity during the 24-hr dietary recall. Poultry was defined as chicken and/or turkey.
Arsenobetaine, DMA (dimethylarsinate) and total arsenic are median (interquartile range). DMA and total arsenic are recalibrated to remove contribution of dietary and smoking sources of arsenic.
Participants who reported their primary tap water source is from a "community supply."
Participants who reported their primary tap water source is from a "well or rain cistern."
Trends in urine arsenic concentrations over time
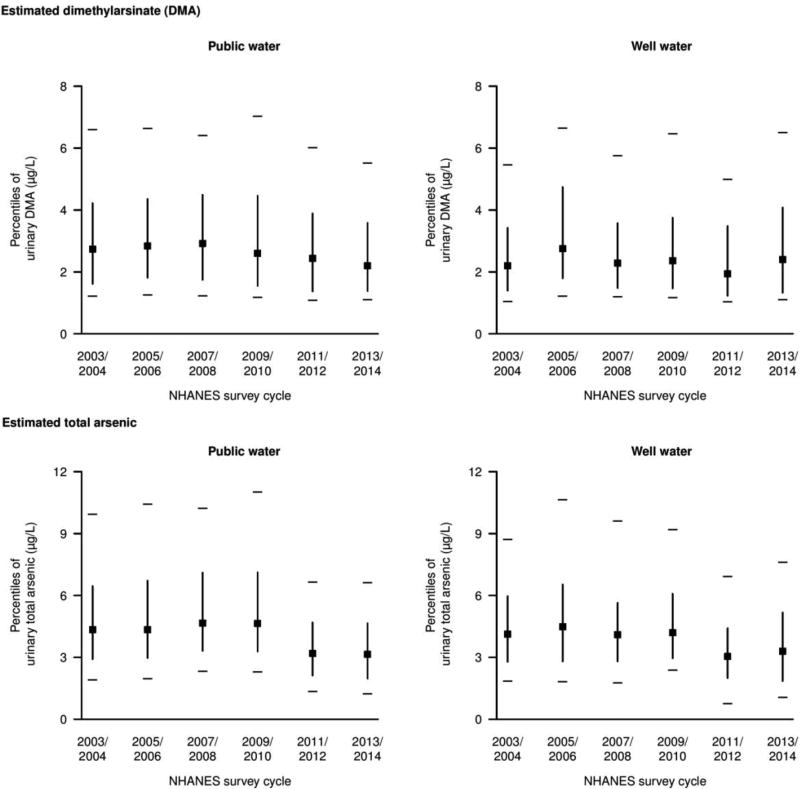
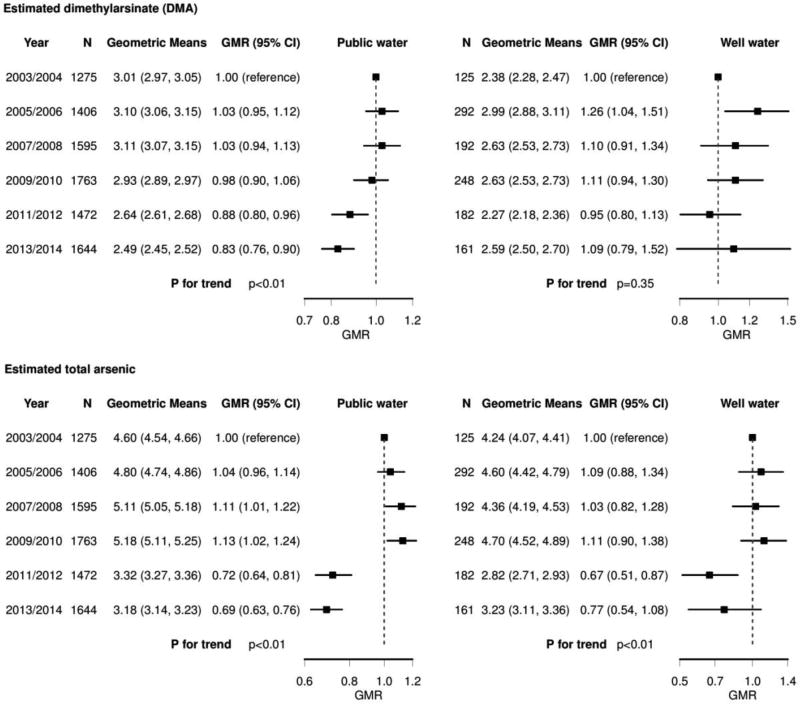
The distribution of urinary DMA and total arsenic for both public water and private well users stratified by NHANES survey cycle are presented in Figure 1. After adjustment, the estimated geometric mean of urinary DMA for public water users likely related to drinking water remained similar between NHANES 2003–2004 (3.01 µg/L) and 2009–2010 (2.93 µg/L) but declined in NHANES 2011–2012 (2.64 µg/L) and NHANES 2013–2014 (2.49 µg/L) on both the absolute and relative scales (Figure 2, p-trend <0.01). For private well users, the estimated geometric mean of urinary DMA increased from NHANES 2003–2004 (2.38 µg/L) to 2013–2014 (2.59 µg/L) but the overall trend was not significant (p-trend 0.35). The estimated geometric mean of total arsenic was more heterogeneous among both public water and well water users, slightly increasing from 2003–2004 (4.60 µg/L) to 2009–2010 (5.18 µg/L), but declining in 2011–2012 (3.32 µg/L) (p-trend<0.01) for public water users. Among private well users, total arsenic was lower in 2011–2012 (2.82 µg/L) compared to 2003–2004 (4.24 µg/L), but it was higher again in 2013–2014 (3.23 µg/L).
Figure 1. Percentiles of urine dimethylarsinate (DMA) and total arsenic recalibrated to reflect non-dietary and non-smoking sources of arsenic stratified by 2-year NHANES cycle.
