Abstract
Nowadays, minimal residual disease (MRD) is accepted as the strongest independent prognostic factor in acute lymphoblastic leukemia (ALL). It can be detected by molecular methods that use leukemia-specific or patient-specific molecular markers (fusion gene transcripts, or immunoglobulin/T-cell receptor [IG/TR] gene rearrangements), and by multi-parametric flow cytometry. The sensitivity and specificity of these methods can vary across treatment time points and therapeutic settings. Thus, knowledge of the principles and limitations of each technology is of the utmost importance for correct interpretation of MRD results. Time will tell whether new molecular and flow cytometric high-throughput technologies can overcome the limitations of current standard methods and eventually bring additional benefits. MRD during standard ALL chemotherapy is the strongest overall prognostic indicator and has therefore been used for refining initial treatment stratification. Moreover, MRD positivity after the maintenance phase of treatment may point to an impending relapse and thus enable salvage treatment to be initiated earlier, which could possibly improve treatment results. The prognostic relevance of pretransplantation MRD was shown by several studies, and MRD high-risk patients were shown to benefit from stem cell transplantation (SCT). Also, MRD positivity after SCT correlates with worse outcomes. In addition, MRD information is very instructive in current clinical trials that test novel agents to evaluate their treatment efficacy. Although conventional clinical risk factors lose their independent prognostic significance when combined with MRD information, recently identified genetic markers may further improve the treatment stratification in ALL.
Introduction
Acute lymphoblastic leukemia (ALL) is a clonal disease that affects early lymphoid progenitors in bone marrow. It is a heterogeneous malignancy in terms of genetic background and also in clinical manifestation and prognosis. Implementing pediatric ALL treatment algorithms led to substantial improvements in adult ALL. Nevertheless, 40% to 50% of adult patients relapse.1,2 This can be attributed partly to the higher incidence of high-risk molecular aberrations in older patients and also to the fact that older patients are less able to tolerate treatment intensification.
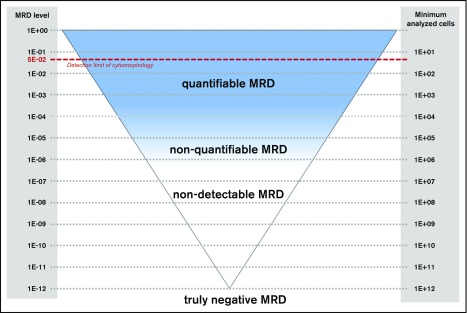
The term minimal residual disease (MRD) is used to describe the low-level disease which is not detectable by conventional cytomorphology (Figure 1). Compared with the classical microscopic detection of residual leukemic cells, MRD is assessed by sensitive molecular and flow cytometric methods to more precisely monitor disease kinetics during and after treatment.
Figure 1.
MRD detection in ALL. Schematic diagram for detection of MRD. The red dashed line indicates the detection limit of cytomorphology (5%). Note the difference between nondetectable and truly negative MRD.
Many trials have confirmed that MRD is the strongest prognostic factor in both children and adults with ALL, independent of traditional pretherapeutic risk factors. MRD detection is not only useful for the assessment of initial treatment response and subsequent definition of MRD-based risk groups, but also to monitor disease burden in the setting of stem cell transplantation (SCT), for early recognition of impending relapse, and as potential end point in clinical trials. MRD is used for guiding clinical decisions in current treatment protocols. This review highlights relevant methodologic aspects for correct interpretation of MRD results and the most important data on the significance of MRD quantification and its application to tailoring treatment in adult ALL.
Techniques for MRD assessment
Classical methods for MRD quantification
Methods for MRD quantification are based either on the discrimination of ALL cells from normal physiological counterparts and the identification of the leukemia-associated immunophenotype (LAIP) by multiparametric flow cytometry (MFC) or on the detection of leukemia-specific rearrangements of immunoglobulin and T-cell receptor (IG/TR) genes and/or fusion gene transcripts by real-time quantitative polymerase chain reaction (RT-qPCR).
IG/TR RT-qPCR can be used for MRD detection in >95% of patients with ALL. Sensitivity is determined separately for each assay and routinely reaches 10−4 to 10−5 (1 leukemic cell in 10 000 to 100 000 healthy cells). Applying this method requires initial characterization of IG/TR rearrangements in each diagnostic sample with a panel of screening PCRs and Sanger or next-generation sequencing (NGS) of PCR products and subsequent optimization of RT-qPCR assays specific for each rearrangement. Extensive optimization and standardization of RT-qPCR–based MRD detection have been achieved within the EuroMRD Consortium (http://www.euromrd.org), which now consists of 57 MRD PCR laboratories all over the world. The Consortium organizes quality-control rounds for the members twice each year, develops guidelines for interpretation of RT-qPCR–based MRD results, and collaborates on development of new techniques for MRD detection. Clonal IG/TR rearrangements are not directly related to the oncogenic process and can undergo clonal evolution in immature leukemic blasts with still active IG/TR recombination machinery, which might lead to the loss of leukemia-specific IG/TR sequence and false-negative MRD results. Conversely, massive bone marrow regeneration after treatment can cause unspecific primer annealing and false-positive MRD results.3,4
Only about 30% to 40% of B-cell precursor ALL (BCP-ALL) and 10% to 20% of T-cell ALL (T-ALL) patients have specific chromosome aberrations that can be used for MRD detection, generally on a transcript level. The advantage of this approach over IG/TR rearrangement detection is that the same primer sets for RT-qPCR analysis can be used for all patients, thus keeping the costs and laboriousness for the individual analyses lower. However, interpreting RNA-based results is more challenging than interpreting DNA-based results because the transcript levels can be influenced by the changes in the transcription activity of leukemic cells.
