Abstract
Long-term use of opioid analgesics may be ineffective or associated with significant negative side effects for some people. At present, there is no sound method of identifying optimal opioid candidates. Individuals with chronic low back pain (n=89) and healthy controls (n=102) underwent ischemic pain induction under placebo, opioid blockade (naloxone), and morphine in counterbalanced order. They completed the Spielberger Anger-out subscale. Endogenous opioid function × anger-out × pain status (chronic pain; healthy control) interactions were tested for morphine responses to ischemic threshold, tolerance and pain intensity (McGill Sensory and Affective subscales) and side effects. For individuals with chronic pain and healthy controls, those with low endogenous opioid function and low anger-out scores exhibited the largest morphine analgesic responses, whereas those with high anger-out and low endogenous opioid function showed relatively weaker morphine analgesic responses. Further, individuals with chronic pain with low endogenous opioid function and low anger-out scores also reported the fewest negative effects to morphine, whereas those with low endogenous opioid function and high anger-out reported the most. Findings point toward individuals with chronic pain who may strike a favorable balance of good analgesia with few side effects, as well as those who have an unfavorable balance of poor analgesia and many side effects.
Keywords: Endogenous opioid function, anger-out, morphine analgesic responses, side effects
Use of opioid analgesic medications for management of chronic nonmalignant pain has become increasingly common.2,10 Whereas long-term use of opioid analgesics may be effective for some, it may be ineffective or not well tolerated by others (10–23% in long-term trials25). In either case, long-term use of opioid analgesics can be associated with significant negative side effects13,25, and may be associated with worse rather than improved function.18,20 Moreover, opioids entail risks for misuse, abuse, or dependence.15,19,26 At present, however, there is no well-validated method of identifying optimal candidates – those who experience high levels of analgesia but few side effects – for opioid pain management.11,32
Recent evidence suggests that psychosocial factors may predict responses to opioid analgesics. Elevated negative affect (NA; e.g., depression, anxiety) has been linked to lower opioid analgesic response as indicated by greater post-surgical opioid analgesic requirements.12,16,27 A prospective study of analgesic response to opioid medication found that chronic low back pain patients with high NA reported significantly lower reduction in pain intensity over 6.5 months than low NA patients.35 Two placebo-controlled laboratory studies found that elevated NA predicted smaller opioid analgesic responses to acute pain stimuli, in both individuals with chronic back pain34 and healthy controls.14 Taken together, results suggest that NA-related factors may be important phenotypic predictors of responses to opioid analgesics.
One limitation of these studies is that they examined simple univariate relationships. Examining linear relationships between separate predictors and opioid responses (i.e., one variable at a time) has the potential to reveal factors related to analgesic reactions to opioids. However, combining factors in interactive (i.e., multivariate moderation) relationships may help identify more precisely those individuals who reveal especially poor or especially salutary analgesic responses. One challenge is to identify such factors a priori to facilitate hypothesis testing.
Results of our prior work suggest that elevated trait anger expressiveness (trait anger-out) is associated with deficient endogenous opioid function assessed via opioid blockade methods.3,4,7,8,9 Trait anger-out is the tendency for an individual to verbally express or physically display anger when anger is aroused. Results of our other work suggest that high levels of endogenous opioid function predict relatively low analgesic response to morphine to both acute and chronic pain.5,6 These latter two reports were based on a study in which healthy controls and individuals with chronic low back pain underwent acute pain induction under three (counterbalanced) conditions: 1) placebo, 2) intravenous naloxone to block endogenous opioids; 3) intravenous morphine. This method gave measures of both endogenous opioid function and opioid analgesic response. We found that endogenous opioid function was inversely related to analgesic effects of opioid medication on acute pain stimuli among all participants, and on the intensity of spontaneous low back pain among those with chronic low back pain.5,6
In the present study, we report additional analyses of the parent study described above,5,6 but with a larger sample of both healthy controls and individuals with chronic low back pain. We integrate our past findings by proposing that the relationships between endogenous opioid function and morphine analgesic response may be moderated by trait anger expressiveness. Specifically, the strength of these relationships may depend on whether individuals report high or low levels of anger-out. We also tested whether endogenous opioid function interacted with trait anger-out to predict side effects during morphine infusion. We expected results to reveal individuals characterized simultaneously by variation in anger expressiveness and endogenous opioid function, who showed particularly high and/or low levels of morphine analgesia to pain stimuli and high and/or low levels of side effects to morphine.
Method
Design
The study used a three session, double-blind, crossover design with administration of an opioid antagonist (naloxone), an opioid analgesic (morphine), and saline placebo. Order of drug administration was randomized and counterbalanced. Identical data collection procedures and equipment were employed in a closely coordinated fashion at two sites (Vanderbilt University Medical Center and Rush University Medical Center). The protocol for this study was approved by the Institutional Review Boards at Rush University Medical Center and Vanderbilt University School of Medicine.