Urinary arsenicals are in the original scale (non log-transformed). Squares represent medians. Vertical lines represent the interquartile range (25th – 75th percentile). Horizonal dashes represent the 10th and 90th percentile values. Recalibrated urine DMA and total arsenic were obtained from residuals regressing each log-transformed arsenic variable (DMA and total arsenic) on smoking status (never/ever/current), natural log-transformed arsenobetaine, and natural log-transformed intake of rice, cereals, juices, wine, chicken, and turkey in g/kg bodyweight/day (See Methods).
Figure 2. Geometric means (GMs) and geometric mean ratios (GMRs) and 95% confidence intervals (CIs) of urine dimethylarsinate (DMA) and total arsenic recalibrated to reflect non-dietary and non-smoking sources of arsenic stratified by 2-year NHANES cycle.
Squares and lines represent GMR estimates and 95% CIs. Recalibrated urine DMA and total arsenic were obtained from residuals regressing each log-transformed arsenic variable (DMA and total arsenic) on smoking status (never/ever/current), natural log-transformed arsenobetaine, and natural log-transformed intake of rice, cereals, juices, wine, chicken, and turkey in g/kg bodyweight/day (See Methods). Geometric means were further adjusted for age, race/ethnicity, education, and body mass index. P for trend was estimated by entering each NHANES 2-year cycle in the model as an ordinal variable.
Among Mexican-Americans using public water, geometric means of DMA declined from 4.06 µg/L in 2003–2004 to 2.58 µg/L in 2013–2014 (36% reduction; 95% CI 25%, 46%; p-trend<0.01) and of total arsenic declined from 6.05 µg/L in 2003–2004 to 3.18 µg/L in 2013–2014 (47% reduction; 95%CI 40%, 54%; p-trend< 0.01) (Table 2).
Table 2. Geometric means (GMs) and geometric mean ratios (GMRs) and 95% confidence intervals (CIs) of urine dimethylarsinate (DMA) and total arsenic recalibrated to reflect non-dietary and non-smoking sources of arsenic among Mexican-Americans using public water (N=1569).