The detection of aberrant LAIP by MFC is less laborious and faster compared with the above-mentioned molecular methods. This allows prompt reporting of the results, which is particularly useful in making therapeutic decisions. By using the classical 4- to 6-color MFC, LAIP can be identified in more than 90% of patients with ALL. Flow-based detection of MRD requires a cluster of 10 to 40 events and therefore needs the acquisition of higher cell numbers compared with PCR-based MRD techniques. Sensitivity of classical MFC MRD detection is about 1 log lower than that for the molecular methods.5,6 The sensitivity and specificity of the method are influenced by the similarities between leukemic lymphoblasts and nonmalignant lymphoid precursors. In addition, phenotypic shifts frequently occur in MRD cells as well as in normal cells during therapy. When antibody-based therapies (eg, targeting CD19, CD20, or CD22) are used, classical gating strategies might be hampered because completely unknown marker shifts may occur, which would influence the detectability of residual leukemic cells.
High-throughput methods for MRD quantification
Thanks to the development of new high-throughput technologies, novel software tools, and increased computing capacities, advanced molecular and immunophenotypic methods for quantifying MRD have become increasingly available. Amplicon-based NGS of IG/TR gene rearrangements has the potential to overcome some of the limitations of RT-qPCR and can enhance sensitivity provided that sufficient numbers of cells are analyzed. Just like RT-qPCR, IG/TR NGS requires a diagnostic sample to identify the leukemia-specific index rearrangements that are monitored throughout therapy; however, design and testing of patient-specific oligonucleotides is avoided because the same multiplex PCR assay can be used for identification and follow-up of index sequences. Recent reports have shown that MRD by NGS tends to be more specific for relapse prediction than MRD by RT-qPCR.3,7 In addition, NGS provides information on the physiological B- and T-cell repertoire during and after treatment, which has been shown to be prognostically relevant.7,8 However, multicenter standardization for all phases of analysis, including the use of calibrators, quality controls, and guidelines for correct interpretation of NGS data, is still lacking and is one of the topics being considered by the EuroClonality NGS Consortium.
Flow cytometric immunophenotyping has undergone an impressive evolution. A significant increase in the number of different parameters that can be simultaneously assessed in individual cells, the development of new antibodies and fluorochromes, and an enormous acceleration of analysis speed have contributed to improved sensitivity and specificity of flow cytometric MRD detection.6 The EuroFlow Consortium, which is focused on development, standardization, and validation of MFC assays for MRD detection, has recently introduced new, fully standardized, high-throughput concepts in flow cytometric MRD detection.9-11
Practical issues: what must be known for adequate sampling and data interpretation
Source of material
MRD can be quantified in peripheral blood or in bone marrow; however, MRD levels in BCP-ALL tend to be 1 to 3 logs lower in peripheral blood than in bone marrow.12,13 Therefore, bone marrow assessments might be replaced by analysis of blood samples in T-ALL but not in BCP-ALL.
Time point of MRD assessment
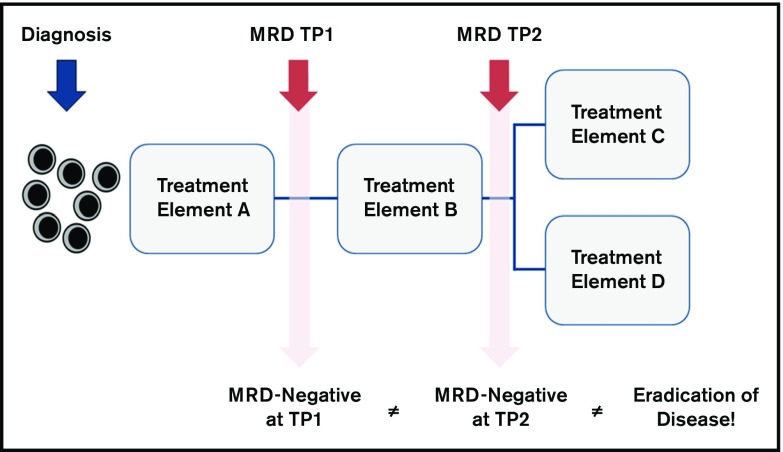
MRD is a time point–dependent variable. MRD levels at different time points have different prognostic value for relapse (Figure 2): early MRD assessment identifies patients with a rapid tumor clearance and a very low risk of relapse, whereas any persisting MRD at the end of consolidation therapy is associated with a particularly poor prognosis.
Figure 2.
General considerations for correct interpretation of MRD results. This schematic illustrates that MRD is a time point (TP)–dependent variable. Therefore, the prognostic meaning of MRD negativity is different when measured very early during therapy (Treatment Element A) compared with late time points (Treatment Element B). For example, MRD assessment at the end of induction therapy is useful for recognizing patients with low risk of relapse, and MRD measurement at the end of consolidation therapy is useful for identifying patients at high risk of relapse. In addition, the prognostic significance of MRD might be influenced by subsequent treatment elements (Treatment Elements C and D). MRD negativity does not necessarily indicate the eradication of the disease but does indicate a decrease to a level below the detection limit of the respective assay. Therefore, the knowledge of the sensitivity of the method is important for correct interpretation of the results.