Participants
Participants were 93 individuals with chronic low back pain and 106 healthy controls who were recruited through on-line advertisements on the Vanderbilt University e-mail recruitment system, the Rush University Pain Clinic, advertisements in local print media, or posted flyers. Inclusion criteria for all participants were: 1) age between 18–55; 2) no self-reported history of cardiovascular disease, hypertension, liver, kidney disorders; diabetes, or seizure disorder; 3) no self-reported history of posttraumatic stress disorder, bipolar disorder, psychotic disorder, alcohol or drug dependence; 4) no use of anti-hypertensive medications; and 5) no daily use of opioid analgesics. Additional inclusion criteria for the chronic low back pain participants were: 1) chronic daily low back pain of at least 3 months duration, and 2) an average past month severity of at least 3 on a 0–10 verbal numeric pain intensity scale. Individuals with chronic pain related to malignancy, autoimmune disorders, or fibromyalgia (based on self-report) were excluded. Potential participants who were pregnant (determined by urine pregnancy screens) were excluded to avoid unknown effects of naloxone on the fetus. No participants in the healthy group were taking antidepressants, neuroleptics, or as-needed opioid analgesics. No participants in the chronic low back pain group were taking neuroleptic adjuvant therapy for pain control, and only 3.4% were taking antidepressants. In addition, no participants in either group reported use of any opioid analgesics in the 3 days prior to each study session study (confirmed by opioid urine screen). Participants were compensated $375 for their time upon completion of the three study sessions. Of the 199 subjects who started the study (i.e., were administered a drug in session 1), three subjects dropped out prior to session 2, and five additional subjects dropped out prior to session 3. These drop-outs left a final sample of 89 individuals with chronic low back pain, and 102 healthy controls. See Table 1 for sample characteristics. Individuals with chronic pain and the healthy controls did not differ significantly on number of women or racial composition. However, the chronic pain group was slightly older on average [F(1,190) = 4.97; p < .03].
Table 1.
Sample Characteristics
| Characteristic | Chronic Pain (n=89) | Controls (n=102) |
|---|---|---|
| Gender (% female) | 62.9% | 52.0% |
| Race: | ||
| Caucasian | 60.7% | 55.9% |
| African-American | 32.6% | 38.2% |
| Ethnicity | ||
| Non-Hispanic | 95.5% | 94.1% |
| Mean Age (years) | 36.93 (11.5) | 33.43 (9.2) |
| Mean Pain Duration (months) | 116.53 (104.3) | – |
Study Drugs
Blockade of opioid receptors was achieved by administration of naloxone, an opioid antagonist with a brief half-life (1.1 hours;22). As in our past work,4 an 8 mg dose in 20 ml normal saline was infused intravenously over a 10-minute period through an intravenous cannula placed in the non-dominant arm. At this dosage, naloxone provides effective blockade of all three major opioid receptor subtypes.21
The opioid analgesic medication examined in this study was morphine sulfate, the prototypic mu opioid receptor agonist. As in similar laboratory pain studies with morphine,14 the current study employed a dosage of 0.08 mg/kg (in 20ml normal saline), which was infused in the same manner as naloxone. This dosage (approximately 6mg for a 74 kg individual) was selected because it was judged to be sufficient to produce analgesia, but low enough to avoid ceiling effects that might obscure key individual differences in morphine responding. Peak naloxone and morphine activity are both achieved within approximately 15 min.1 Normal saline (20ml) was infused in an identical manner during the placebo condition.
Measures of Trait Anger-Out and Negative and Positive Affect
Trait anger-out was assessed using the anger-out subscale of the Spielberger Anger Expression Inventory, which demonstrates good psychometric characteristics.30 High scores on the anger-out scale reflect a strong tendency to manage anger when it is experienced via direct verbal and physical expression.
Because trait anger-out overlaps conceptually and empirically with the broader construct of negative affect, we statistically controlled for general affect in all analyses to help isolate the unique effects of trait anger-out on morphine analgesia and side effects. To this end, we used the Positive Affect (PA) and Negative Affect (NA) scales from the Positive and Negative Affect Schedule (PANAS;36). The PANAS is a reliable and valid instrument.36
Measure of Side Effects from Morphine Infusion
Participants were asked to describe opioid-related side effects using the VAS Opioid Effects Questionnaire developed by Zacny.37 This questionnaire consists of 26 items that reflect known opioid side effects and each item is rated on a 100mm VAS scale (anchored with “not at all” and “extremely”).24 As we have done previously,17 we conducted a principal components analysis (PCA) to reduce the number of variables examined and substantially reduce the number of analyses performed. PCA (with varimax rotation) indicated that three components, each with eigenvalues > 1.9, accounted for 50.1% of the total variance in side effects. We used the regression method to compute three subscale scores. Three items, “tingling”, “ability to control thoughts”, and “ability to control body” did not meet criteria for inclusion in any of the subscales. Based on item content, the three subscales were labeled as: Sedation (three highest loading items were dreamy, coasting, floating), Unpleasantness (three highest loading items were down, anxious, feeling bad), and Euphoria (three highest loading items were stimulated, elated, pleasant thoughts). These derived subscale scores were used in analyses.
Acute Pain Intensity Measures
Participants completed the McGill Pain Questionnaire-Short Form (MPQ;24) at each session after the ischemic pain task (see below). The MPQ is a well-validated measure that allows separate assessment of the sensory (MPQ-S) and affective (MPQ-A) qualities of pain.24
Acute Pain Induction
Acute pain was induced using an ischemic task based on procedures described by Maurset and colleagues23. Participants first engaged in 2 minutes of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength, as determined before beginning the laboratory procedures. Then they were asked to raise their dominant arm for 15 seconds so that their forearm remained above their head. A blood pressure cuff was then inflated over the participant’s dominant biceps to 200mmHg, and the arm was lowered. Participants were then instructed to indicate when they first experienced pain, with this ischemic pain threshold defined as the time elapsed from task onset to when the sensation was first described as “painful.” Ischemic pain tolerance was defined as the time elapsed between onset of the pain task and participants’ expressed desire to terminate the task or a preset maximum exposure time of 8 minutes was reached. Participants then completed the MPQ.