Recalibrated urine DMA and total arsenic were obtained from residuals regressing each log-transformed arsenic variable (DMA and total arsenic) on smoking status (never/ever/current), natural log-transformed arsenobetaine, and natural log-transformed intake of rice, cereals, juices, wine, chicken, and turkey in g/kg bodyweight/day (See Methods). Geometric means were further adjusted for age, education, and body mass index. P for trend was estimated by entering each NHANES 2-year cycle in the model as an ordinal variable.
| Dimethylarsinate (DMA) | Total arsenic | ||||
|---|---|---|---|---|---|
| N | Geometric Mean | Geometric Mean Ratio | Geometric Mean | Geometric Mean Ratio | |
| 2003/2004 | 257 | 4.06 (3.93, 4.19) | 1 (reference) | 6.05 (5.86, 6.24) | 1 (reference) |
| 2005/2006 | 300 | 3.37 (3.27, 3.49) | 0.83 (0.70, 0.98) | 5.23 (5.07, 5.40) | 0.87 (0.75, 1.00) |
| 2007/2008 | 263 | 3.67 (3.55, 3.79) | 0.90 (0.75, 1.09) | 5.79 (5.61, 5.98) | 0.96 (0.82, 1.12) |
| 2009/2010 | 327 | 3.23 (3.12, 3.33) | 0.79 (0.66, 0.95) | 5.39 (5.22, 5.57) | 0.89 (0.78, 1.01) |
| 2011/2012 | 160 | 2.69 (2.60, 2.78) | 0.66 (0.54, 0.81) | 3.54 (3.43, 3.65) | 0.59 (0.52, 0.66) |
| 2013/2014 | 262 | 2.58 (2.50, 2.67) | 0.64 (0.54, 0.75) | 3.18 (3.08, 3.28) | 0.53 (0.46, 0.60) |
| P for trend | P<0.01 | P<0.01 | |||
Risk analysis
Based on our estimates of arsenic exposure reduction in the US population served by public water systems, we estimated the actual number of cancer cases avoided by lowering the arsenic MCL to 10 µg/L (Table 3) using exposure measurements from NHANES combined with the current17 and proposed18 cancer slope factors from the IRIS program, as well as with drinking water unit cancer risk factors employed by the EPA in its benefit-cost analysis in the year 2000. We report results to one significant figure to acknowledge the uncertainty in the appropriate cancer dose-response metric used. These results are reported to three significant figures in Supplemental Table 2. When using changes in urinary DMA to approximate reductions in drinking water arsenic exposure, the annual reduction in lung and bladder cancer was 200 cases avoided based on the 2000 EPA benefit-cost analysis approach versus 900 cases avoided using the 2010 EPA proposed slope factor. Using the current EPA slope factor, the estimated annual reduction in skin cancer cases was 50. Estimates derived using changes in urinary total arsenic are also presented in Table 3.
Table 3. Estimated annual number of cancer cases avoided by lowering the arsenic MCL from 50 to 10 µg/L based on estimated arsenic exposure reduction (using DMA or total arsenic) in the US population served by public water systems comparing NHANES 2013–2014 vs. NHANES 2003–2004 (post and prior to the 2006 MCL).
The original EPA analysis (2000) had estimated 37.4 to 55.7 annual cases of lung and bladder cancer avoided in the overall US population. The number of annual skin cancer cases avoided was not estimated in the original EPA analysis (2000). Results are reported to one significant figure.
