Abstract
Purpose of Review
Tuberculous meningitis is the most devastating manifestation of infection with Mycobacterium tuberculosis and represents a medical emergency. Approximately one half of tuberculous meningitis patients die or suffer severe neurologic disability. The goal of this review will be to review the pathogenic, clinical, and radiologic features of tuberculous meningitis and to highlight recent advancements in translational and clinical science.
Recent Findings
Pharmacologic therapy includes combination anti-tuberculosis drug regimens and adjunctive corticosteroids. It is becoming clear that a successful treatment outcome depends on an immune response that is neither too weak nor overly robust, and genetic determinants of this immune response may identify which patients will benefit from adjunctive corticosteroids. Recent clinical trials of intensified anti-tuberculosis treatment regimens conducted in Indonesia and Vietnam, motivated by the pharmacologic challenges of treating M. tuberculosis infections of the central nervous system, have yielded conflicting results regarding the survival benefit of intensified treatment regimens. More consistent findings have been observed regarding the relationship between initial anti-tuberculosis drug resistance and mortality among tuberculous meningitis patients.
Summary
Prompt initiation of anti-tuberculosis treatment for all suspected cases remains a key aspect of management. Priorities for research include the improvement of diagnostic testing strategies and the optimization of host-directed and anti-tuberculosis therapies.
Keywords: Tuberculosis, Meningitis, Tuberculous meningitis, Mycobacterial infections, Central nervous system infections
Introduction
Descriptions of clinical presentations of meningitis, with proposed mechanisms of disease, are found in ancient Greek and Roman medical texts. The “Canon of Medicine,” written by Ibn Sina (980–1037), provided a detailed approach to the diagnosis and treatment of meningitis, including bloodletting and the application of roses and vinegar to the forehead, and became the standard medical textbook across Europe and Asia for several centuries [1]. Thomas Willis (1621–1675) was the first to relate meningeal tubercles to the signs and symptoms of meningitis, observing an association between cerebral edema and “plaques and tubercles” of the meninges in patients with “fatal and incurable headaches,” which he attributed to abnormal activities of animal spirits [2]. The first comprehensive clinical description of tuberculous meningitis was provided by the Scottish physician Robert Whytt (1714–1766), in a case series of 20 patients entitled “On the Dropsy on the Brain” [3]. Although the infectious nature of tuberculous meningitis was established following Robert Koch’s (1843–1910) discovery of the tubercle bacillus in 1882, it was not until the work of Arnold Rich (1893–1968) and Howard McCordock (1895–1938) several decades later, discussed below, that the unique pathogenic features of tuberculous meningitis were recognized, distinguishing this disease from other types of bacterial meningitis [4].
Prior to the modern chemotherapy era, tuberculous meningitis was an almost uniformly fatal condition [5]. In 1948, The British Medical Research Council published, “Streptomycin Treatment of Tuberculous Meningitis,” which described 105 consecutive cases of tuberculous meningitis treated with intrathecal streptomycin, reporting a 6-month mortality rate of 64% [5]. The later adoption of combination treatment regimens for tuberculous meningitis was based on clinical trials of pulmonary tuberculosis conducted by the British Medical Research Council over several decades [6]. Although the routine use of rifamycin-based combination regimens has led to significant improvements in the survival of tuberculous meningitis patients, the disease once known as “dropsy on the brain” continues to present significant challenges with regard to diagnostic testing and therapeutic strategies. The goal of this review will be to review the pathogenic, clinical, and radiologic features of tuberculous meningitis and to highlight recent advancements in translational and clinical science.
Epidemiology
Despite a recent worldwide decline of incident cases, tuberculosis remains a leading cause of death on a global scale [7]. One third of the world’s population is infected with Mycobacterium tuberculosis, which progresses to active disease in approximately 10% of individuals. In 2015, the World Health Organization estimated that 10.4 million incident cases of tuberculosis occurred worldwide, with 10% among children and 11% among individuals living with human immunodeficiency virus (HIV) [7]. Overall, tuberculous meningitis comprises 1–2% of incident cases of active tuberculosis. In settings with concurrent epidemics of HIV and tuberculosis, M. tuberculosis is now a leading cause of bacterial meningitis, alongside pathogens such as Neisseria meningitidis, Haemophilus influenzae, or Streptococcus pneumoniae [8, 9]. Approximately one half of all tuberculous meningitis infections lead to severe disability or death [10].
Pathogenesis
Tuberculosis infections, regardless of clinical presentation, are established with the inhalation of bacilli into distal airways, in the form of droplet nuclei. After escaping from the airspace into the interstitium, M. tuberculosis spreads first to the local draining lymph nodes in the lung and then to distant sites via the bloodstream. During hematogenous dissemination, mycobacteria may be deposited adjacent to the ventricles or subarachnoid space, leading to granuloma formation at those sites of deposition [11]. Granulomas in the central nervous system are often detected in post-mortem studies of tuberculosis patients, even among individuals without suspected disease at that site [12]. In their landmark autopsy study of tuberculous meningitis patients, Rich and McCordock observed granulomas adjacent to the subarachnoid space that had ruptured into that space in nearly all cases [4]. They proposed that the rupture of this granuloma (known as a “Rich focus”) led to the robust inflammatory response and therefore provides the initiating event of tuberculous meningitis. Although Rich and McCordock’s broad framework of pathogenesis remains in place, recent work has identified potential modulators of this process, including variability in the host immunologic response [13] and diversity of M. tuberculosis strains [14].
M. tuberculosis infections of the central nervous system most commonly present as subacute or chronic meningitis. There may also be discrete granulomas (tuberculomas), and these lesions may enlarge or coalesce sufficiently to present with the signs and symptoms of space-occupying lesions [11, 15]. Central nervous system infection may be the only clinical manifestation of tuberculosis, or may occur in conjunction with pulmonary or disseminated disease, and the latter is known as miliary tuberculosis (named for the millet seed appearance of the granulomas) [16]. The widespread anatomic involvement of miliary tuberculosis promotes the development of meningitis by increasing the probability of granuloma deposition in the central nervous system [17].
The patient’s immune response is triggered by this rupture event, and the downstream consequence is the collection of a tuberculous exudate at the basal brain. Histologically, this exudate includes erythrocytes, mononuclear cells, neutrophils, and bacilli [11]. Another downstream consequence may be the development of vasculitis within the cerebral arterial system including branches of the middle cerebral artery, the vertebrobasilar system, and the circle of Willis vessels [18]. Infarctions in the areas supplied by these vessels are a major contributor to long-term neurologic deficits among survivors [19]. Cranial nerve impairment can result from either infarction or compression by the exudate [11]. In a mouse model of tuberculous meningitis, increasing local production of TNF-alpha within the exudate was closely related to pathogenesis [20]. Hydrocephalus among tuberculous meningitis patients is more common in children than adults and more progressive than the hydrocephalus that accompanies other types of bacterial meningitis [21].