Procedure
After providing informed consent, participants completed a packet of questionnaires, including information regarding demographics and chronic pain. Individuals then participated in three identical experimental sessions (placebo, naloxone, morphine) that were scheduled approximately one week apart, at the same time of day to control for variance due to circadian rhythms. See Figure 1 for a flow chart of the study procedure.
Figure 1.
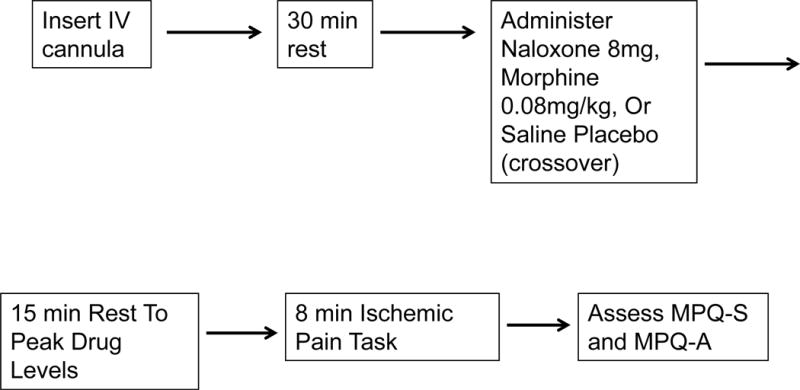
Study Flowchart
Participants remained seated upright in a comfortable chair throughout all laboratory procedures. During each session, participants initially completed a 10-min seated rest period, after which an indwelling venous cannula was inserted into the non-dominant arm by a trained research nurse under physician supervision. After a 30-min resting adaptation period, participants received (via the cannula) saline placebo, naloxone, or morphine, with order of drug administration across the three sessions randomly determined and counterbalanced. The investigational pharmacy at each institution prepared and provided the study drugs in blinded fashion to the study nurses.
After a 15-min rest period to allow peak drug activity to be achieved, participants engaged in the ischemic pain task, as detailed above. After the pain task was completed, participants were asked to describe the medication-related side effects they experienced using the VAS Opioid Effects Questionnaire (only morphine condition side effects were examined in the analysis given the focus of this study). All participants remained in the lab under observation for 2 hours after peak drug activity had been achieved to allow drug effects to remit, after which they were released to a responsible adult.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 21 (SPSS Inc., Chicago, IL). In preparation for conducting analyses, opioid blockade effects (as an index of endogenous opioid inhibition of evoked acute pain) were derived separately for threshold, tolerance, and MPQ-S and MPQ-A scores. For threshold and tolerance, blockade effects were calculated by subtracting values when endogenous opioids were blocked (naloxone condition) from comparable values when endogenous opioids were intact (placebo condition), such that the greater the positive blockade effect values, the greater the endogenous opioid analgesic function. For MPQ-S and MPQ-A scores, the procedure was reversed so that resulting endogenous opioid function effects could be interpreted consistently across all four pain indexes. Thus, blockade effects were calculated by subtracting MPQ-S and MPQ-A values when endogenous opioids were intact (placebo condition) from comparable values when endogenous opioids were blocked (naloxone condition). Again, the greater the positive difference between placebo and naloxone pain responses, the greater the endogenous opioid analgesic function.
Similarly, morphine analgesic effects (as an index of exogenous opioid inhibition of ischemic pain) were also derived for the threshold, tolerance, MPQ-S and MPQ-A measures. For threshold and tolerance, morphine effects were calculated by subtracting values during placebo administration when endogenous opioids were intact from comparable values after morphine administration, such that the greater the positive morphine effect values, the greater the morphine analgesic response. For MPQ-S and MPQ-A scores, the procedure was reversed so that resulting morphine response values could be interpreted consistently across all four pain indexes. Thus, morphine effects were calculated by subtracting MPQ-S and MPQ-A values after morphine administration from comparable values when endogenous opioids were intact (placebo condition). Again, the greater the positive difference between placebo and morphine pain responses, the greater the morphine analgesic response.
Morphine side effects were also derived for the Sedation, Unpleasantness and Euphoria subscales. These effects were calculated by subtracting each subscale score recorded following placebo administration from the corresponding subscale score recorded following morphine administration. Thus, larger positive scores indicated greater side effects with morphine relative to placebo.
Primary analyses used hierarchical multiple regression to examine moderation effects. Two-way interaction terms were computed by multiplying endogenous opioid blockade effect variables (differences on pain indexes between placebo and naloxone administration) of each acute pain index by trait anger-out scores (e.g., tolerance blockade effect × anger-out score), blockade effects by pain status (1 = healthy; 2 = chronic back pain), and trait anger-out scores × pain status. To assess whether effects differed in individuals with chronic back pain versus healthy controls, three-way interaction terms were computed by multiplying the two-way interaction terms by pain status. Regression analyses proceeded by entering PA and NA scores in the first block (as control variables for affective state), blockade effect variable, anger-out scores and pain status in the second block (main effects), two-way interaction terms in the third block, and the three-way interaction term in the fourth block. The morphine analgesia response variables for each pain index (differences on pain indexes between placebo and morphine administration) were the dependent measures. If a three-way interaction term produced a significant increase in R2, then the two-way interactions of blockade effect variables by trait anger-out scores were tested separately for individuals with back pain and healthy controls. If a blockade effect × anger-out two-way interaction term produced a significant increase in R2 for either group, then the procedure described by Preacher, Curran, and Bauer28 for dissecting interaction terms for two continuous variables was used. Namely, regression equations for four combinations of hypothetical values (−1SD, +1SD) for the blockade variable and anger-out scores were conducted with morphine analgesic response variables as the dependent measures, and slopes tested for significance. A similar procedure was used to test interaction effects on the three side effects subscales. Note that to stem a possible proliferation of analyses and given our focus on the blockade effect × anger-out effects, other significant two-way interactions were not dissected for further analysis.