| Overall population | Mexican-Americans | |||
|---|---|---|---|---|
| Method | Skin cancer |
Lung and bladder cancer |
Skin cancer | Lung and bladder cancer |
| Based on measured urine DMA reduction | ||||
| aEPA 2000 benefit-cost analysis | -- | 200 | -- | 40 |
| bEPA current cancer slope factor | 50 | -- | 10 | -- |
| cEPA 2010 proposed cancer slope factor | -- | 900 | -- | 200 |
|
| ||||
| Based on measured urine total arsenic reduction | ||||
| aEPA 2000 benefit-cost analysis | -- | 400 | -- | 60 |
| bEPA current cancer slope factor | 100 | -- | 20 | -- |
| cEPA 2010 proposed cancer slope factor | -- | 2000 | -- | 300 |
The EPA 2000 benefit-cost analysis uses the unit cancer risk factor (also called drinking water unit risk) to estimate the number of cases avoided per year instead of a cancer slope factor.19
The EPA current cancer slope factor was established in 1995 for skin cancer only, and it is the only cancer slope factor that has been finalized in the Integrated Risk Information System (IRIS).17
The EPA proposed cancer slope factor was proposed by the EPA for combined lung and bladder cancer in 2010 but it has never been finalized.18
Discussion
The decline in urinary arsenic among public water users in NHANES 2003–2014 supports the hypothesis that the implementation of the current arsenic MCL has reduced arsenic exposure for the general US population. The decline was only observed following the 2009–2010 cycle, consistent with the evaluation of MCL violations in the state of California and the compliance determination process of the drinking water arsenic rule, which required time for testing and time to address an MCL exceedance (e.g. change source or install water treatment).20,21 Exceedance of the MCL for the drinking water arsenic rule is based on a running annual average of quarterly samples. Public water systems were allowed up to one year of additional sampling time since the required initial sample (which had to be collected by December 2006 for surface water systems and by December 2007 for groundwater systems) before a compliance determination was made. This time lag can be observed in California’s public water supply arsenic violation data, which indicate that it took several years for California public water supply systems to first identify and then comply with the Arsenic Rule, with violations gradually decreasing after 2008.21 Given the built-in time delay of full enforcement of the 10 µg/L MCL, the similarity in GMs for urinary DMA and total arsenic concentrations between 2003–2004 and 2007–2008 among public water users provides an indication of the amount of natural variability in water arsenic exposure over time, enhancing our confidence in the significant changes observed in the later years.
For public water users, we estimate a reduction in water arsenic exposure of 17% from 2003–2004 to 2013–2014, which represents a significant exposure reduction when applied at the population level. It is unknown if these positive changes in arsenic prevention have occurred across all US geographic and geological regions, as this spatial analysis is not possible with the NHANES public database. The decline, however, was markedly stronger among Mexican-Americans compared to the overall population. These findings support that the recent infrastructure investments in many cities in the Southwest that focused on ensuring water arsenic below 10 µg/L has reduced arsenic exposure in the population.5
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program has estimated a total of 301,530 new combined lung and bladder cancer cases for 2017. In its benefit-cost analysis in support of revising the arsenic in drinking water rule, EPA estimated an annual reduction of 37.4 to 55.7 combined lung and bladder cancer cases at an MCL of 10 µg/L.19 The EPA did not quantify the reduction in number of skin cancer cases at the time. Given our estimated reductions in urinary DMA derived from NHANES, reflecting reductions in water arsenic exposure, the estimated annual reduction of cancer cases was 50 for skin cancer (using the current EPA slope factor17) and ranged from 200 (using the unit cancer risk from EPA’s benefit-cost analysis approach19) to 900 (using the EPA 2010 proposed slope factor18) for lung and bladder cancer. Using the same unit cancer risk factor employed in the 2000 EPA benefit-cost analysis for the arsenic MCL, we estimated an avoidance of 200 lung and bladder cancer cases using our measured exposure reductions from NHANES, as compared to EPA’s original estimate of 37.4 – 55.7 avoided cases per year. One explanation for this difference may lie in EPA’s assumptions regarding exposure reduction; in its benefit-cost analysis, EPA assumed that for water systems with arsenic levels in excess of 10 µg/L prior to the implementation of the MCL, post-implementation concentrations would be 8 µg/L.19 In reality, investments made across US public water systems may have been more effective than EPA originally assumed, possibly resulting in reductions in arsenic in water much lower than 8 µg/L.
