Introduction
When explaining the risks of allogeneic hematopoietic cell transplantation (allo-HCT) for malignant disease to patients, providers typically emphasize potentially life-threatening complications, including graft-versus-host disease (GVHD), opportunistic infection, organ damage, hemorrhage, and graft rejection. After learning of the potential morbidity and mortality associated with allo-HCT in such consent sessions, transplant candidates are sometimes surprised that they also face the risk of disease recurrence despite transplantation. Indeed, over the past 30 years, funding for clinical- and laboratory-based transplant research has focused far more on prevention and treatment of GVHD, rather than on addressing the issue of relapse. This may be understandable given the still somewhat mysterious nature of GVHD and its devastating consequences, yet disease recurrence remains the single leading cause for failure after allo-HCT.1 Indeed, a review of investigational studies listed on ClinicalTrials.gov reveals a relative paucity of interventional trials devoted to the prevention and/or treatment of disease relapse after allo-HCT.
Outcomes for patients who relapse after allo-HCT have been historically dismal, particularly for patients with acute leukemia. In a single institution study of 351 patients who relapsed after allo-HCT, the 3-year postrelapse overall survival (OS) rate was only 19%. Risk factors for mortality after relapse included shorter time to relapse, high disease risk index at HCT, myeloablative conditioning (MAC), high pre-HCT comorbidity index, and onset of GVHD occurring prior to relapse. Important prognostic factors did not vary by underlying disease type. Receiving a donor lymphocyte infusion (DLI) or second HCT was associated with superior postrelapse survival as was the development of GVHD after relapse.2 The importance of restoring graft-versus-tumor activity to treat recurrence is further underscored by the observations that remissions can be induced in some cases with immune suppression withdrawal alone.3 While cell-mediated allo-immunity certainly contributes to tumor eradication, pharmacologic agents during and after HCT may also play a role in addressing relapse either directly by killing malignant cells or indirectly by stimulating engrafted donor immune effectors. This review will address the applications of these medications to prevent or treat recurrence.
The challenge of addressing post-HCT relapse
One of the challenges in conducting either retrospective analyses or prospective randomized trials to assess strategies that might mitigate relapse rates is the inherent biologic susceptibility of the underlying malignancy. It has been well established that karyotypic abnormalities in acute leukemia impact not only outcomes of conventional therapy but also those after allo-HCT.4 A CIBMTR (Center for International and Marrow Transplant Research) analysis of >13 000 patients suggested that the disease risk index rather than any one specific therapeutic intervention influenced relapse rates and outcomes after allo-HCT.5 A more granular detailed assessment of mutational profiles clearly indicates the impact of specific molecular abnormalities on the likelihood of relapse after allo-HCT.6,7 Thus, even randomized trials must be interpreted with caution if modern biological parameters are not available or well balanced.
Another practical impediment is the lack of access to investigational agents to test in the post–allo-HCT setting. Early-phase studies involving new drugs for relapse often exclude patients who have undergone allo-HCT given the myriad complications that such patients may experience. Trials using agents administered as maintenance after allo-HCT are usually only permitted to be performed when such agents have been approved for other indications or settings. This dynamic often permits off-protocol access to these agents, possibly hindering enrollment into prospective randomized trials when they are finally conducted in the post allo-HCT setting (Table 1).
Table 1.
Challenges of conducting clinical trials to prevent or treat disease relapse after allo-HCT
| Agent specific |
| Off-target toxicities including cytopenias/additional immunosuppression |
| Induction of GVHD |
| Impairment of effective graft-vs-malignancy effect |
| Drug-drug interactions |
| Disease specific |
| No singular target for the majority of diseases |
| No validated reliable MRD assays for majority of diseases to act preemptively |
| Competition with other trials/modalities |
| Population specific |
| Competing risk of opportunistic infection |
| Competing risk of GVHD |
| Trials conducted will have inherent selection bias given early dropout after allo-HCT |
| Industry specific |
| Reluctance to conduct early-phase trials in the post–allo-HCT setting |
| Access to agents inhibits enrollment in randomized trials |
| Small market |
Timing of intervention
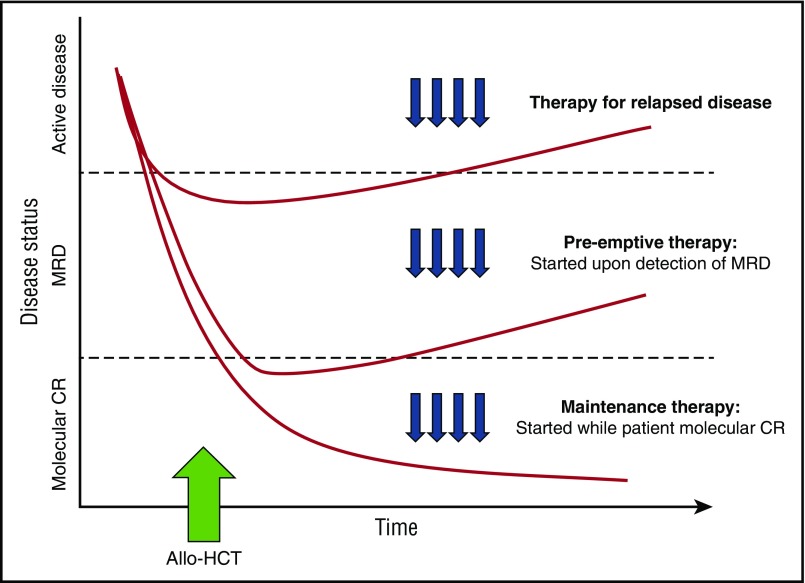
Pharmacologic intervention can be introduced at the time of HCT intercalated with or as part of conditioning. However, the incorporation of additional agents with conditioning runs the risk of heightening the toxicities inherent to the transplant procedure itself. Alternatively, therapy can be initiated in a maintenance strategy, while still in remission, to prevent disease relapse once acute toxicities of HCT have resolved. An ideal maintenance approach does not compromise graft integrity, induce significant GVHD, or interfere with the metabolism of other essential pharmacologic agents such as calcineurin inhibitors. Although maintenance leads to the treatment of all patients, many of whom may not be destined to relapse, a preemptive approach targets only patients with warning signs of impending relapse, such as those with minimal residual disease (MRD) detected by multiparameter flow cytometry, polymerase chain reaction or more sensitive molecular techniques, or falling donor hematopoietic chimerism. A preemptive strategy, however, will only be effective if the kinetics of relapse allow sufficient time from MRD detection to initiate therapy before morphologic relapse and clinical consequences.8 (Figure 1)
Figure 1.