Results
Endogenous Opioid Function × Anger-Out Effects for Morphine Analgesic Responses
Hierarchical multiple regressions were performed for ischemic pain threshold, tolerance, MPQ-S and MPQ-A morphine analgesic response values. As described above, PA and NA scores were entered first, followed by the endogenous opioid blockade effect variables, anger-out and pain status variables, followed by the two-way interaction terms, and finally followed by the three-way interaction term.
For ischemic pain threshold the three-way interaction was nonsignificant for morphine analgesic responses, (t = .56; p > .10). The threshold blockade effect × anger-out interaction term, for the entire sample (patients and healthy controls) was also nonsignificant (t = −.94; p > .10).
For ischemic pain tolerance, the three-way interaction was nonsignificant for morphine analgesic responses (t = .34; p > .10); however, the tolerance blockade effect × anger-out interaction term, for both patients and healthy controls, was a significant predictor (R2 change = .02; t = 2.11; p < .036). The blockade effect simple slope for participants with low anger-out (−1 SD below the mean) was significant and negative (beta = −.52, t = −3.48, p < .001), whereas the simple slope for participants with high anger-out (+1 SD above the mean) was non-significant (beta = −.28, t = −1.14, p > .10). These effects are graphically depicted in Figure 2, with endogenous opioid blockade effect values on the x-axis, low and high anger-out values represented by the two lines, and morphine analgesic response values on the y-axis. Results suggest that only individuals low in anger-out who also exhibited small endogenous opioid blockade effects (showed deficits in endogenous opioid function) experienced strong analgesic responses to morphine. Individuals high in anger-out who also showed small endogenous opioid effects experienced much weaker morphine analgesic responses than the former set of individuals. Of note, both individuals with low and high anger-out who also revealed large endogenous opioid responses experienced the weakest analgesic response to morphine for pain tolerance.
Figure 2.
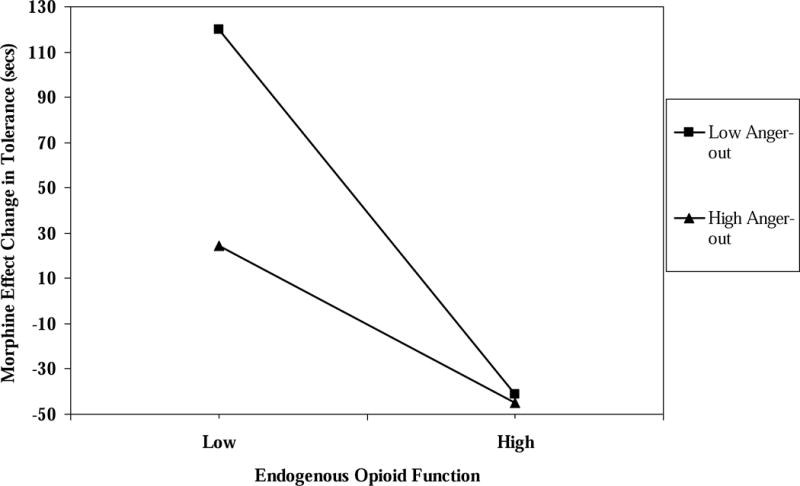
The interaction of endogenous opioid function values for pain tolerance × anger-out scale scores predicting morphine analgesic responses for pain tolerance.
For ischemic pain MPQ-S, the three-way interaction was nonsignificant for morphine analgesic responses. Again, the MPQ-S blockade effect × anger-out interaction term was a significant predictor (R2 change = .02; t = 2.87; p < .005) for patients and healthy controls alike. The blockade effect simple slope for participants with low anger-out (−1 SD below the mean) was significant and negative (beta = −.46, t = −4.38, p < .001), whereas the simple slope for participants with high anger-out (+1 SD above the mean) was non-significant (beta = −.11, t = −.99, p > .10). These effects are graphically depicted in Figure 3. The pattern of effects is similar to those observed for the analgesic effects of morphine on ischemic tolerance.
Figure 3.
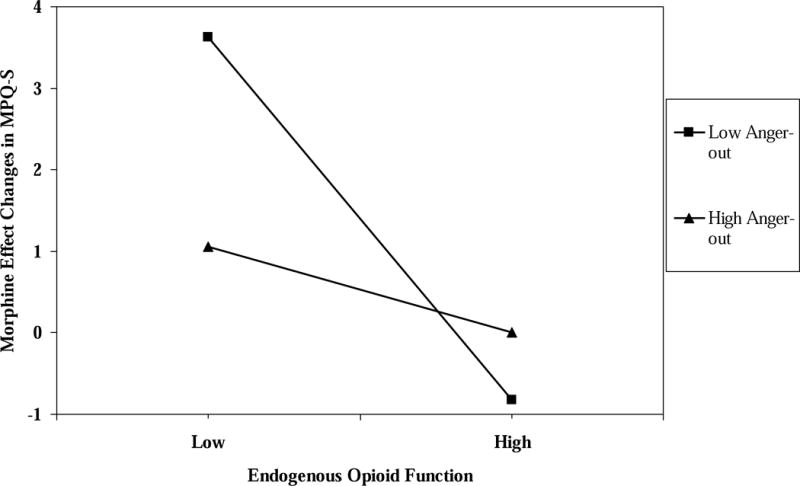
The interaction of endogenous opioid function values for MPQ-S scores × anger-out scale scores predicting morphine analgesic responses for MPQ-S scores.