Clinical Presentation
The reported duration of symptoms of tuberculous meningitis ranges between a single day and 6 months, presenting as acute, subacute, or chronic meningitis. Pulmonary tuberculosis may also be present [22]. The non-specific, fluctuating prodrome of tuberculous meningitis includes malaise and fatigue, anorexia and vomiting, headache, and fever [23, 24]. Acute presentations may be difficult to distinguish from other forms of bacterial meningitis. Less commonly, tuberculous meningitis presents as a progressive dementia, characterized by changes to personality and social withdrawal. There may be cranial nerve palsies at the time of initial presentation. Sixth nerve palsies are most common, but the second, third, fourth, and eighth nerves can also be involved [25]. Seizures may occur at any time throughout the initial diagnostic period, as well as during the entire treatment period. Pyramidal or cerebellar signs are a consequence of brainstem infarctions or cerebral edema, and hydrocephalus is a common finding in patients with these severe manifestations, due to the underlying pathogenic effects of the tuberculous exudate collecting at the basal brain [26]. In its final stages, tuberculous meningitis is characterized by coma, spasticity, and posturing.
The clinical staging system for tuberculous meningitis was developed by the British Medical Research Council (Table 1). Patients satisfy criteria for stage 1 if they are alert and oriented and free of focal neurologic deficits. Patients with stage 2 disease have either a focal neurologic deficit or a Glasgow Coma Score between 10 and 14, and stage 3 patients have a Glasgow Coma Score less than 10, indicating severe brain injury. The clinical stage on presentation is the strongest predictor of clinical outcome, with a high mortality risk for patients presenting with stage 3 disease [23, 24, 27, 28]. Diagnostic delays are related to inferior outcomes, including delays as short as 3 days in some reports, reinforcing the critical need to initiate anti-tuberculosis therapy as soon as the diagnosis is suspected [29, 30].
Table 1.
British Medical Research Council staging of tuberculous meningitis
| Stage | Level of consciousness | Focal neurologic deficits | Other findings |
|---|---|---|---|
| 1 | Normal | None | Fever, irritability, headache |
| 2 | Lethargy | Minor | Nuchal rigidity, seizures, cranial nerve palsies |
| 3 | Stupor or coma | Major | Posturing, hemodynamic instability |
Source: [5]
Diagnostic Approach
Cerebrospinal Fluid Studies
Analysis of cerebrospinal fluid is a key step in the diagnostic approach to tuberculous meningitis. Typical findings include lymphocytic pleocytosis, increased protein, and decreased glucose [31–33]. Prediction rules based on clinical characteristics and cerebrospinal fluid analysis have been developed in different settings but are challenged by the overlapping clinical features of cryptococcal and tuberculous meningitis among patients living with HIV infection [34, 35]. The prediction rule initially developed in a Vietnamese population includes scoring for age, disease duration, white blood cell counts in blood and cerebrospinal fluid, and neutrophil percentage in cerebrospinal fluid [31] (Table 2) and has been subsequently evaluated in diverse settings with a low incidence of HIV co-infection [35–39]. Generally, the positive and negative predictive values of this prediction rule will vary based on the proportion of meningitis cases caused by M. tuberculosis versus other causes. As one example, in Malawi, with a higher HIV prevalence and a greater proportion of meningitis cases caused by other bacterial pathogens as compared to Vietnam, the positive predictive value decreased to 14%, with a negative predictive value of 94% [35].
Table 2.
Clinical prediction rule to support the diagnosis of tuberculous meningitis [31]
| Clinical feature | Scorea |
|---|---|
| Age | |
| ≥36 years | + 2 |
| <36 years | 0 |
| White blood cell count in blood | |
| ≥15 × 103 cells/mL | + 4 |
| <15 × 103 cells/mL | 0 |
| Duration of illness | |
| ≥6 days | − 5 |
| <6 days | 0 |
| White blood cell count in cerebrospinal fluid | |
| ≥900 × 103 cells/µL | + 3 |
| <900 × 103 cells/µL | 0 |
| Percentage of neutrophils in cerebrospinal fluid | |
| ≥75 | + 4 |
| <75 | 0 |
Total score ≤ 4 suggests tuberculous meningitis; total score > 4 suggests alternate diagnosis
Imaging Studies
The typical neuroradiologic findings in tuberculous meningitis include hydrocephalus, enhancement of the basilar meninges, and infarctions. Both computerized tomography (CT) and magnetic resonance imaging (MRI) may be used to assess possible tuberculous meningitis [40]. While neuroimaging studies may appear normal in early stages of disease, most patients will develop one or more neuroimaging abnormalities [41]. Meningeal enhancement in the basal brain and communicating hydrocephalus are the most common radiographic findings in patients with tuberculous meningitis [42–45]. Infarctions often occur despite anti-tuberculosis treatment, with the basal ganglia and internal capsule being most affected [41, 46]. Abnormalities on magnetic resonance angiography, either localized or disseminated stenosis of intracranial arteries, predict the risk of subsequent cerebral infarctions and related clinical outcomes [47, 48]. Among HIV-uninfected tuberculous meningitis patients in China, the two patterns of observed abnormalities in magnetic resonance angiography studies were irregular calibers of intracranial arteries, widely disseminated, and localized stenosis at the basal brain, with the middle cerebral artery most frequently involved overall [47].
Tuberculomas, which can be seen in tuberculosis patients both with and without meningitis, appear as round, lobulated masses, either homogenous or ring-enhancing, with irregular walls of varying thickness [46]. A patient may have solitary or multiple tuberculomas, with some demonstrating central calcification and surrounding hypoattenuation (the “target sign”) [49]. The obstruction of cerebrospinal fluid outflow by enlarging tuberculomas can lead to a non-communicating hydrocephalus. Additionally, patients may develop tuberculous meningitis affecting the spine, characterized by cerebro-spinal fluid loculations, obliteration of the spinal subarachnoid space, and loss of visible spinal cord in the cervicothoracic region [41, 50].
AFB Smear and Culture
There is considerable variability in the reported sensitivity of cerebrospinal fluid smear and culture in cases of tuberculous meningitis [10]. The sensitivity is improved by several technical factors, including the volume of cerebrospinal fluid cultured (at least 6 mL) and examination of the cerebrospinal fluid smear for at least 30 min [51]. A modification of the Ziehl-Neelsen stain, using cytospin slides with Triton processing, was reported to dramatically improve sensitivity among 48 cerebrospinal fluid samples collected from 29 Chinese tuberculous meningitis patients [52].
Nucleic Acid Amplification Testing
Rapid molecular diagnostic tests can assist with tuberculous meningitis diagnostic strategies. The Xpert M. tuberculosis/ RIF assay (Cepheid, CA) is based on real-time polymerase chain reaction to detect M. tuberculosis DNA in clinical samples, simultaneous with the presence or absence of mutations in the genetic locus corresponding to rifampin resistance. Among 379 suspected tuberculous meningitis patients in Vietnam, Xpert MTB/RIF demonstrated 59% sensitivity and 99% specificity; by comparison, the sensitivity of AFB smear of cerebrospinal fluid was 79% in that study [53]. Interestingly, the sensitivity of Xpert was increased among HIV-infected tuberculous patients, reflecting an increased bacterial load in cerebrospinal fluid. The Xpert assay also identified four isolates with mutations conferring rifampin resistance; three of these isolates were confirmed rifampin-resistant by culture and one was culture negative. The sensitivity of Xpert for the diagnosis of tuberculous meningitis is improved by centrifugation of cerebrospinal fluid [54], yet remains insufficient to exclude (“rule out”) the diagnosis. Therefore, empiric anti-tuberculosis treatment should be initiated for patients with possible or probable tuberculous meningitis, even with a negative Xpert cerebrospinal fluid assay result [55].