Given the lack of consensus (and associated uncertainty) regarding the appropriate cancer dose-response metric, it is probably better to interpret estimations with just one significant figure, e.g. 200 to 900 lung and bladder cancers estimated based on either the unit cancer risk factor from the 2000 EPA benefit-cost analysis or the 2010 EPA proposed slope factor, respectively. The difference between these estimations largely depends on the dose-response metric used. While the 2010 EPA proposed slope factor has been controversial, it assumes a linear-dose response, which is consistent with the approach used by EPA for most carcinogens, and is supported by the findings of recent studies of arsenic and lung and bladder cancer at low-to-moderate arsenic exposure levels in US populations.22,23 While arsenic is toxic for multiple organs and systems, we did not consider additional non-cancer endpoints, which EPA considered qualitatively. Additionally, EPA’s risk assessment approach does not address the synergistic effects of tobacco smoking status and inorganic arsenic exposure on cancer risk. Given that the proportion of never smokers increased in the US population throughout the study period, it is possible that the current approach overestimates the effect of arsenic reduction on cancer risk. A more sophisticated risk assessment approach could evaluate slope factors separately for smokers and non-smokers and could consider changes in the population smoking status over time.
Additional analyses are needed to fully evaluate the potential economic benefits associated with the implementation of the current MCL. In 2007, SEER estimated that lung and bladder cancer together result in 2,523,000 years of life lost (YLL) and a loss of productivity cost of 40 billion dollars for the US population.24 It is likely that the indirect economic benefit of avoiding 200–900 excess cancer cases per year over several generations experiencing reduced exposure outweighs the initial capital costs and continuing operation and maintenance costs of implementing arsenic-reduction initiatives for public water systems.
We observed no consistent changes in urinary arsenic levels among private well users between 2003 and 2014. However, the results for well water users should be interpreted cautiously given the small sample size within each survey cycle and the possibility that well water users sampled in NHANES are not geographically representative of the underlying population of well users in the US as NHANES did not intentionally oversample for this population subgroup, which is markedly smaller in size as compared to the public water users. While estimated levels of urinary total arsenic for well users did decrease in the 2011–2012 cycle compared to the 2003–2004 cycle, these results were not consistent for urinary DMA, the primary metabolite of inorganic arsenic. Although the EPA MCL does not apply to private wells, testing and treatment for arsenic in drinking water among US residents relying on private well water differs widely by state and by socioeconomic status.25 In NHANES, urinary DMA and total arsenic levels were lower among well water users compared to public water users. No previous study has compared arsenic exposure levels in populations served by public water systems or private wells in the United States. The geographic clustering of high-arsenic wells throughout the US and the challenges to adequately sample the vast US rural areas in NHANES could explain higher survey to survey variability and wider confidence intervals in urine arsenic levels in the population served by private wells compared to the population served by community water systems. It has been estimated that 1.7 million Americans are at risk of exposure to arsenic >10 µg/L and 3.8 million to arsenic > 5 µg/L in household well water.26 Efforts are needed to protect affected private well water users from arsenic exposure. In New Jersey, for instance, all wells need to be tested for water contaminants (including arsenic in northern counties) as part of any real estate transaction via the Private Well Testing Act,27 but only approximately one quarter of private wells in the northern parts of New Jersey have been tested for arsenic since the passage of this act in 2002. For affected wells, families can receive a no-interest loan to pay for the purchase and installation of a water treatment system. Although the Private Well Testing Act has resulted in the installation of more arsenic treatment systems in northern New Jersey, no state government requires homeowners to install treatment systems to reduce arsenic if test results for arsenic exceed the MCL. Moreover, many private well owners who test for arsenic continue to experience drinking water arsenic levels greater than the MCL due to incorrect or improperly maintained treatment systems. Additional state and federal initiatives are needed to help families sample, test, and address arsenic exposure from unregulated private wells.28 Nationally representative studies of private well water users are needed to evaluate whether testing and treatment behaviors have changed over time for the US population.
Additional limitations include the lack of directly measured water arsenic in the study participants and the possibility that our water arsenic estimation method may incompletely remove other sources (e.g. airborne arsenic, which is likely minimal).29 In estimating water arsenic via our residual-based method, we accounted for the contribution of past 24-hour intake of arsenic containing foods (rice, poultry, juices, wine, and cereals) to both DMA and total arsenic. Urinary arsenic levels reflect exposure from the previous 1–3 days. In addition to dietary recall bias, it is possible that the 24-hour dietary recall method did not fully remove the contribution of arsenic from these dietary sources.