For ischemic pain MPQ-A, the three-way interaction term was nonsignificant for morphine analgesic responses, whereas the MPQ-A blockade effect × anger-out interaction term was a significant predictor (R2 change = .02; t = 2.09; p < .04). As for tolerance and MPQ-S measures, the blockade effect simple slope for participants with low anger-out (−1 SD below the mean) was significant and negative (beta = −.45, t = −4.65, p < .001), whereas the simple slope for participants with high anger-out (+1 SD above the mean) was non-significant (beta = −0.15, t = −1.3, p > .10). These effects are graphically depicted in Figure 4, and again reveal a pattern of effects similar to those for tolerance and MPQ-S values.
Figure 4.
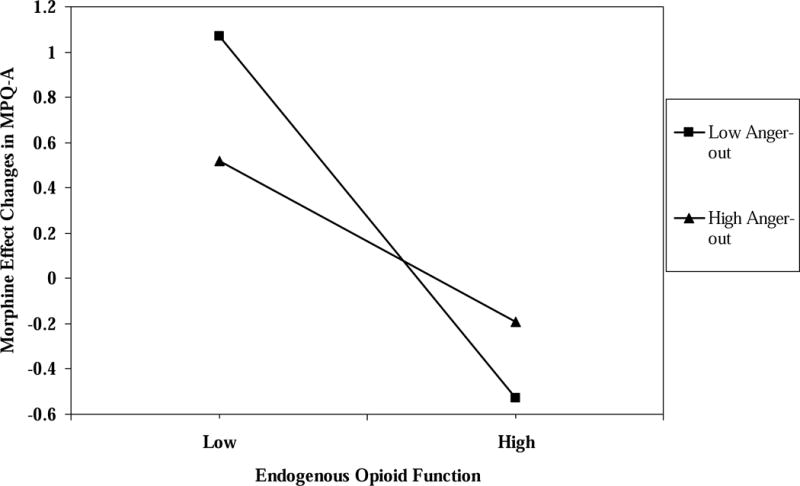
The interaction of endogenous opioid function values for MPQ-A scores × anger-out scale scores predicting morphine analgesic responses for MPQ-A scores.
As a whole, these results imply that individuals with an elevated tendency to express anger and who exhibit relative deficits in endogenous opioid function may experience weaker analgesic responses from morphine than individuals low in anger-out who exhibit similar relative deficits in endogenous opioid function. Indeed, individuals with both low anger-out and low endogenous opioid function showed the strongest morphine analgesic responses of any set of individuals and across all three pain indexes showing significant interactions. Lastly, both individuals with low and high anger-out with strong endogenous opioid responses may derive the weakest analgesic responses to morphine.
Endogenous Opioid Function × Anger-Out Effects for Morphine Side Effects
Hierarchical multiple regressions were performed for Sedation, Unpleasantness, and Euphoria subscales of the Opioid Effects Questionnaire. Because it had the largest interaction effects on analgesia outcomes and to decrease the number of analyses, we analyzed only the MPQ-S blockade effect × anger-out interaction effects on the three subscales. Similar to the procedure described above, PA and NA scores were entered first, followed by the MPQ-S blockade effect, anger-out and pain status variables, followed by the two-way interaction terms, and finally followed by the three-way interaction term. The three-way and two-way MPQ-S blockade effect × anger-out interaction terms were non-significant for the Sedation (t = .96; p > .10) and Euphoria subscales (t = .53; p > .10). For Unpleasantness subscale scores, the three-way interaction term was significant (R2 change = .03; t = 2.06; p < .03). The MPQ-S blockade effect × anger-out interaction terms were evaluated for the healthy controls and individuals with chronic pain separately. For healthy controls, the MPQ-S blockade effect × anger-out interaction term was nonsignificant (t = .91; p > .10), whereas this interaction was a significant predictor among those with chronic pain (R2 change = .12; t = −2.88; p < .005). The blockade effect simple slope for participants with chronic pain with low anger-out (−1 SD below the mean) was significant and positive (beta = .05, t= 2.26, p=0.02), whereas the simple slope for patients with high anger-out (+1 SD above the mean) was significant and negative (beta = −.05, t = −2.17, p < .03). These effects are graphically depicted in Figure 4.
In sum, these results suggest that individuals with chronic low back pain, an elevated tendency to express anger, and weak endogenous opioid responses may experience relatively high levels of unpleasant emotional and cognitive side effects when given morphine. In contrast, those who tend not to express anger may experience relatively low levels of unpleasant emotional and cognitive side effects with morphine if they also have relatively low endogenous opioid function. Finally, both individuals with low and high anger-out and with strong endogenous opioid responses appear to have only mild unpleasant side effects with morphine. These effects were not significant for healthy control participants.