Treatment of Tuberculous Meningitis
Anti-tuberculosis Drug Regimens
Treatment regimens for tuberculous meningitis have been extrapolated from pulmonary tuberculosis clinical trials. Recently updated guidelines from the American Thoracic Society, Infectious Disease Society of America, and the US Centers for Disease Control and Prevention continue to recommend initial four-drug therapy with isoniazid, rifampin, pyrazinamide, and ethambutol [56] (Table 3). Pyrazinamide is discontinued after the first 2 months of therapy, corresponding to the intensive phase, and ethambutol is discontinued once susceptibility to isoniazid has been confirmed by susceptibility testing, for a total duration of 9 to 12 months. Isoniazid and pyrazinamide have excellent cerebrospinal fluid penetration [57–59], with lesser penetration for ethambutol [60, 61]. Importantly, cerebrospinal fluid concentrations of rifampin barely exceed the minimum inhibitory concentration against M. tuberculosis [62–64], a notable finding given the role of rifampin in driving tuberculosis treatment response. Even among pulmonary tuberculosis patients, the guideline-recommended rifampin dose sizes lead to pharmacokinetic exposures at the lower end of the dose response curve [65]. Among second-line agents, fluoroquinolones demonstrate favorable distribution across the blood-brain barrier [66], achieving cerebrospinal fluid concentrations greater than the minimum inhibitory concentration with standard dosing [67].
Table 3.
ATS/CDC/IDSA guidelines for dosages of anti-tuberculosis drugs for the treatment of drug-susceptible tuberculous meningitis in children and adults
| Anti-tuberculosis drug | Daily dose in children | Daily dose in adults |
|---|---|---|
| Isoniazid | 10–15 mg per kg | 5 mg per kg (typically 300 mg) |
| Rifampin | 10–20 mg per kg | 10 mg per kg (typically 600 mg) |
| Ethambutol | 20 (15–25) mg per kg | Weight 40–55 kg: 800 mg |
| Weight 56–75 kg: 1200 mg | ||
| Weight 76–90 kg: 1600 mg | ||
| Pyrazinamide | 35 (30–40) mg per kg | Weight 40–55 kg: 1000 mg |
| Weight 56–75 kg: 1500 mg | ||
| Weight 76–90 kg: 2000 mg |
Adult dosing starting at age 15 years or weight > 40 kg in younger children. Source: [56]
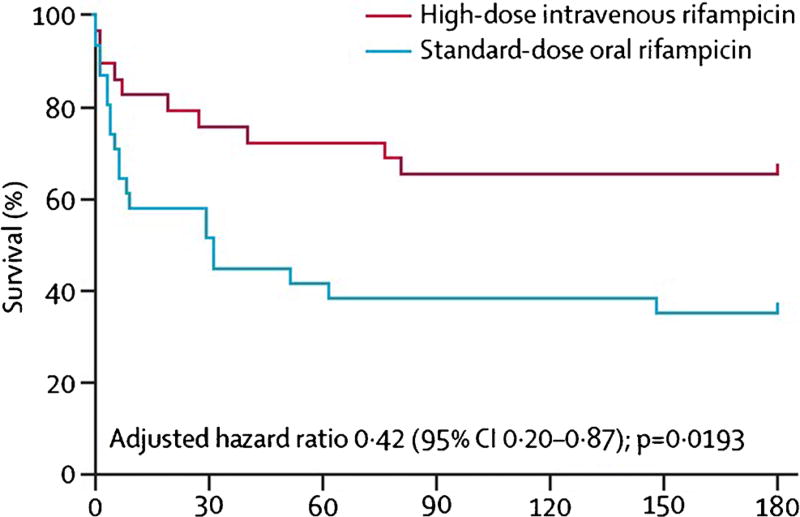
Two recent clinical trials have evaluated intensified anti-tuberculous treatment regimens for tuberculous meningitis, with a combination of increased rifampin dosing and a fluoroquinolone added to the first-line drugs. A clinical trial of 60 Indonesian adults evaluated standard-dose oral rifampicin (450 mg daily, corresponding to 9.4 mg per kg) versus higher-dose intravenous rifampicin (600 mg daily, 12.5 mg per kg) with or without standard-dose moxifloxacin (400 mg) or high-dose moxifloxacin (800 mg), in an open-label factorial design. The use of high-dose intravenous rifampin for an initial 2-week period was associated with a reduction in 6-month mortality from 65 to 34% [68•] (Fig. 1). The change in rifampin dose and mode of delivery was associated with 3-fold increases in plasma area under the time-concentration curve, plasma maximum concentration, and cerebrospinal fluid maximum concentration. Subsequent pharmacokinetic/pharmacodynamics analysis of this study formally demonstrated the relationship between rifampin cerebrospinal fluid exposures and survival [69].
Fig. 1.
Effect of high-dose intravenous rifampin on survival of Indonesian tuberculous meningitis patients (Reprinted from Lancet Infectious Diseases, Vol 13(1), Rovina Ruslami, A Rizal Ganiem, Sofiati Dian, Lika Apriani, Tri Hanggono Achmad, Andre J van der Ven, George Borm, Rob E Aarnoutse, Reinout van Crevel, Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial, page nos. 27–35, Copyright (2013), with permission from Elsevier)
A randomized, double-blind, placebo-controlled trial of 817 adult tuberculous meningitis patients was conducted in Vietnam, comparing high-dose oral rifampin (15 mg/kg/day) and levofloxacin (20 mg/kg/day), administered in combination with pyrazinamide and ethambutol during the first 8 weeks of treatment, with standard treatment regimen according to national guidelines. In notable contrast to the Indonesian trial, intensified anti-tuberculosis treatment was not associated with improved survival compared to standard treatment over 9 months of follow-up (28% mortality in both arms) [70•]. The reasons for the disparate findings between these two intensified treatment trials are a subject of continued debate, with regard to the relative contributions of anti-tuberculosis drug exposures and detrimental host inflammatory responses in determining tuberculous meningitis treatment outcomes. Follow-up pharmacokinetic studies of the Vietnamese study patients may further elucidate the exposure-response relationships of high-dose rifampin and levofloxacin.
Adjunctive Corticosteroids
Adjunctive dexamethasone is recommended for all individuals with suspected tuberculous meningitis. The benefit of adjunctive, concurrent, treatment with dexamethasone was demonstrated in a randomized double-blind clinical trial of Vietnamese adolescents and adults [27]. In subanalyses, the overall benefit of adjunctive corticosteroids was driven by improved survival among HIV-uninfected adults with early stage disease, without impacting rates of neurologic disability among survivors. In the subset of HIV-infected adults enrolled in this trial, there was no benefit of adjunctive corticosteroids on survival or rates of neurologic disability. In a follow-up evaluation of participants in this trial, the survival benefit of dexamethasone continued over a 2-year period from the time of enrollment but was again concentrated among patients with early stage disease [71].