Correct interpretation of MRD results
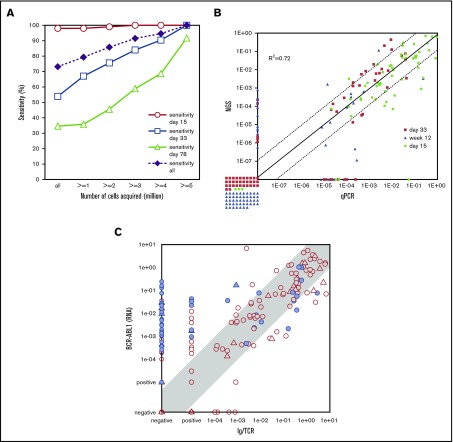
Like other quantitative methods, MRD quantification techniques have a lower limit of detection and a lower limit of quantification. Therefore, MRD negativity is not synonymous with the absence of residual disease (Figure 1), which is why several authors use the term “measurable residual disease” instead of “minimal residual disease.” Sensitivity of measurements is determined by the respective technique and the amount of cell correlates analyzed (Figures 1 and 3A). Current treatment protocols require a sensitivity of at least 10−4. Some recent studies that use commercial approaches for NGS MRD detection claim to reach sensitivities down to 10–7.14,15 However, it is important to note that the amount of input DNA is crucial for reaching a particular sensitivity. This often represents a serious limitation in the aplastic samples during treatment.16 An MRD assessment using 100 000 cells can never reach a sensitivity of 10−6, even if the readout suggests that it did. The EuroFlow network demonstrated a clear relation between sensitivity and number of cells acquired for MFC-based MRD analysis11 (Figure 3A). Therefore, knowledge of the respective technology is of the utmost importance for correct interpretation of MRD results. Studies comparing different techniques have uncovered significant discrepancies (Figure 3), especially those between BCR-ABL1 MRD and IG/TR MRD. This might be related to non–lymphoid cells bearing the BCR-ABL1 fusion17,18 but not having rearranged IG/TR genes. It seems that IG/TR and BCR-ABL1 MRD may provide distinct insights into MRD kinetics of different leukemic subpopulations in response to tyrosine kinase inhibitors, chemotherapy, SCT, and possibly also immunotherapies. To allow a correct interpretation of the MRD results, the MRD report must provide information on the MRD technique used and on MRD markers as well as on the theoretical limit of detection and the limit of quantification of the assay.
Figure 3.
Comparison of MRD results assessed with different techniques. (A) MFC vs IG/TR RT-qPCR.11 The sensitivity of MFC for MRD detection relative to RT-qPCR (y-axis) is shown for certain time points (days 15, 33, and 78) and for all samples together. Data are presented for variable numbers of acquired cells (x-axis): all samples (independent of cell counts; n = 377) and samples with at least 1 (n = 330), 2 (n = 287), 3 (n = 255), 4 (n = 227) and 5 (n = 191) million cells acquired. Sensitivity is calculated as the number of samples positive by both FCM and PCR divided by the total number of samples positive by PCR. The sensitivity significantly increases when larger numbers of cells are acquired. (B) Immunoglobulin heavy chain RT-qPCR vs immunoglobulin heavy chain NGS.7 The comparison of MRD as detected by RT-qPCR and NGS is shown for different follow-up time points (days 15, 33, and 78) by qPCR (x-axis) and NGS (y-axis). The correlation of both methods is good (R2 = 0.72) with the majority of the noncorrelating samples below the sensitivity thresholds of the methods. (C) IG/TR RT-qPCR vs BCR-ABL1 RT-qPCR.17 Comparison of MRD levels in ALL patients as detected by IG/TR RT-qPCR (x-axis) vs BCR-ABL1 genomic transcript quantification (y-axis). A significant number of samples (23%) have quantifiable BCR-ABL1 levels, whereas IG/TR MRD is negative. Panel C adapted from Hovorkova et al.17
Prognostic significance of MRD
MRD in the setting of chemotherapy
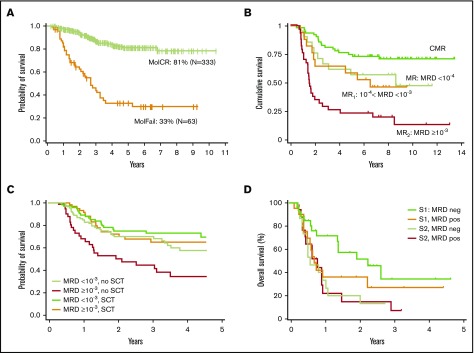
Several large studies in both childhood and adult ALL have shown that the initial MRD response is a highly relevant prognostic factor, and therefore MRD has been used for the refinement of initial treatment stratification.19 Analysis of the largest cohort of adult ALL data so far was performed by the German Multicenter Study Group for Adult ALL (GMALL), which assessed MRD in Philadelphia chromosome–negative (Ph–) patients with standard-risk and high-risk features. Molecular response to standard induction and consolidation treatment was the only significant prognostic factor for remission duration and survival throughout both risk groups in a multivariable analysis20 (Figure 4A). Patients with molecular failure undergoing SCT in first complete remission (CR1) had significantly better probability of continuous CR than those without SCT (66% vs 11%).
Figure 4.