Conclusion
Following the implementation of the 2006 EPA MCL, we found a reduction in arsenic exposure among public water users but no changes among private well users. Our study supports that residents who rely on public water systems have experienced reductions in drinking water arsenic exposure and confirms the critical role of federal drinking water regulations in reducing toxic exposures and protecting human health.30,31,32
Supplementary Material
Research in context.
Evidence before this study
We searched for articles published in English in Pubmed, Medline, and EMBASE up until July 27, 2017 that included the following combination of terms: “arsenic” AND “EPA” or “Environmental Protection Agency” AND “MCL” or “Maximum Contaminant Level.” No studies have evaluated the impact of the implementation of the 2006 MCL on reducing arsenic exposure at the individual level or by using biomarker data. One study identified outside of our search criteria did not evaluate individual exposure, but found that arsenic MCL violations declined in California after 2010, indicating that arsenic levels in drinking water decreased in California several years after implementation of the current MCL in 2006.
Added value of this study
Our analysis presents nationally representative estimates of drinking water arsenic exposure reductions due to changes in the EPA’s arsenic MCL, and evaluates the impact separately for residents reliant on community water supplies and private wells. Moreover, this study estimates avoided cases of skin or lung and bladder cancer based on exposure reduction estimates derived from NHANES and EPA’s risk assessment approaches.
Implications of all the available evidence
Implementation of the current, more health-protective arsenic MCL likely reduced drinking water arsenic exposure in the US population for residents reliant on community drinking water supplies. Well water users remain inadequately protected against drinking water arsenic exposure, particularly residents of lower socioeconomic status who are less likely to test for arsenic and maintain treatment systems. We were unable to directly measure water arsenic or to evaluate changes in arsenic exposure geographically. Subsequent research should evaluate changes in drinking water exposure geographically.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (1R01ES025216, R01ES021367, 5P30ES009089 and P42ES010349). A. E. Nigra was supported by 5T32ES007322.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The views and opinions of authors expressed in this article do not necessarily state or reflect those of the U.S. Government.
Declaration of Interest:
The authors declare that they have no actual or potential competing financial interests.
Contributors
AEN, KEN, SNC, JHG, and ANA contributed to study design. AEN, TRS, KEN, and ANA contributed to data analysis. AEN, TRS, KEN, DH, SNC, JHG, and ANA contributed to data interpretation and manuscript writing.
References
- 1.National Resource Council. Critical aspects of EPA's IRIS assessment of inorganic arsenic: interim report. National Academies Press; 2013. [Google Scholar]
- 2.Maupin MA, Kenny JF, Hutson SS, Lovelace JK, Barber NL, Linsey KS. Estimated use of water in the United States in 2010: US Geological Survey. 2014 [Google Scholar]
- 3.Morales KH, Ryan L, Kuo T-L, Wu M-M, Chen C-J. Risk of internal cancers from arsenic in drinking water. Environmental Health Perspectives. 2000;108(7):655. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Barrera A, Lopez MH. A demographic portrait of Mexican-origin Hispanics in the United States. Washington, DC: Pew Hispanic Center; 2013. [Google Scholar]
- 5.United States Environmental Protection Agency. Drinking water requirements for states and public water systems: Arsenic Rule compliance success stories. [accessed 26 March 2017];2016 Nov 2; https://www.epa.gov/dwreginfo/arsenic-rule-compliance-success-stories.
- 6.National Center for Health Statistics. About the National Health and Nutrition Examination Survey. [accessed 1 March 2017];2014 https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 7.National Center for Health Statistics. Laboratory Procedure Manual: Urine arsenic speciation. [accessed 10 March 2017];2014 https://www.cdc.gov/Nchs/Data/Nhanes/Nhanes_13_14/UAS_UASS_H_MET.pdf.
- 8.National Center for Health Statistics. 2013–2014 Data Documentation, Codebook, and Frequencies: Urinary speciated arsenics. [accessed 15 September 2017];2016 https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UAS_H.htm.