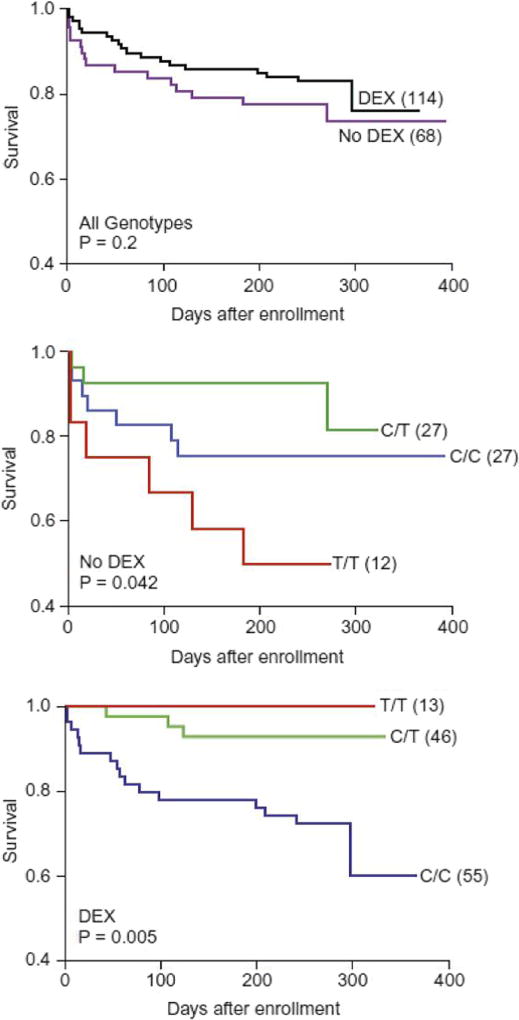
Recent translational science has elucidated mechanisms for the protective effect of adjunctive corticosteroids during tuberculous meningitis treatment. Studies of mycobacterial infections in zebrafish identified mutations in the gene encoding leukotriene A4 hydrolase (LTA4H) as a key mediator of disease susceptibility, and the importance of this pathway was later validated in human M. tuberculosis infection [72]. When re-analyzing the clinical data from the randomized trial of dexamethasone in Vietnam, a surprising finding was that both the hypoinflammatory genotype and hyperinflammatory genotypes (the CC and TT genotypes of the LTA4H promoter SNP rs17525494, respectively) were related to inferior outcomes, when compared to the heterozygote genotype [73]. Importantly, the survival benefit of dexamethasone was concentrated among patients with the hyperinflammatory genotype (Fig. 2). Follow-up studies of tuberculous meningitis patients in Vietnam [74] and Indonesia [75], with all patients receiving adjunctive dexamethasone, have yielded conflicting results regarding the impact of LTA4H promoter mutations on tuberculous meningitis outcomes, and more work remains to untangle these relationships. Yet it is becoming clear that both the susceptibility to tuberculosis and the outcomes of treatment are dependent on an appropriate immune response that is neither ineffectually weak nor inappropriately robust.
Fig. 2.
Differential efficacy of adjunctive dexamethasone among tuberculous meningitis patients based on LTA4H promoter genotype (Reprinted from Cell, Vol 148(3), David M. Tobin, Francisco J. Roca, Sungwhan F. Oh, Ross McFarland, Thad W. Vickery, John P. Ray, Dennis C. Ko, Yuxia Zou, Nguyen D. Bang, Tran T.H. Chau, Jay C. Vary, Thomas R. Hawn, Sarah J. Dunstan, Jeremy J. Farrar, Guy E. Thwaites, Mary-Claire King et al., Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections, Pages No. 434–46, Copyright (2012), with permission from Elsevier). a Effect of adjunctive dexamethasone on survival across all genotypes. b Survival of patients not treated with dexamethasone, stratified by LTA4H promoter genotype. c Survival of patients treated with dexamethasone, stratified by LTA4H promoter genotype
Aspirin
Given the role of stroke in the adverse outcomes of tuberculous meningitis and the failure of dexamethasone to reduce neurologic disability, investigators have looked at adjunctive aspirin therapy. Open-label clinical trials in children [76] and adults [77] have examined the role of aspirin during tuberculous meningitis treatment, with conflicting results. An ongoing double-blind, randomized trial of aspirin in Vietnamese tuberculous meningitis patients may shed light in this area.
Special Populations
Pediatric Disease
At a population level, the highest incidence of tuberculosis meningitis is found among children aged 2 to 4 years old [78]. Establishing the diagnosis early in disease, before permanent neurologic damage has occurred, is challenging given the young age and non-specific symptoms [79]. Among 204 Romanian tuberculous meningitis patients, children were less likely to develop mental status changes but more likely to develop other complications such as hydrocephalus [80]. Neuro-ophthalmic findings were present in two thirds of Indian children with tuberculous meningitis, most commonly retrobulbar neuritis and optic atrophy [81]. In addition to the indirect effects of raised intracranial pressure on the optic nerve [81], M. tuberculosis may directly invade the proximal portion of the optic nerve, or the accumulating tuberculous exudate may strangulate the optic nerve and chiasma [82]. Hyponatremia is also more likely in children [78], due to either the syndrome of inappropriate anti-diuretic hormone or cerebral salt wasting [78, 83], which can be distinguished by extracellular fluid status [84]. Cerebrospinal fluid biomarkers of neuronal and astroglial injury predict survival at 6 months in South African children, with early changes (during the first 3 weeks) providing the greatest prognostic information [42].
There are few pediatric tuberculosis clinical trials to guide dosing decisions, and the dosing of tuberculosis drugs for treating tuberculous meningitis has been largely extrapolated from adult experiences, although World Health Organization guidelines now recommend higher doses of rifampin (15 mg per kg), isoniazid (10 mg per kg), and pyrazinamide (35 mg per kg) based on pharmacokinetic data [85–88]. In an observational study of 100 Vietnamese children with suspected tuberculous meningitis, treated with weight-based dosing for isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin according to national guidelines, an inverse relationship was observed between age and drug clearance, leading to lower drug exposures among the youngest children [89]. While cerebrospinal fluid concentrations of isoniazid and pyrazinamide exceeded the minimum inhibitory concentration for M. tuberculosis in most children, nearly all children demonstrated rifampin cerebrospinal fluid exposures below this threshold. Notably, weight-based dosing regimens employed in Cape Town, South Africa, for many years have used higher doses of first-line drugs, including up to 20 mg per kg of rifampin [78]. The superior outcomes among children in this setting have been attributed to this practice [90].
HIV Infection
Infection with HIV increases the risk of progression from latent tuberculosis infection to active tuberculosis disease, as well as the risk of primary progressive disease immediately following the acquisition of infection [91]. HIV-associated tuberculosis is also more likely to present with extrapulmonary manifestations, with an increasing likelihood in relation to the degree of immunosuppression [92]. This increased risk of extrapulmonary disease is reflected in the increased risk of meningitis among HIV/tuberculosis patients, compared to tuberculosis patients without HIV infection [93].
A number of studies have compared the clinical presentation of tuberculous meningitis patients in the presence and absence of HIV co-infection. Generally, symptoms such as fever, headache, weight loss, and vomiting are similar in both groups of patients, while HIV-infected patients are more likely to demonstrate hepatosplenomegaly and lymphadenopathy on examination [93–100]. Some studies have reported a greater proportion of HIV-associated cases with an altered level of consciousness [95, 101], but others have found no difference [93, 96]. Laboratory findings among HIV-associated tuberculous meningitis cases may be more likely to include elevated liver transaminases, anemia, and hyponatremia [99, 102, 103]. In addition, lower cerebrospinal fluid leukocytes and protein levels, and higher opening pressures, have been reported [31, 95, 98]. On neuroimaging studies, HIV-infected patients are more likely to demonstrate meningeal enhancement, cerebral infarctions, and mass lesions and less likely to have obstructive hydrocephalus [29, 93, 95, 96, 100].