Prognostic value of MRD in Ph–adult ALL. (A) Probability of OS for patients in the standard-risk and high-risk groups according to molecular response (MR) status (leukemia-associated IG/TR rearrangements detected by RT-qPCR) after induction or early consolidation chemotherapy (week 16) excluding SCT in CR1.20 Results of the GMALL 06/99 and 07/03 trials. Complete molecular remission (MolCR) is defined as MRD negativity with an assay sensitivity of at least 10–4. Molecular failure (MolFail) is defined as quantifiable MRD positivity ≥10–4. (B) OS according to different molecular levels of postinduction MRD (detected by IG/TR RT-qPCR). Results of the NILG ALL 09/00 trial. Complete molecular remission (CMR) is defined as MRD negativity at weeks 10, 16, and 22. MR is defined as MRD <10–4 at week 10 and/or week 16 and/or week 22. Molecular resistance type 1 (MR1) is defined as measurable MRD ≥10–4 and <10–3. MR2 is defined as measurable MRD ≥10–3. Panel B adapted from Bassan et al31 with permission. (C) Effect of SCT on OS. Results of the GRAALL-2003/2005 trials. Simon-Makuch plots of SCT time-dependent analysis of OS according to molecular MRD response (≥10–3or <10–3) and type of postremission therapy (SCT vs no SCT) in high-risk ALL. MRD was detected by IG/TR-based RT-qPCR. Panel C adapted from Dhédin et al.24 (D) OS for patients with relapsed or refractory BCP-ALL receiving salvage immunotherapy (inotuzumab ozogamicin or blinatumomab) by MRD response (MRD positive or MRD negative by 6-color MFC) and salvage status (Salvage 1 [S1] vs Salvage 2 [S2]). Retrospective analysis at MD Anderson Cancer Center. Includes data from 4 Ph+ patients. Panel D adapted from Jabbour et al46 with permission.
The French Group for Research on Adult ALL (GRAALL),21 the Northern Italian Study Group (NILG),22 and the Spanish Programa Español de Tratamientos en Hematología (PETHEMA)23 study group confirmed the strong and independent prognostic impact of MRD after induction and early consolidation treatment. However, different study groups used different cutoff values, depending on the MRD technique, timing of MRD analysis, and the target patient population. Within the NILG, crucial time points were week 16 (cutoff, 1 × 10−4) and week 22 (absence of detectable MRD), PETHEMA used a cutoff of 5 × 10−4 at weeks 16 to 18, and GRAALL focused on week 6 with a cutoff of 1 × 10−4 for all Ph– ALL and 1 × 10−3 for high-risk patients.21-24 Apart from these differences, all studies confirmed the strong independent prognostic effect of MRD response in adult ALL. Ravandi and colleagues25 performed MFC-based MRD quantification at the time of CR and at 3 and 6 months in an elderly patient population (age 15 to 84 years) being treated with a backbone regimen of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyperCVAD) at the MD Anderson Cancer Center. MRD negativity was associated with a significant improvement in disease-free survival (DFS) and overall survival (OS) at all time points.
Early achievement of MRD negativity during induction therapy correlated with a particular good outcome in adult ALL.26 Earlier studies identified a small subset of ALL patients with a very rapid tumor clearance (low-level or undetectable MRD after 2 weeks of therapy) and an excellent prognosis,27 which is in line with reports on childhood ALL.28 In an MFC-based trial, high levels or disease at day 14 of treatment (>30%) identified a small subgroup of 10% of patients with a particularly poor prognosis and median event-free survival (EFS) and OS of only 9 and 21 months, respectively. However, MRD at this early time point lost its independent prognostic impact when MRD at later time points was incorporated into a multivariable analysis.29
MRD in the setting of SCT
Relapse still remains the major cause of treatment failure after SCT in ALL, even in patients who received transplantation during hematologic remission. Several studies showed the prognostic relevance of pretransplantation MRD in adults.20,22,30,31 Bassan et al31 highlighted the fact that MRD is a quantitative variable and MRD levels correlate with post-SCT outcome. Patients with MRD levels of ≥10−3 at week 16 and/or week 22 had a worse posttransplantation outcome with a 6-year relapse incidence of 64% compared with 23% in patients with MRD levels <10−3 (Figure 4B). In contrast, the French GRAALL-2003 and -2005 trials did not observe this difference when analyzing MRD at an earlier time point at week 6. Among a cohort of 522 high-risk patients, 282 (54%) received SCT after 3 or 6 blocks of consolidation on the basis of the availability of a related or unrelated donor. Two hundred seventy-eight patients were studied for MRD after first induction (154 SCT and 124 non-SCT patients). SCT benefitted patients with MRD levels ≥10−3 at week 6 (hazard ratio, 0.4) compared with nontransplantation patients, and SCT erased the unfavorable impact of poor MRD response in this cohort. In contrast, SCT did not show a beneficial effect in MRD good responders, with a lower cumulative incidence of relapse observed in the SCT cohort being counterbalanced by a higher nonrelapse mortality24 rate (Figure 4C).
MRD monitoring is much less frequently used after SCT because chimerism monitoring provides an alternative for early relapse detection. A study by Terwey et al32 provided evidence that the higher sensitivity and better specificity of IG/TR-based MRD testing enables earlier and more specific detection of impending relapse compared with chimerism analysis. In multivariable analysis, MRD positivity was an independent significant predictor of risk of relapse. Another MFC-based trial33 showed that patients with evidence of MRD after SCT (and in particular, early after SCT) had significantly worse outcomes in comparison with patients without evidence of MRD. Zhao et al34 had previously demonstrated that MRD positivity after SCT is correlated with poor EFS and high cumulative incidence of relapse in both high-risk and standard-risk groups of ALL patients.