- 9.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research. 2011;111(1):110–8. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MR, Tellez-Plaza M, Vaidya D, et al. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: Evidence from MESA and NHANES. American Journal of Epidemiology. 2016;184(8):590–602. doi: 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigra AE, Nachman KE, Love DC, Grau-Perez M, Navas-Acien A. Poultry consumption and arsenic exposure in the U.S. population. Environmental Health Perspectives. 2016;125(3):370. doi: 10.1289/EHP351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumley T. Analysis of complex survey samples. Journal of Statistical Software. 2004;9(1):1–19. [Google Scholar]
- 13.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 14.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environmental Health Perspectives. 1999;107(8):663. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. Journal of Exposure Science and Environmental Epidemiology. 2009;19(4):343–8. doi: 10.1038/jes.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Environmental Protection Agency. Exposure Factors Handbook 2011 Edition (Final) [accessed 1 February 2017];2011 https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=23625220.
- 17.United States Environmental Protection Agency. Arsenic, inorganic (CASRN 7440-38-2) [accessed 14 May 2017];2017 http://www.epa.gov/iris/subst/0278.htm.
- 18.United States Environmental Protection Agency. IRIS Toxicological Review of Inorganic Arsenic (Cancer) (2010 External Review Draft) Washington, DC: 2010. [Google Scholar]
- 19.United States Environmental Protection Agency. [accessed 1 May 2017];Arsenic in drinking water rule economic analysis (EPA 815-R-00-026) 2000 https://yosemite.epa.gov/ee/epa/ria.nsf/vwAN/W200012A.pdf/$file/W200012A.pdf14.
- 20.United States Environmental Protection Agency. Arsenic and clarifications to compliance and new source monitoring rule: A quick reference guide. US EPA Washington, DC: 2001. [Google Scholar]
- 21.Grooms KK. Does water quality improve when a Safe Drinking Water Act violation is issued? A study of the effectiveness of the SDWA in California. The BE Journal of Economic Analysis & Policy. 2016;16(1):1–23. [Google Scholar]
- 22.Garcia-Esquinas E, Pollan M, Umans JG, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the Strong Heart Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):1944–53. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baris D, Waddell R, Beane Freeman LE, et al. Elevated bladder cancer in northern New England: The role of drinking water and arsenic. Journal of the National Cancer Institute. 2016;108(9) doi: 10.1093/jnci/djw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2007. [accessed 15 September 2017]. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 25.Flanagan SV, Spayd SE, Procopio NA, et al. Arsenic in private well water part 3 of 3: Socioeconomic vulnerability to exposure in Maine and New Jersey. Science of the Total Environment. 2016;562:1019–30. doi: 10.1016/j.scitotenv.2016.03.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Flanagan SV. The case for universal screening of private well water quality in the US and testing requirements to achieve it: Evidence from arsenic. Environmental Health Perspectives. 2017 doi: 10.1289/EHP629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.New Jersey Department of Environment. Private Well Testing Act rules proposed readoption and proposed amendment. [accessed 24 May 2017];2007 http://www.nj.gov/dep/rules/proposals/100107a.pdf.
- 28.Fox MA, Nachman KE, Anderson B, Lam J, Resnick B. Meeting the public health challenge of protecting private wells: Proceedings and recommendations from an expert panel workshop. Science of the Total Environment. 2016;554–555:113–8. doi: 10.1016/j.scitotenv.2016.02.128. [DOI] [PubMed] [Google Scholar]
- 29.Nordberg GF, Fowler BA, Nordberg M. Handbook on the Toxicology of Metals. Academic Press; 2014. [Google Scholar]
- 30.Samet JM, Burke TA, Goldstein BD. The Trump Administration and the environment - Heed the science. The New England Journal of Medicine. 2017;376(12):1182–8. doi: 10.1056/NEJMms1615242. [DOI] [PubMed] [Google Scholar]
- 31.Focazio MJ, Tipton D, Dunkle Shapiro S, Geiger LH. The chemical quality of self- supplied domestic well water in the United States. Groundwater Monitoring & Remediation. 2006;26(3):92–104. [Google Scholar]
- 32.DeSimone LA, Hamilton PA, Gilliom RJ. Quality of water from domestic wells in principal aquifers of the United States, 1991–2004: Overview of major findings: US Department of the Interior, US Geological Survey. 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.