Randomized clinical trials have established the benefit of early anti-retroviral therapy initiation among HIV/ tuberculosis patients, particularly in the setting of advanced HIV disease (e.g., CD4 T cell count less than 50 cells/µL) [104, 105]. However, a notable exception was a randomized trial of early versus deferred anti-retroviral therapy among 253 Vietnamese tuberculous meningitis patients [106]. There was no difference in survival when anti-retroviral therapy was initiated within 1 week or deferred until after 8 weeks of tuberculosis treatment, and a greater number of severe adverse events occurred in the early anti-retroviral therapy arm of the study. The lack of benefit of early anti-retroviral therapy may reflect detrimental effects of the immune reconstitution inflammatory syndrome in the confines of the central nervous system. In this context, it is noteworthy that early anti-retroviral therapy increased the mortality risk in a randomized controlled trial of early versus deferred anti-retroviral therapy among African cryptococcal meningitis patients [107], perhaps via a similar mechanism [108].
Drug-Resistant Tuberculous Meningitis
The global challenge of drug-resistant tuberculosis is magnified by the particular threats of tuberculous meningitis, which has a higher case-fatality rate overall compared to other forms of tuberculosis. Treatment of known or suspected drug-resistant tuberculous meningitis can be supported by therapeutic drug monitoring [109], given the variable pharmacokinetics and narrower therapeutic index of many second-line anti-tuberculosis drugs [110].
Isoniazid Resistance
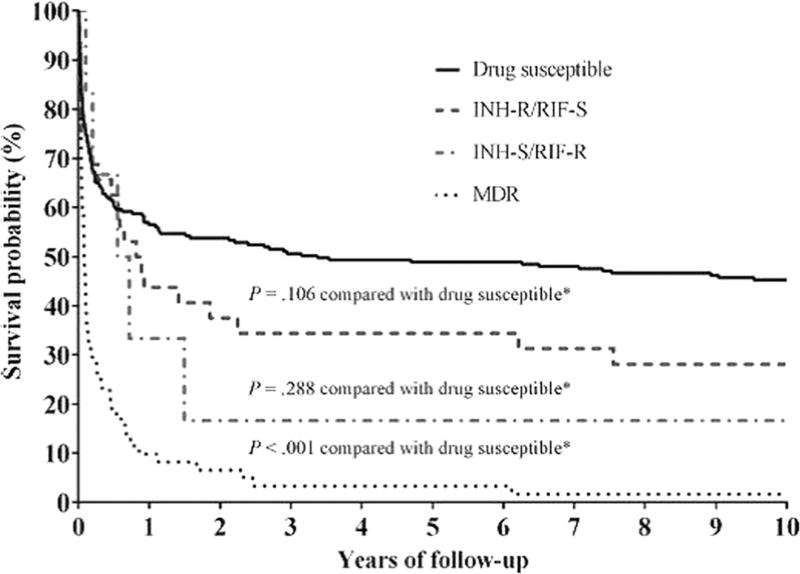
Isoniazid freely distributes into cerebrospinal fluid, both in the settings of inflamed and uninflamed meninges, and demonstrates bactericidal activity against M. tuberculosis. These pharmacokinetic and pharmacodynamic properties point to a central role in the treatment of tuberculous meningitis. Globally, isoniazid monoresistance is the most prevalent form of drug-resistant tuberculosis [111]. Among 1614 tuberculous meningitis patients in the USA over a 12-year period, there was a 2-fold increase in the odds of death before completing treatment among patients with isoniazid-resistant disease [22]. The magnitude of the association between initial isoniazid resistance and death was similar among 186 HIV-infected tuberculous meningitis patients in Vietnam, with the additional finding that excess mortality due to isoniazid resistance did not emerge until after the first 60 days of treatment [112]. Interestingly, this time-dependent relationship between isoniazid resistance and death was also observed among tuberculous meningitis patients in New York City, USA [113•] (Fig. 3).
Fig. 3.
Survival of New York City, USA, tuberculous meningitis patients according to initial drug susceptibility testing (C Vinnard et al., Long-term mortality of patients with tuberculous meningitis in New York City: a cohort study, Clinical Infectious Diseases, 2017, volume 64, issue 4, pages 401 to 407, by permission of Oxford University Press). (Fig. 2, panel C, from [113•])
Thus, isoniazid-resistant tuberculous meningitis may provide a window of opportunity to impact the course of disease with early intensification of therapy. Although intensified therapy with high-dose rifampin and a fluoroquinolone failed to detect a survival benefit overall among Vietnamese patients, there was a survival benefit among HIV-uninfected tuberculous meningitis patients with isoniazid-resistant disease [114•]. Importantly, the survival benefit of intensified treatment was greatest when intensification occurred at the time of initiation of tuberculosis treatment, rather than in response to drug susceptibility testing. Implementation of these findings into clinical practice would require either a rapid diagnostic test for isoniazid resistance in tuberculous meningitis patients or the use of risk factors for isoniazid resistance, including tuberculosis contact history or the epidemiology of isoniazid-resistant disease in the underlying population [115].
Multidrug Resistance
Multidrug-resistant tuberculous meningitis, defined as resistance to both isoniazid and rifampin with or without resistance to other agents, carries a poor prognosis. The marked increase in mortality of tuberculous meningitis when rifampin resistance is added to isoniazid resistance demonstrates the importance of rifampin in the treatment regimen, despite its limited penetration into cerebrospinal fluid compared to isoniazid. Over a 12-year period in the USA, 19 of 26 (73%) patients with multidrug-resistant tuberculous meningitis died before completing tuberculosis treatment [116]. Among 16 patients with multidrug-resistant tuberculous meningitis in the intensified therapy trial in Vietnam (including a single patient with rifampin-resistant, isoniazid-susceptible disease), 11 died before completing treatment, with a median time to death of 27 days from treatment initiation [70•]. Descriptions of successful treatment of multidrug-resistant tuberculous meningitis are limited to case reports [117], including the use of intrathecal administration of levofloxacin and amikacin [118]. Linezolid may also be a useful second-line drug in this context based on recently published clinical experiences in Chinese children [119] and adults [120].
Long-Term Neurologic Outcomes Among Survivors
Neurologic sequelae resulting from tuberculous meningitis include hydrocephalus, stroke, cranial nerve palsies, seizures, and mass lesions, and the risk of these sequelae increases with diagnostic delays [29]. Among participants in the clinical trial of dexamethasone in Vietnam, 14% demonstrated severe disability at 5 years of follow-up, with no difference between the dexamethasone and placebo arms [71]. Among HIV-uninfected tuberculous meningitis patients in New York City who successfully completed treatment, there was no additional mortality burden when compared to population age- and sex-matched controls over a 10-year follow-up period, although differences in neurologic morbidities that did not impact survival were not measured [113•]. A multistate cohort study of 806 US tuberculous meningitis patients, conducted using administrative claims data, identified rates of neurologic complications at 1 year following diagnosis for stroke (15%), seizures (12%), and visual impairment (19%) [121]. Most of these complications occurred during the index hospitalization. A systematic review of 19 pediatric tuberculous meningitis studies, including a total of 1636 children treated for tuberculous meningitis, reported a mortality rate of 19%, with a 54% risk of neurologic complications among survivors [28]. More work is needed to understand the total burden of tuberculous meningitis in high-incidence settings, with particular attention to the consequences of permanent disabilities among childhood survivors of this disease.