Subjects’ Accuracy in Guessing Drug Condition and Effects on Endogenous Opioid and Morphine Responses
On average (across the three lab sessions), 54% of subjects correctly guessed when they had received naloxone. On average (across the three lab sessions), 80% of subjects correctly guessed when they had received morphine. Because these results suggested that many subjects were at least somewhat aware of the session condition, we compared the groups who guessed correctly and incorrectly on endogenous opioid function and morphine response variables for the four pain indexes. For naloxone opioid blockade effects on pain responses, all comparisons between the correct and incorrect guesser groups were nonsignificant [F’s < 2.13; p’s > .10]. For morphine effects on pain responses, all comparisons between the correct and incorrect guesser groups were also nonsignificant [F’s < 1.69; p’s > .10]. On the one hand, many subjects did accurately guess the drug they received. On the other hand, this belief did not significantly affect responses to ischemic pain stimuli.
Discussion
At present, there is no well-validated means of identifying optimal candidates for opioid pain management; that is, individuals who experience high levels of analgesia but low levels of side effects.11,32 In an attempt to identify better predictors of opioid responses, we combined factors in interactive (i.e., multivariate moderation) relationships, selected based on past findings, to identify more precisely the characteristics of individuals who display different profiles of analgesic and side effect responses to morphine. The present findings suggest that, regardless of chronic pain status, individuals with low endogenous opioid function and an elevated tendency to express anger derive little analgesic response from morphine yet suffer the highest level of unpleasant cognitive and emotional side effects. In contrast, both healthy individuals and those with chronic pain who have low endogenous opioid function and low anger expressiveness derive relatively high levels of morphine analgesia, with chronic pain participants fitting this profile least likely to suffer unpleasant morphine side effects. Taken together, these findings point toward individuals with chronic pain who may experience a favorable balance of good analgesia with few unpleasant side effects (low endogenous opioids and low anger expressiveness), as well as individuals who may experience an unfavorable balance of poor analgesia and many unpleasant side effects (low endogenous opioids and high anger expressiveness). Such multivariate profiles may improve accuracy over univariate approaches in understanding distinctions between opioid responders and non-responders.
Results of simple effects coupled with inspection of figures point toward potentially important conclusions regarding the identification of optimal opioid treatment candidates. First, the morphine response for both healthy controls and chronic pain participants who reported both high levels of anger-out and exhibited low endogenous opioid function was smaller than the morphine response for healthy controls and chronic pain participants who reported low high levels of anger-out and exhibited low endogenous opioid function. In previous work,5,6 we showed that individuals with low endogenous opioid function exhibited high levels of morphine analgesic responses to both induced acute pain and spontaneous low back pain. We surmised that people with poor endogenous opioid function could compensate for their low endogenous analgesic capacity by supplementing deficient endogenous analgesia with exogenous opioids, like morphine. In contrast, the present findings suggest that this may not be the case for people with high anger expressiveness.
Second, the morphine response for both healthy controls and chronic pain participants who reported both low levels of anger-out and exhibited low endogenous opioid function was higher – again, across three of four acute pain indexes – than for individuals fitting all other profiles. Thus, individuals low in the tendency to express anger may benefit greatly from administration of morphine if their innate capacity to generate endogenous opioid analgesia is also low. The findings we reported5,6 – that individuals with low endogenous opioid function exhibit high levels of morphine analgesic responses – may pertain most strongly to those low in anger-out. Thus, only individuals low in endogenous opioid function and anger expressiveness may benefit from supplementation of deficient endogenous opioid analgesia by use of exogenous opioid analgesics.
Third, both healthy controls and chronic pain participants, regardless of anger-out levels, who also exhibited high endogenous opioid function reported less morphine analgesia– again, across three of four acute pain indexes – than did individuals fitting all other profiles. We previously reported5,6 that individuals with high endogenous opioid function exhibited low levels of morphine analgesic responses. The current results indicate that these relationships may exist irrespective of individuals’ relative tendency to express anger, and so these individuals may benefit least from use of exogenous opioid analgesics.
Fourth, chronic low back pain subjects characterized by low endogenous opioid function and high anger-out reported the highest levels of negative emotional and cognitive side effects following morphine administration. Note that the interaction between endogenous opioid function and anger-out was nonsignificant for healthy controls. This finding suggests that individuals with chronic low back pain who have both low endogenous opioid function and high anger expressiveness tend to experience high levels of unpleasant cognitive and emotional reactions to morphine. Taken together with findings for morphine analgesic responses, it appears that chronic pain patients characterized by both low endogenous opioid function and high anger expressiveness experience an unfavorable balance of relatively low morphine analgesic responses and relatively high levels of unpleasant side effects. From a precision medicine perspective, this pattern of findings suggests that individuals with chronic low back pain who exhibit low endogenous opioid function and report high anger expressiveness may derive few benefits but many costs from morphine, and so may not represent optimal candidates for opioid therapy.
In contrast, low back pain subjects characterized by both low endogenous opioid function and low anger-out reported the lowest levels of side effects. Again, note that the interaction between endogenous opioid function and anger-out was nonsignificant for healthy controls. This finding suggests that individuals with chronic low back pain who exhibit both low endogenous opioid function and low anger expressiveness tend to experience low levels of unpleasant cognitive and emotional side effects during morphine administration. Taken together with findings for morphine analgesic responses, patients characterized by both low endogenous opioid function and low anger-out may experience a favorable balance of relatively high morphine analgesic responses coupled with relatively low levels of unpleasant side effects. Again, from a precision medicine perspective, it may be the case that individuals with chronic low back pain who have low endogenous opioid function and low anger expressiveness may derive many benefits and few costs from morphine, and so may represent optimal candidates for opioid therapy.