MRD in the era of novel agents
The survival rates for adult patients with ALL treated on current risk-adapted multiagent chemotherapy protocols have reached a plateau. Novel therapies, such as monoclonal antibodies, bispecific T-cell engagers, or chimeric antigen receptor T cells (CART), are an exciting advance in the immunotherapeutic treatment of BCP-ALL.
Approximately 30% to 50% of BCP-ALL blasts express CD20, which prompted the incorporation of rituximab into chemotherapy regimens. The GMALL trial added 8 doses of rituximab to induction/consolidation treatment of standard-risk patients and 3 doses for high-risk patients. A total of 117 CD20+ patients received rituximab, and they were compared with 70 CD20+ patients recruited earlier. MRD course between both patient cohorts differed substantially. The decrease in MRD load in the patients who received rituximab was faster (MRD <10−4 in rituximab-treated patients was 60% vs 19% on day 21 and 89% vs 57% at week 16). This difference in MRD kinetics translated into a significantly better outcome in the rituximab group (continuous CR at 3 years, 64% vs 48%; OS, 75% vs 54%).35 Intriguingly, this was not confirmed by the GRAALL study group, which recently published the results of a randomized trial on adding 16 to 18 infusions of rituximab to all treatment phases until month 11 of maintenance. Rituximab improved outcome for younger adults with CD20+Ph– ALL compared with the control group36 (2-year EFS, 65% vs 52%), but there was no significant difference in MRD response after first induction and/or first consolidation phase between the 2 groups. Potentially, the prolonged administration of rituximab played a role in the beneficial outcome of the rituximab-treated group that was not reflected by MRD assessments during induction and early consolidation.
More recently, novel immunotherapeutics against CD19 and CD22 were tested in clinical trials. In relapsed/refractory ALL, blinatumomab led to morphological response in 43% to 69% of patients37-39 with 76% to 88% of responding patients being MRD negative. Patients who achieved negative molecular MRD status had longer survival than patients who remained MRD positive.38,40 Regarding CART19, multiple groups have shown that this approach can induce CRs in 60% to 90% of BCP-ALL patients in clinical trials, with most of the responding patients (both children and adults) becoming MRD negative.41-43 Relapsed or refractory ALL patients being treated with inotuzumab ozogamicin reached response rates of 58% to 81%,44,45 with 72% to 78% of these patients having MRD results below 0.01%.44,45 Data on the prognostic value of MRD in this setting are still preliminary; however, different from first-line chemotherapeutic approaches, relapse rate is high even in patients reaching MRD negativity. Therefore, MRD response in this setting seems to be an essential but not sufficient criterion for long-term remissions. Higher sensitivities or earlier MRD assessments might be necessary to identify a subgroup of patients with a particularly rapid and deep MRD response and a better prognosis. The prognostic value of MRD assessment is also influenced by the type of subsequent treatment being used after MRD assessment (Figure 2). Only a portion of the relapsed or refractory ALL patients received intensive therapy (allogeneic SCT [allo-SCT]) after treatment with blinatumomab or inotuzumab ozogamicin, and single-drug antibody treatment is probably not sufficient to cure the majority of relapsed or refractory ALL. In addition, the prognostic impact of MRD seems to be influenced by the salvage status: in a single-center analysis of 130 patients with relapsed or refractory ALL being treated with blinatumomab or inotuzumab ozogamicin, the achievement of MRD negativity was associated with a better prognosis only for patients in the first salvage, whereas after further salvages, the outcomes were poor regardless of MRD.46
MRD in Ph+ ALL
The Philadelphia chromosome, characterized by the presence of a BCR-ABL1 fusion gene, is the most common chromosomal abnormality in adult BCP-ALL. It is found in approximately 25% of patients, and incidence increases with age.47 MRD is accepted as the most important prognostic factor in Ph– ALL, but the utility of MRD assessment in Ph+ ALL is less well defined. However, several recent studies of chemotherapy plus tyrosine kinase inhibitors provided evidence that deeper molecular responses before transplantation are associated with improved outcomes.48,49 A retrospective analysis of the Japan Society for Hematopoietic Cell Transplantation evaluated BCR-ABL RT-qPCR MRD before transplantation in 432 adult Ph+ ALL patients in CR1. OS and leukemia-free survival at 4 years were 67% and 60% in MRD-negative patients compared with 55% (P = .0001) and 46% (P = .0002) in MRD-positive patients, respectively. Cumulative incidence of nonrelapse mortality rate was comparable between both groups.49 In contrast, the prospective French GRAAPH-2003 trial showed that early MRD did not significantly influence outcome. A lower incidence of relapse was almost completely abrogated by a higher treatment-related mortality.50 The GRAAPH-2005 trial showed that patients achieving molecular CR do not benefit from allo-SCT, whereas patients with persistent MRD do benefit.51 Short et al52 reported on 85 Ph+ patients who received hyper-CVAD plus a tyrosine kinase inhibitor and did not undergo allo-SCT. MRD negativity at 3 months was associated with a superior median OS and relapse-free survival. Prospective trials that use MRD-based risk stratification may elucidate optimal postremission management in Ph+ ALL.