Summary
The previous decade has witnessed advances in our understanding of several aspects of tuberculous meningitis, but much work remains. Prompt initiation of anti-tuberculosis treatment for all suspected cases remains a key aspect of management, given the powerful relationship between disease stage at treatment initiation and long-term outcomes. Ongoing research efforts will refine the optimal treatment regimen, targeting the pathogen itself as well as deleterious inflammatory responses, with the goal of reducing the morbidity and mortality of a disease that targets the most vulnerable members of society.
Footnotes
This article is part of the Topical Collection on Infection
Conflict of Interest Alyssa Mezochow, Kiran Thakur, and Christopher Vinnard declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Ramdani H, Hajjioui A, Fourtassi M. Brain lesions in Ibn Sina’s “Canon of Medicine”: ancient theories and current medical concepts. J Med Surg Res. 2014;1(2):73–6. [Google Scholar]
- 2.Eadie MJ. A pathology of the animal spirits—the clinical neurology of Thomas Willis (1621–1675). Part II—disorders of intrinsically abnormal animal spirits. J Clin Neurosci. 2003;10(2):146–57. doi: 10.1016/s0967-5868(02)00164-9. [DOI] [PubMed] [Google Scholar]
- 3.Whytt R. Observations on the dropsy in the brain. The Works of Robert Whytt. 1768 [Google Scholar]
- 4.Rich AR, McCordock HA. Pathogenesis of tubercular meningitis. Bull Johns Hopkins Hosp. 1933;52:5–13. [Google Scholar]
- 5.STREPTOMYCIN treatment of tuberculous meningitis. Lancet. 1948;1(6503):582–96. [PubMed] [Google Scholar]
- 6.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10 Suppl 2):S231–79. [PubMed] [Google Scholar]
- 7.Organization. W.H. Global tuberculosis report. 2016. [Google Scholar]
- 8.Britz E, et al. The epidemiology of meningitis among adults in a South African Province with a high HIV prevalence, 2009–2012. PLoS One. 2016;11(9):e0163036. doi: 10.1371/journal.pone.0163036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergemann A, Karstaedt AS. The spectrum of meningitis in a population with high prevalence of HIV disease. QJM. 1996;89(7):499–504. doi: 10.1093/qjmed/89.7.499. [DOI] [PubMed] [Google Scholar]
- 10.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi: 10.1016/S1474-4422(13)70168-6. [DOI] [PubMed] [Google Scholar]
- 11.Rock RB, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21(2):243–61. doi: 10.1128/CMR.00042-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacGregor G. Tuberculosis of the central nervous system, with special reference to tuberculous meningitis. J Pathol Bacteriol. 1937;45:613–45. [Google Scholar]
- 13.Thuong NT, et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8(5):422–8. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 14.Thwaites GE, et al. Isoniazid resistance, mycobacterial genotype and outcome in Vietnamese adults with tuberculous meningitis. Int J Tuberc Lung Dis. 2002;6(10):865–71. [PubMed] [Google Scholar]
- 15.Leonard JM. Central nervous system tuberculosis. Microbiol Spectr. 2017;5(2) doi: 10.1128/microbiolspec.TNMI7-0044-2017. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135(5):703–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Donald PR, Schaaf HS, Schoeman JF. Tuberculous meningitis and miliary tuberculosis: the rich focus revisited. J Inf Secur. 2005;50(3):193–5. doi: 10.1016/j.jinf.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Misra UK, Kalita J, Maurya PK. Stroke in tuberculous meningitis. J Neurol Sci. 2011;303(1–2):22–30. doi: 10.1016/j.jns.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Chan KH, et al. Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis. 2005;19(6):391–5. doi: 10.1159/000085568. [DOI] [PubMed] [Google Scholar]
- 20.Tsenova L, et al. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci U S A. 1999;96(10):5657–62. doi: 10.1073/pnas.96.10.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57(4):368–74. doi: 10.4103/0028-3886.55572. [DOI] [PubMed] [Google Scholar]
- 22.Vinnard C, et al. Isoniazid resistance and death in patients with tuberculous meningitis: retrospective cohort study. BMJ. 2010;341:c4451. doi: 10.1136/bmj.c4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kent SJ, et al. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17(6):987–94. doi: 10.1093/clinids/17.6.987. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy DH, Fallon RJ. Tuberculous meningitis. JAMA. 1979;241(3):264–8. [PubMed] [Google Scholar]
- 25.Sharma P, et al. Incidence, predictors and prognostic value of cranial nerve involvement in patients with tuberculous meningitis: a retrospective evaluation. Eur J Intern Med. 2011;22(3):289–95. doi: 10.1016/j.ejim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Raut T, et al. Hydrocephalus in tuberculous meningitis: incidence, its predictive factors and impact on the prognosis. J Inf Secur. 2013;66(4):330–7. doi: 10.1016/j.jinf.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Thwaites GE, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 28.Chiang SS, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–57. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 29.Verdon R, et al. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis. 1996;22(6):982–8. doi: 10.1093/clinids/22.6.982. [DOI] [PubMed] [Google Scholar]
- 30.Delage G, Dusseault M. Tuberculous meningitis in children: a retrospective study of 79 patients, with an analysis of prognostic factors. Can Med Assoc J. 1979;120(3):305–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Thwaites GE, et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet. 2002;360(9342):1287–92. doi: 10.1016/s0140-6736(02)11318-3. [DOI] [PubMed] [Google Scholar]
- 32.Solari L, et al. The validity of cerebrospinal fluid parameters for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2013;17(12):e1111–5. doi: 10.1016/j.ijid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Youssef FG, et al. Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagn Microbiol Infect Dis. 2006;55(4):275–8. doi: 10.1016/j.diagmicrobio.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Vidal JE, et al. Is it possible to differentiate tuberculous and cryptococcal meningitis in HIV-infected patients using only clinical and basic cerebrospinal fluid characteristics? S Afr Med J. 2017;107(2):156–9. doi: 10.7196/SAMJ.2017.v107i2.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Checkley AM, et al. Sensitivity and specificity of an index for the diagnosis of TB meningitis in patients in an urban teaching hospital in Malawi. Tropical Med Int Health. 2008;13(8):1042–6. doi: 10.1111/j.1365-3156.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 36.Saavedra JS, et al. Validation of Thwaites Index for diagnosing tuberculous meningitis in a Colombian population. J Neurol Sci. 2016;370:112–8. doi: 10.1016/j.jns.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Torok ME, et al. Validation of a diagnostic algorithm for adult tuberculous meningitis. Am J Trop Med Hyg. 2007;77(3):555–9. [PubMed] [Google Scholar]
- 38.Kurien R, et al. Tuberculous meningitis: a comparison of scoring systems for diagnosis. Oman Med J. 2013;28(3):163–6. doi: 10.5001/omj.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunbul M, et al. Thwaites’ diagnostic scoring and the prediction of tuberculous meningitis. Med Princ Pract. 2005;14(3):151–4. doi: 10.1159/000084631. [DOI] [PubMed] [Google Scholar]
- 40.Bomanji JB, et al. Imaging in tuberculosis. Cold Spring Harb Perspect Med. 2015;5(6) doi: 10.1101/cshperspect.a017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg RK, Malhotra HS, Jain A. Neuroimaging in tuberculous meningitis. Neurol India. 2016;64(2):219–27. doi: 10.4103/0028-3886.177608. [DOI] [PubMed] [Google Scholar]