The present findings may also hint at some factors underlying opioid responsiveness. Our prior results showed that individuals with low endogenous opioid function may obtain high levels of morphine analgesia, thus suggesting highly responsive opioid receptors when exogenous opioids are available. Based on findings described above, it may be the case that this effect applies most strongly to individuals who are also low in anger expressiveness. That is, individuals with poor endogenous opioid function and who report low anger-out may still possess highly responsive opioid receptors, given their positive analgesic responses to morphine. Conversely, we found here that individuals with low endogenous opioid function and high anger-out revealed weaker analgesic responses, suggesting that these individual may exhibit poor opioid receptor function even in the presence of adequate levels of opioid agonists. These findings also shed further light on the nature of previously reported endogenous opioid deficits among individuals high in anger-out4,7, implying these deficits may derive more from opioid receptor-related differences than differences in levels of endogenous opioids.
In our past work, we have reported that people with a strong tendency to express anger may experience high levels of acute and chronic pain intensity because of these deficits in endogenous opioid function4,7. These deficits, we have speculated, may also underlie their difficulty regulating strong anger without verbal or physical expression. People with difficulty regulating strong emotions and who have intense chronic pain may gravitate toward opioid-based medications to, perhaps, find relief from both problems. Here, we have identified individuals with high anger-out for whom little analgesic benefit is gained from morphine, but who also may exhibit little endogenous opioid analgesia. It is reasonable to posit that these individuals may be at elevated risk for opioid abuse because they use narcotic analgesics more frequently and at higher doses in an effort to achieve some kind of relief, which may be elusive.
Study limitations are noted. First, the participants with chronic low back pain comprised a large number of people with moderate pain who were relatively functional, and so this sample may have differed to some degree from typical pain clinic samples. These participants were also distinct from a typical pain clinic sample in that none of the participants was taking daily opioid analgesic medication. We intentionally excluded daily opioid users because administration of naloxone to people taking daily opioid analgesics would trigger acute withdrawal symptoms. Second, only a single opioid analgesic agent was tested. It is unknown whether endogenous opioid function and/or anger expressiveness are related differently to responses to opioid analgesic medications other than morphine, which is a conceivable outcome given that different opioid analgesic agents are known to activate distinct signaling pathways.28 Third, the present results were generated in the context of a single dose of morphine in individuals not taking daily opioids. The degree to which these findings may generalize to the clinical situation of predicting long-term responses to daily opioids is unknown.
In sum, we made a focused effort to identify optimal candidates for opioid pain management by combining two factors in interactive (i.e., multivariate moderation) relationships. From a clinical standpoint, the present results hint that low back pain patients who tend to regulate anger through verbal and physical expression and also have deficient endogenous opioid function may be poor candidates for opioid therapy. In contrast, the present results imply that low back patients who tend not to regulate anger via direct expression and who also have deficient endogenous opioid function may make optimal candidates for opioid therapy. To our knowledge, this is the first study to directly examine the cost (side effects) and benefit (analgesia) balance of morphine administration in humans, and points to characteristics of two distinct kinds of morphine responders. Note, too, that the analgesic and side effect response profiles described here emerged only by identification through multivariate moderation analyses – they were not apparent in univariate analyses. We chose endogenous opioid function and anger expressiveness because of the relationships we found in previous work. The results of this laboratory study should guide future work on identifying other, and perhaps more narrowly defined, sets of characteristics of individuals for whom opioid therapy is indicated or not.
Figure 5.
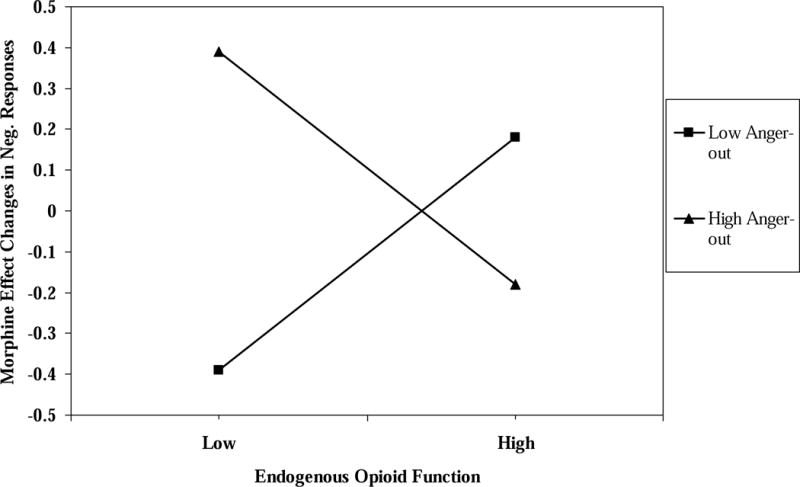
Low back pain subjects only. The interaction of endogenous opioid function values for MPQ-S scores × anger-out scale scores predicting Unpleasantness factor scores.
Perspective.
We sought to identify optimal candidates for opioid pain management. Low back pain patients who express anger and also have deficient endogenous opioid function may be poor candidates for opioid therapy. In contrast, low back patients who tend not to express anger and who also have deficient endogenous opioid function may make optimal candidates for opioid therapy.
Highlights.