Recently, a new ALL subgroup called BCR-ABL1–like ALL has been identified. This subgroup has been recognized on the basis of an expression profile similar to that of BCR-ABL1-positive ALL. Regardless of the patient’s age, this novel subtype was shown to be associated with inferior survival and persistence of MRD in several retrospective analyses.53-56 However, a more recent study of 442 unselected pediatric patients with newly diagnosed ALL and MRD-directed therapy did not show an adverse prognosis of BCR-ABL1–like ALL. A high proportion of patients with BCR-ABL1–like ALL in this study were classified as having higher-risk leukemia on the basis of MRD and therefore received intensified treatment.57
Postremission MRD monitoring in MRD-negative patients: is it worthwhile?
In pediatric ALL, the risk of relapse in patients with MRD negativity after first-line induction treatment is extremely low, and relapses are dispersed over several years, which does not justify repeated invasive procedures of bone marrow aspiration over a broad time range and the associated costs of MRD assessment. In addition, a low pretest probability of real MRD positivity increases the risk of false-positive results. In adults, the situation is different: even patients with MRD negativity after induction and early consolidation treatment have a relapse risk of 20% to 30%,20,27,58 and relapse in adults is associated with a very unfavorable prognosis. An MRD-based prediction of an impending relapse may enable the initiation of salvage treatment before hematologic relapse, which might improve the treatment results. The power of MRD monitoring as an indicator of an impending relapse was prospectively evaluated in 105 MRD-negative patients during the early post-consolidation phase of treatment within the GMALL trials.59 The reoccurrence of MRD that was measurable within the quantitative range of RT-qPCR (ie, molecular relapse) was proven to be significantly associated with subsequent hematologic relapse, which followed after a median time of 4.1 months. These data were recently confirmed by Pemmaraju et al60 who performed MFC-based postremission MRD monitoring in a series of 546 MRD-negative patients. Fifty-five patients re-converted to MRD positivity, and 44 (80%) of 55 patients subsequently developed morphologic relapse after a median of 3 months. Only 6 patients remained alive in CR1 despite MRD recurrence, all of them after having received MRD-triggered therapy. On the basis of these data, we propose 3-month intervals for postremission MRD monitoring until the end of maintenance treatment in Ph– ALL because this approach identifies the majority of patients who subsequently relapse and seems to be a good trade-off between costs and benefit. For patients who convert to quantifiable MRD positivity, a salvage treatment should be pursued.59
The nonquantifiably positive RT-qPCR MRD results are another story. Remarkably, not all the patients with very low MRD levels in the above-mentioned GMALL study59 relapsed, which confirms that nonquantifiable MRD positivity is a kind of gray zone. This is in agreement with recent observations in the post-SCT setting in relapsed pediatric ALL,61 in which only MRD burden of ≥10−4 was associated with a subsequent clinical relapse. One possible explanation of this phenomenon is that some of the nonquantifiable RT-qPCR positivities may be false, caused by the unspecific amplification in RT-qPCR.3,4 It is also possible that very-low-level MRD is more prone to being eliminated over time, perhaps thanks to the graft-versus-leukemia effect.
MRD-driven therapeutic concepts in adult ALL
The association between MRD and relapse risk has provided the impetus to tailor treatment according to MRD. MRD-based treatment algorithms may lead to treatment de-escalation in case of MRD good response, or to treatment intensification in case of MRD persistence. Both concepts are investigated within clinical studies on adult patients with ALL, albeit not yet in a randomized fashion, mainly focusing on the therapeutic decision to provide transplantation or not.
The Spanish PETHEMA trial avoided SCT in high-risk adult ALL patients with good cytologic and MRD response, whereas patients with high-level disease were allocated to allo-SCT. Five-year DFS and OS probabilities were 32% and 37% for patients assigned to allo-SCT and 55% and 59% for those assigned to chemotherapy, respectively.23 Within the GMALL 07/2003 trial, patients with persistent MRD >10−4 after first consolidation were allocated to the MRD high-risk group and they qualified for allo-SCT. Allo-SCT was performed in 47% of these patients, which resulted in a significantly higher 5-year OS compared with those without allo-SCT in whom MRD persisted.20 The Italian NILG study allocated Ph– and t(4;11)-negative patients to allo-SCT, autologous SCT (auto-SCT), or maintenance therapy on the basis of MRD response at weeks 16 and 22. MRD good responders showed a 5-year DFS of 72% compared with 33% in those with persistent MRD. Altogether, 36 of 54 patients with persistent MRD received allo- or auto-SCT with a higher 4-year DFS of 33% vs 0% in 18 of 54 patients with persistent MRD who did not undergo SCT.22
Although treatment was stratified according to MRD, MRD remained the most significant risk factor for relapse in all 3 trials, thereby indicating a therapeutic dilemma: SCT seems to improve prognosis of those with persistent MRD, but outcome is still unfavorable. Within the NILG trial, patients with high pretransplant MRD levels had a worse posttransplant outcome despite commitment to the SCT procedure that was intended to overcome treatment resistance.31 Further intensification of chemotherapy is not expected to reduce tumor load before SCT, given the saturation of MRD response toward the intensive GMALL treatment scheme.20 Therefore, innovative approaches with an alternative mode of action for patients with MRD persistence after induction or consolidation chemotherapy are warranted. In 2 phase 2 trials that included 20 and 116 MRD-positive ALL patients (with cutoff levels of 10−4 and 10−3, respectively), blinatumomab led to the clearance of MRD in 78% to 80% of patients treated in MRD-positive CR.62,63 MRD response was associated with a superior relapse-free survival and OS.63
MRD vs pretherapeutic factors: does MRD tell the whole story?