- 42.Rohlwink UK, et al. Imaging features of the brain, cerebral vessels and spine in pediatric tuberculous meningitis with associated hydrocephalus. Pediatr Infect Dis J. 2016;35(10):e301–10. doi: 10.1097/INF.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uysal G, et al. Magnetic resonance imaging in diagnosis of childhood central nervous system tuberculosis. Infection. 2001;29(3):148–53. doi: 10.1007/s15010-001-2014-9. [DOI] [PubMed] [Google Scholar]
- 44.Andronikou S, et al. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr Radiol. 2004;34(11):876–85. doi: 10.1007/s00247-004-1237-1. [DOI] [PubMed] [Google Scholar]
- 45.Ozates M, et al. CT of the brain in tuberculous meningitis. A review of 289 patients. Acta Radiol. 2000;41(1):13–7. doi: 10.1034/j.1600-0455.2000.041001013.x. [DOI] [PubMed] [Google Scholar]
- 46.Burrill J, et al. Tuberculosis: a radiologic review. Radiographics. 2007;27(5):1255–73. doi: 10.1148/rg.275065176. [DOI] [PubMed] [Google Scholar]
- 47.Lu TT, et al. Magnetic resonance angiography manifestations and prognostic significance in HIV-negative tuberculosis meningitis. Int J Tuberc Lung Dis. 2015;19(12):1448–54. doi: 10.5588/ijtld.15.0113. [DOI] [PubMed] [Google Scholar]
- 48.Kalita J, et al. MR angiography in tuberculous meningitis. Acta Radiol. 2012;53(3):324–9. doi: 10.1258/ar.2012.110712. [DOI] [PubMed] [Google Scholar]
- 49.Sanei Taheri M, et al. Central nervous system tuberculosis: an imaging-focused review of a reemerging disease. Radiol Res Pract. 2015;2015:202806. doi: 10.1155/2015/202806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasundra GM, et al. Distal cord-predominant longitudinally extensive myelitis with diffuse spinal meningitis and dural abscesses due to occult tuberculosis: a rare occurrence. J Pediatr Neurosci. 2016;11(1):77–9. doi: 10.4103/1817-1745.181268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42(1):378–9. doi: 10.1128/JCM.42.1.378-379.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen P, et al. A highly efficient Ziehl-Neelsen stain: identifying de novo intracellular Mycobacterium tuberculosis and improving detection of extracellular M. tuberculosis in cerebrospinal fluid. J Clin Microbiol. 2012;50(4):1166–70. doi: 10.1128/JCM.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nhu NT, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52(1):226–33. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahr NC, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis. 2015;19(10):1209–15. doi: 10.5588/ijtld.15.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mai NT, Thwaites GE. Recent advances in the diagnosis and management of tuberculous meningitis. Curr Opin Infect Dis. 2017;30(1):123–8. doi: 10.1097/QCO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 56.Nahid P, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–95. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donald PR, et al. Cerebrospinal fluid isoniazid concentrations in children with tuberculous meningitis: the influence of dosage and acetylation status. Pediatrics. 1992;89(2):247–50. [PubMed] [Google Scholar]
- 58.Ellard GA, Humphries MJ, Allen BW. Cerebrospinal fluid drug concentrations and the treatment of tuberculous meningitis. Am Rev Respir Dis. 1993;148(3):650–5. doi: 10.1164/ajrccm/148.3.650. [DOI] [PubMed] [Google Scholar]
- 59.Donald PR. The chemotherapy of tuberculous meningitis in children and adults. Tuberculosis (Edinb) 2010;90(6):375–92. doi: 10.1016/j.tube.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Pilheu JA, et al. Concentrations of ethambutol in the cerebrospinal fluid after oral administration. Tubercle. 1971;52(2):117–22. doi: 10.1016/0041-3879(71)90017-1. [DOI] [PubMed] [Google Scholar]
- 61.Gundert-Remy U, Klett M, Weber E. Concentration of ethambutol in cerebrospinal fluid in man as a function of the non-protein-bound drug fraction in serum. Eur J Clin Pharmacol. 1973;6(2):133–6. doi: 10.1007/BF00562440. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, et al. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55(1):83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 63.Mindermann T, Zimmerli W, Gratzl O. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob Agents Chemother. 1998;42(10):2626–9. doi: 10.1128/aac.42.10.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Oliveira JJ. Cerebrospinal fluid concentrations of rifampin in meningeal tuberculosis. Am Rev Respir Dis. 1972;106(3):432–7. doi: 10.1164/arrd.1972.106.3.432. [DOI] [PubMed] [Google Scholar]
- 65.Boeree MJ, et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191(9):1058–65. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 66.Pea F, et al. Levofloxacin disposition in cerebrospinal fluid in patients with external ventriculostomy. Antimicrob Agents Chemother. 2003;47(10):3104–8. doi: 10.1128/AAC.47.10.3104-3108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thwaites GE, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother. 2011;55(7):3244–53. doi: 10.1128/AAC.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Ruslami R, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. This study reports the results of a randomized, non-blinded, clinical trial of high-dose intravenous rifampin and levofloxacin, in combination with the other first-line anti-tuberculosis drugs, for the intensified treatment of tuberculosis meningitis in Indonesia, reporting a significant survival benefit in the intensified treatment arm. [DOI] [PubMed] [Google Scholar]
- 69.Te Brake L, et al. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents. 2015;45(5):496–503. doi: 10.1016/j.ijantimicag.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 70•.Heemskerk AD, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374(2):124–34. doi: 10.1056/NEJMoa1507062. This study reports the results of a randomized, blinded, clinical trial of high-dose rifampin and levofloxacin, in combination with the other first-line anti-tuberculosis drugs, for the intensified treatment of tuberculosis meningitis in Vietnam, with no overall survival benefit observed for the intensified treatment arm. [DOI] [PubMed] [Google Scholar]
- 71.Torok ME, et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6(12):e27821. doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140(5):717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tobin DM, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148(3):434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thuong NTT, et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J Infect Dis. 2017;215(7):1020–8. doi: 10.1093/infdis/jix050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Laarhoven A, et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J Infect Dis. 2017;215(7):1029–39. doi: 10.1093/infdis/jix051. [DOI] [PubMed] [Google Scholar]
- 76.Schoeman JF, et al. The role of aspirin in childhood tuberculous meningitis. J Child Neurol. 2011;26(8):956–62. doi: 10.1177/0883073811398132. [DOI] [PubMed] [Google Scholar]
- 77.Misra UK, Kalita J, Nair PP. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci. 2010;293(1–2):12–7. doi: 10.1016/j.jns.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 78.van Toorn R, Solomons R. Update on the diagnosis and management of tuberculous meningitis in children. Semin Pediatr Neurol. 2014;21(1):12–8. doi: 10.1016/j.spen.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 79.van Well GT, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123(1):e1–8. doi: 10.1542/peds.2008-1353. [DOI] [PubMed] [Google Scholar]
- 80.Miftode EG, et al. Tuberculous meningitis in children and adults: a 10-year retrospective comparative analysis. PLoS One. 2015;10(7):e0133477. doi: 10.1371/journal.pone.0133477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amitava AK, Alarm S, Hussain R. Neuro-ophthalmic features in pediatric tubercular meningoencephalitis. J Pediatr Ophthalmol Strabismus. 2001;38(4):229–34. doi: 10.3928/0191-3913-20010701-10. [DOI] [PubMed] [Google Scholar]