At present, there is no sound method of identifying optimal opioid candidates.
Endogenous opioid function × anger-out interactions identified subgroups with different patterns of morphine analgesia and side effects.
Low endogenous opioid function plus low anger-out showed largest morphine analgesic responses.
High anger-out plus low endogenous opioid function showed weaker morphine analgesic responses
High anger-out plus low endogenous opioid function showed highest side effects.
Acknowledgments
Disclosures.
This study described in this manuscript was supported by Grants R01 DA031726 and R01 DA037891 from the National Institute of Drug Abuse/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Contributor Information
John W. Burns, Rush University Medical Center.
Stephen Bruehl, Vanderbilt University Medical Center.
Christopher R. France, Ohio University.
Erik Schuster, Rush University Medical Center.
Daria Orlowska, Rush University Medical Center.
Melissa Chont, Vanderbilt University Medical Center.
Rajnish K. Gupta, Vanderbilt University Medical Center.
Asokumar Buvanendran, Rush University Medical Center.
References
- 1.Berkowitz BA, Ngai SH, Hempstead J, Spector S. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975;195:499–504. [PubMed] [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell CI, Merrill JO, Silverberg MJ, Banta-Green C, Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009 Dec;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 5.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Kinner E, Schuster E, Passik S, France CR. Endogenous opioid function mediates the association between laboratory evoked pain sensitivity and morphine analgesic responses. Pain. 2013;154:1856–1864. doi: 10.1016/j.pain.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Schuster EM, France CR. Endogenous opioid inhibition of chronic low back pain influences degree of back pain relief following morphine administration. Reg Anesthesia Pain Med. 2014;39:120–125. doi: 10.1097/AAP.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl S, Chung OY, Burns JW, Biridepalli S. The association between anger expression and chronic pain intensity: evidence for partial mediation by endogenous opioid dysfunction. Pain. 2003;106:317–324. doi: 10.1016/S0304-3959(03)00319-1. [DOI] [PubMed] [Google Scholar]
- 8.Bruehl S, Chung OY, Burns JW, Diedrich L. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JW, Bruehl S, Chont M. Anger regulation style, anger arousal and acute pain sensitivity: evidence for an endogenous opioid “triggering” model. J Behav Med. 2014;37:642–653. doi: 10.1007/s10865-013-9511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 12.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient controlled analgesia. Clin J Pain. 2008;24:399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 13.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 16.Geha H, Nimeskern N, Beziat JL. Patient-controlled analgesia in orthognathic surgery: evaluation of the relationship to anxiety and anxiolytics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e33–36. doi: 10.1016/j.tripleo.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Bruehl S, Burns JW, Buvanendran A, Chont M, Schuster ME, France CR. Relationship between endogenous opioid function and opioid analgesic side effects. Reg Anesthesia Pain Med. 2014;39:219–224. doi: 10.1097/AAP.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harden RN, Bruehl S. Opioid prophylaxis of chronic pain: An examination of the ongoing controversy. J Back Musculoskel Rehab. 1997;9:155–180. doi: 10.3233/BMR-1997-9207. [DOI] [PubMed] [Google Scholar]
- 19.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004 Dec;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–159. [PubMed] [Google Scholar]
- 22.Martin WR. Drugs five years later: naloxone. Ann Intern Med. 1976;85:765–768. doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 23.Maurset A, Skoglung LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1991;13:643–647. [PubMed] [Google Scholar]
- 24.Melzack R. The short form of the McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 25.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. ECRI Institute, 5200 Butler Pike, Plymouth Meeting, PA, USA, 19462. Cochrane Database Syst Rev. 2010;20:CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc. 2009;84:593–601. doi: 10.1016/S0025-6196(11)60748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry F1, Parker RK, White PF, Clifford PA. Role of psychological factors in postoperative pain control and recovery with patient-controlled analgesia. Clin J Pain. 1994;10:57–63. doi: 10.1097/00002508-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- 29.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharm. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders, 1985. Hemisphere Publishing Corp.; Washington, D.C.: pp. 5–30. [Google Scholar]
- 31.Tseng LF, Lin JJ, Collins KA. Partial antinociceptive cross-tolerance to intracerebroventricular [beta]-endorphin in mice tolerant to systemic morphine. Euro J Pharmac. 1993;241:63–70. doi: 10.1016/0014-2999(93)90933-9. [DOI] [PubMed] [Google Scholar]
- 32.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 33.Wardlaw SL, Kim J, Sobieszczyk S. Effect of morphine on proopiomelanocortin gene expression and peptide levels in the hypothalamus. Molecular Brain Res. 1996;41:140–147. doi: 10.1016/0169-328x(96)00084-8. [DOI] [PubMed] [Google Scholar]
- 34.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Wasan AD, Michna E, Edwards RR, Katz JN, Nedeljkovic SS, Dolman AJ, Janfaza D, Isaac Z, Jamison RN. Psychiatric Comorbidity Is Associated Prospectively with Diminished Opioid Analgesia and Increased Opioid Misuse in Patients with Chronic Low Back Pain. Anesthesiology. 2015;123:861–72. doi: 10.1097/ALN.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Personal Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. (1988) [DOI] [PubMed] [Google Scholar]
- 37.Zacny JP. A possible link between sensation-seeking status and positive subjective effects of oxycodone in healthy volunteers. Pharmacol Biochem Behav. 2010 Mar;95(1):113–120. doi: 10.1016/j.pbb.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