Several prospective trials indicated that conventional risk factors lose their independent prognostic significance when combined with MRD assessment. The GRAALL21 confirmed this observation on 423 adult ALL patients, claiming that most conventional pretherapeutic risk factors can be safely abandoned in trials that rely on prospective MRD monitoring. However, this study also analyzed newly defined genetic markers: focal deletions of the IKZF1 gene in BCP-ALL and NOTCH1 pathway mutations in combination with additional mutations in N-RAS/K-RAS or PTEN in T-ALL. The statistical independence of these genetic factors and MRD response in predicting relapse was confirmed in this patient cohort, especially for T-ALL. The authors concluded that MRD levels combined with these new genetic markers may predict relapse more efficiently than conventional risk factors. However, in an analysis that focused on high-risk patients, the same study group showed that IKZF1 gene deletions predicted a positive SCT effect but were strongly related to poor early MRD response. Conversely, in T-ALL patients, unfavorable genetic profiles did not predict any positive SCT effect and were not significantly related to poor early MRD response.24 For this reason, the study group decided to base the decision for SCT in CR1 on early MRD response only.
Even though MRD has the potential to integrate different aspects of treatment efficacy, it cannot assess the effect of an intervention after MRD assessment (Figure 2). Long-term antileukemic or toxic effects of drugs are not reflected by early MRD assessment. For example, in a British study comparing mitoxantrone vs idarubicin in relapsed pediatric patients with ALL, the beneficial effect of mitoxantrone on outcome was not predicted by differences in MRD response between both treatment groups.64
As an additional restriction, MRD assessment is not realizable in all patients because of technical limitations (eg, lack of identification of leukemia-specific markers and missing or insufficient amounts of follow-up samples), as is also true for all other prognostic factors. Depending on the minimum technical requirements, the MRD methodology, and adherence to the protocol, MRD-based stratification using stringent criteria seems to be feasible in about 80% to 90% of patients.
In conclusion, MRD has emerged as a powerful and independent predictor of outcome in ALL and has surpassed other widely used and accepted prognostic indicators. However, MRD is a context-dependent variable with different prognostic meanings in first-line treatment compared with salvage therapy, Ph– compared with Ph+ ALL, and for early response assessment vs postremission monitoring. Very early conversion to MRD negativity indicates an excellent prognosis, whereas MRD negativity when reached at a late time point is still associated with a considerable relapse rate. The prognostic relevance in the setting of novel immunotherapies still needs to be evaluated. Therefore, precise MRD cutoff levels, techniques used, and optimal sampling time points must be defined for each treatment protocol before MRD-based treatment stratification can be implemented.
Footnotes
This article was selected by the Blood Advances and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2017. It is reprinted in Hematology Am Soc Hematol Educ Program. 2017;2017:13-21.
Authorship
Contribution: M.B. and M.K. wrote the manuscript.
Conflict-of-interest disclosure: M.B. received honoraria from Amgen, Inc. and Roche Pharma AG; received financial support for reference diagnostics from Amgen, Inc., Affimed, and Regeneron; was a member of the Speakers Bureau for Amgen, Inc., and Pfizer Oncology; received research funding from Amgen, Inc.; and served as a consultant for Incyte. M.K. declares no competing financial interests.
Correspondence: Monika Brüggemann, Department of Hematology, University Hospital Schleswig-Holstein, Campus Kiel, Langer Segen 8-10, 24105 Kiel, Germany; e-mail: m.brueggemann@med2.uni-kiel.de.
References
- 1.Fielding AK, Richards SM, Chopra R, et al. ; Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. [DOI] [PubMed] [Google Scholar]
- 2.Gökbuget N, Stanze D, Beck J, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032-2041. [DOI] [PubMed] [Google Scholar]
- 3.Kotrova M, van der Velden VH, van Dongen JJ, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52(7):962-968. [DOI] [PubMed] [Google Scholar]
- 4.Fronkova E, Muzikova K, Mejstrikova E, et al. B-cell reconstitution after allogeneic SCT impairs minimal residual disease monitoring in children with ALL. Bone Marrow Transplant. 2008;42(3):187-196. [DOI] [PubMed] [Google Scholar]
- 5.Gaipa G, Cazzaniga G, Valsecchi MG, et al. Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica. 2012;97(10):1582-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denys B, van der Sluijs-Gelling AJ, Homburg C, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2013;27(3):635-641. [DOI] [PubMed] [Google Scholar]
- 7.Kotrova M, Muzikova K, Mejstrikova E, et al. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood. 2015;126(8):1045-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sellner L, Brüggemann M, Schlitt M, et al. GvL effects in T-prolymphocytic leukemia: evidence from MRD kinetics and TCR repertoire analyses. Bone Marrow Transplant. 2017;52(4):544-551. [DOI] [PubMed] [Google Scholar]
- 9.Kalina T, Flores-Montero J, van der Velden VH, et al. ; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dongen JJ, Lhermitte L, Böttcher S, et al. ; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theunissen P, Mejstrikova E, Sedek L, et al. ; EuroFlow Consortium. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2017;129(3):347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velden VH, Jacobs DC, Wijkhuijs AJ, et al. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002;16(8):1432-1436. [DOI] [PubMed] [Google Scholar]
- 13.Coustan-Smith E, Sancho J, Hancock ML, et al. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002;100(7):2399-2402. [DOI] [PubMed] [Google Scholar]
- 14.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovorkova L, Zaliova M, Venn NC, et al. Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood. 2017;129(20):2771-2781. [DOI] [PubMed] [Google Scholar]