- 82.Garg RK, et al. Vision loss in tuberculous meningitis. J Neurol Sci. 2017;375:27–34. doi: 10.1016/j.jns.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 83.Vinnard C, Blumberg EA. Endocrine and metabolic aspects of tuberculosis. Microbiol Spectr. 2017;5(1) doi: 10.1128/microbiolspec.TNMI7-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vadivelu S, et al. A review of the neurological and neurosurgical implications of tuberculosis in children. Clin Pediatr (Phila) 2013;52(12):1135–43. doi: 10.1177/0009922813493833. [DOI] [PubMed] [Google Scholar]
- 85.Organization. W.H. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2014. [PubMed] [Google Scholar]
- 86.Thee S, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55(12):5560–7. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donald PR, Maritz JS, Diacon AH. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis (Edinb) 2011;91(3):196–207. doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 88.McIlleron H, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48(11):1547–53. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 89.Pouplin T, et al. Naive-pooled pharmacokinetic analysis of pyrazinamide, isoniazid and rifampicin in plasma and cerebrospinal fluid of Vietnamese children with tuberculous meningitis. BMC Infect Dis. 2016;16:144. doi: 10.1186/s12879-016-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Donald PR. Chemotherapy for tuberculous meningitis. N Engl J Med. 2016;374(2):179–81. doi: 10.1056/NEJMe1511990. [DOI] [PubMed] [Google Scholar]
- 91.Corbett EL, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 92.De Cock KM, et al. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA. 1992;268(12):1581–7. doi: 10.1001/jama.268.12.1581. [DOI] [PubMed] [Google Scholar]
- 93.Berenguer J, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326(10):668–72. doi: 10.1056/NEJM199203053261004. [DOI] [PubMed] [Google Scholar]
- 94.Yechoor VK, et al. Tuberculous meningitis among adults with and without HIV infection. Experience in an urban public hospital. Arch Intern Med. 1996;156(15):1710–6. [PubMed] [Google Scholar]
- 95.Katrak SM, et al. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci. 2000;181(1–2):118–26. doi: 10.1016/s0022-510x(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 96.Schutte CM. Clinical, cerebrospinal fluid and pathological findings and outcomes in HIV-positive and HIV-negative patients with tuberculous meningitis. Infection. 2001;29(4):213–7. doi: 10.1007/s15010-001-1198-3. [DOI] [PubMed] [Google Scholar]
- 97.Karstaedt AS, et al. Tuberculous meningitis in South African urban adults. QJM. 1998;91(11):743–7. doi: 10.1093/qjmed/91.11.743. [DOI] [PubMed] [Google Scholar]
- 98.Dube MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992;93(5):520–4. doi: 10.1016/0002-9343(92)90579-z. [DOI] [PubMed] [Google Scholar]
- 99.Karande S, et al. Tuberculous meningitis and HIV. Indian J Pediatr. 2005;72(9):755–60. doi: 10.1007/BF02734147. [DOI] [PubMed] [Google Scholar]
- 100.van der Weert EM, et al. Comparison of diagnostic criteria of tuberculous meningitis in human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2006;25(1):65–9. doi: 10.1097/01.inf.0000183751.75880.f8. [DOI] [PubMed] [Google Scholar]
- 101.El Sahly HM, et al. Mortality associated with central nervous system tuberculosis. J Inf Secur. 2007;55(6):502–9. doi: 10.1016/j.jinf.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bossi P, et al. Tuberculous meningitis: clinical, biological and x-ray computed tomographic comparison between patients with or without HIV infection. Presse Med. 1997;26(18):844–7. [PubMed] [Google Scholar]
- 103.Thwaites GE, et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192(12):2134–41. doi: 10.1086/498220. [DOI] [PubMed] [Google Scholar]
- 104.Havlir DV, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdool Karim SS, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torok ME, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)—associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boulware DR, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–98. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lawn SD, Meintjes G. Pathogenesis and prevention of immune reconstitution disease during antiretroviral therapy. Expert Rev Anti-Infect Ther. 2011;9(4):415–30. doi: 10.1586/eri.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peloquin C. The role of therapeutic drug monitoring in mycobacterial infections. Mcrobiol Spectr. 2017;5(1) doi: 10.1128/microbiolspec.TNMI7-0029-2016. [DOI] [PubMed] [Google Scholar]
- 110.Ramachandran G, Swaminathan S. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf. 2015;38(3):253–69. doi: 10.1007/s40264-015-0267-y. [DOI] [PubMed] [Google Scholar]
- 111.Stagg HR, et al. Isoniazid-resistant tuberculosis: a cause for concern? Int J Tuberc Lung Dis. 2017;21(2):129–39. doi: 10.5588/ijtld.16.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tho DQ, et al. Influence of antituberculosis drug resistance and Mycobacterium tuberculosis lineage on outcome in HIV-associated tuberculous meningitis. Antimicrob Agents Chemother. 2012;56(6):3074–9. doi: 10.1128/AAC.00319-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Vinnard C, et al. Long-term mortality of patients with tuberculous meningitis in New York City: a cohort study. Clin Infect Dis. 2017;64(4):401–7. doi: 10.1093/cid/ciw763. This study examined 10-year survival of tuberculo us meningitis patients in New York City, with a focus on the role of initial drug resistance. The impact of isoniazid resistance on mortality was not apparent until after the first 60 days of treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114•.Heemskerk AD, et al. Clinical outcomes of patients with drug-resistant tuberculous meningitis treated with an intensified antituberculosis regimen. Clin Infect Dis. 2017 doi: 10.1093/cid/cix230. This study was a subanalysis of patients in the Vietnamese intensified treatment trial, demonstrating a survival benefit of intensified treatment in the subset of patients with initial isoniazid resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vinnard C, et al. Isoniazid-resistant tuberculous meningitis, United States, 1993–2005. Emerg Infect Dis. 2011;17(3):539–42. doi: 10.3201/eid1703.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vinnard C, et al. Multidrug resistant tuberculous meningitis in the United States, 1993–2005. J Inf Secur. 2011;63(3):240–2. doi: 10.1016/j.jinf.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 117.Sullivan RP, et al. Successful treatment of multiple multidrug resistant intracranial tuberculomata. Case Rep Infect Dis. 2016;2016:1841529. doi: 10.1155/2016/1841529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berning SE, Cherry TA, Iseman MD. Novel treatment of meningitis caused by multidrug-resistant Mycobacterium tuberculosis with intrathecal levofloxacin and amikacin: case report. Clin Infect Dis. 2001;32(4):643–6. doi: 10.1086/318698. [DOI] [PubMed] [Google Scholar]
- 119.Li H, et al. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr Infect Dis J. 2016;35(6):607–10. doi: 10.1097/INF.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 120.Sun F, et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob Agents Chemother. 2014;58(10):6297–301. doi: 10.1128/AAC.02784-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Merkler AE, et al. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci. 2017;375:460–3. doi: 10.1016/j.jns.2017.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]