- 18.Nagel I, Bartels M, Duell J, Oberg HH et al. Hematopoietic stem cell involvement in BCR-ABL1-positive ALL as potential mechanism of resistance to blinatumomab therapy [published online ahead of print 21 August 2017]. Blood. doi:10.1182/blood-2017-05-782888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüggemann M, Gökbuget N, Kneba M. Acute lymphoblastic leukemia: monitoring minimal residual disease as a therapeutic principle. Semin Oncol. 2012;39(1):47-57. [DOI] [PubMed] [Google Scholar]
- 20.Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. [DOI] [PubMed] [Google Scholar]
- 21.Beldjord K, Chevret S, Asnafi V, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739-3749. [DOI] [PubMed] [Google Scholar]
- 22.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153-4162. [DOI] [PubMed] [Google Scholar]
- 23.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595-1604. [DOI] [PubMed] [Google Scholar]
- 24.Dhédin N, Huynh A, Maury S, et al. ; GRAALL group. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486-2496. [DOI] [PubMed] [Google Scholar]
- 25.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172(3):392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Šálek C, Folber F, Froňková E, et al. ; Czech Leukemia Study Group - for Life. Early MRD response as a prognostic factor in adult patients with acute lymphoblastic leukemia. Eur J Haematol. 2016;96(3):276-284. [DOI] [PubMed] [Google Scholar]
- 27.Brüggemann M, Raff T, Flohr T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116-1123. [DOI] [PubMed] [Google Scholar]
- 28.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168-5174. [DOI] [PubMed] [Google Scholar]
- 29.Short NJ, Kantarjian HM, Sasaki K, et al. Prognostic significance of day 14 bone marrow evaluation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia. Cancer. 2016;122(24):3812-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Slack R, Jorgensen JL, et al. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2014;14(4):319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassan R, Spinelli O, Oldani E, et al. Different molecular levels of post-induction minimal residual disease may predict hematopoietic stem cell transplantation outcome in adult Philadelphia-negative acute lymphoblastic leukemia. Blood Cancer J. 2014;4(7):e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terwey TH, Hemmati PG, Nagy M, et al. Comparison of chimerism and minimal residual disease monitoring for relapse prediction after allogeneic stem cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20(10):1522-1529. [DOI] [PubMed] [Google Scholar]
- 33.Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. 2014;2014:421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao XS, Liu YR, Zhu HH, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91(2):183-192. [DOI] [PubMed] [Google Scholar]
- 35.Hoelzer D, Gökbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26(1):25-32. [DOI] [PubMed] [Google Scholar]
- 36.Maury S, Chevret S, Thomas X, et al. ; for GRAALL. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016;375(11):1044-1053. [DOI] [PubMed] [Google Scholar]
- 37.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134-4140. [DOI] [PubMed] [Google Scholar]
- 38.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 39.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zugmaier G, Gökbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jabbour E, Short NJ, Jorgensen JL, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. 2017;123(2):294-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoelzer D. Personalized medicine in adult acute lymphoblastic leukemia. Haematologica. 2015;100(7):855-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lussana F, Intermesoli T, Gianni F, et al. Achieving molecular remission before allogeneic stem cell transplantation in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact on relapse and long-term outcome. Biol Blood Marrow Transplant. 2016;22(11):1983-1987. [DOI] [PubMed] [Google Scholar]
- 49.Nishiwaki S, Imai K, Mizuta S, et al. Impact of MRD and TKI on allogeneic hematopoietic cell transplantation for Ph+ALL: a study from the adult ALL WG of the JSHCT. Bone Marrow Transplant. 2016;51(1):43-50. [DOI] [PubMed] [Google Scholar]
- 50.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150-155. [DOI] [PubMed] [Google Scholar]
- 51.Chalandon Y, Thomas X, Hayette S, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719. [DOI] [PubMed] [Google Scholar]
- 52.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herold T, Schneider S, Metzeler KH, et al. Adults with Philadelphia chromosome-like acute lymphoblastic leukemia frequently have IGH-CRLF2 and JAK2 mutations, persistence of minimal residual disease and poor prognosis. Haematologica. 2017;102(1):130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts KG, Pei D, Campana D, et al. Outcomes of children with BCR-ABL1–like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014;32(27):3012-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brüggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120(23):4470-4481. [DOI] [PubMed] [Google Scholar]
- 59.Raff T, Gökbuget N, Lüschen S, et al. ; GMALL Study Group. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910-915. [DOI] [PubMed] [Google Scholar]
- 60.Pemmaraju N, Kantarjian H, Jorgensen JL, et al. Significance of recurrence of minimal residual disease detected by multi-parameter flow cytometry in patients with acute lymphoblastic leukemia in morphological remission. Am J Hematol. 2017;92(3):279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bader P, Kreyenberg H, von Stackelberg A, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol. 2015;33(11):1275-1284. [DOI] [PubMed] [Google Scholar]
- 62.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. [DOI] [PubMed] [Google Scholar]
- 63.Gökbuget N, Dombret H, Bonifacio M, et al. Long-term outcomes after blinatumomab treatment: follow-up of a phase 2 study in patients (Pts) with minimal residual disease (MRD) positive B-cell precursor acute lymphoblastic leukemia (ALL) [abstract]. Blood. 2015;126(23). Abstract 680. [Google Scholar]
- 64.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]