Different approaches to post-transplantation therapies after allogeneic HCT including treatment vs preemptive vs maintenance. (Adapted with permission from Defilipp and Chen.8)
Conditioning regimen dose intensification
Early attempts to tackle the problem of disease recurrence after allo-HCT concentrated on escalating the intensity of conditioning. An early study from Seattle prospectively randomized acute myeloid leukemia (AML) patients to cyclophosphamide (Cy) (120 g/kg) plus either 1200- or 1575-cGy total body irradiation (TBI) in conjunction with cyclosporine/methotrexate GVHD prophylaxis. The 3-year probability of relapse was significantly lower for the 1575-cGy group but was offset by higher rates of moderate to severe acute GVHD and nonrelapse mortality (NRM). OS was identical.9 When additional chemotherapy (high-dose cytarabine) was added to a Cy/TBI regimen, relapse rates were indeed lower but survival was not higher as a consequence of increased NRM.10 Indeed, with the successful introduction of reduced intensity conditioning (RIC) allowing older patients to more safely undergo HCT, investigators began examining whether more gentle preparative regimens were comparable or perhaps superior to MAC by maintaining the graft-versus-leukemia (GVL) response yet sparing patients toxicity and NRM. A number of retrospective institutional and registry studies suggested that although reduced-intensity regimens might compromise disease control, the reduction in toxicity results in comparable OS.11-14 So-called reduced-toxicity MAC regimens (busulfan [Bu]/fludarabine [Flu]) have begun to replace traditional Cy/TBI or Bu/Cy regimens. A randomized study of Bu/Flu vs Bu/Cy in 252 patients with AML 40 to 65 years of age demonstrated a lower 1-year NRM rate of 17.2% vs 7.9% (P = 0.026) for Bu-Flu recipients with no statistically significant differences in relapse rates or OS.15
Despite these reports, manipulating conditioning to improve disease control should not be categorically dismissed. The Pediatric Working Party of the European Society for Blood and Marrow Transplantation (EBMT) recently published a retrospective report comparing 3 regimens (TBI/Cy, Bu/Cy, Bu/Cy/melphalan) for children with AML in complete remission 1 (CR1). Bu/Cy/melphalan was associated with a lower incidence of relapse at 5 years (14.7% vs 31.5% in Bu/Cy vs 30% in TBICy, P < .01) and higher OS (76.6% vs 64% vs 64.5%, P = .04) and leukemia-free survival (74.5% vs 58% vs 61.9%, P < .01), with a comparable NRM (10.8% vs 10.5% vs 8.1%, P = .79).16 More recently, novel approaches incorporating radiolabeled 131I-anti-CD45 antibodies have demonstrated that marrow-directed dose intensification may be combined with other conditioning approaches to selectively deliver antitumor activity without increased off-target toxicity.17,18
The most convincing data supporting the impact of dose intensity on relapse and transplant outcome come from the large prospective randomized phase 3 trial conducted by the Bone Marrow Transplant Clinical Trials Network comparing MAC with RIC in patients with AML or myelodysplastic syndrome (MDS). Patients 18 to 65 years of age were randomly assigned to receive MAC (n = 135) or RIC (n = 137) followed by HCT from HLA-matched related or unrelated donors. At 18 months, relapse rates were significantly lower with MAC vs RIC (13.5% vs 48.3%, P < .001). Despite higher transplant-related mortality in the MAC patients (15.8% vs 4.4%, P = .002), relapse-free survival was higher for MAC patients (67.8% vs 47.3%, P < .01) as was OS (77.5% vs 67.7%, P = .07). Among multiple covariates examined, the diagnosis of AML, the presence of high-risk disease, and a comorbidity index of 0 were found to specifically benefit from MAC.19 Although these results appear convincing, potential interactions with mutational drivers of the underlying malignancy may have influenced outcomes. A recent analysis from The National Marrow Donor Program, CIBMTR, and Dana-Farber Cancer Institute in patients with MDS revealed that high-dose preparative regimens benefited patients with mutations involving the RAS pathway but did not influence relapse rates in patients with p53 mutations.6
Lessons from autologous HCT
The concept of incorporating additional pharmacologic therapy after transplant to prevent disease recurrence began with autologous HCT. The comparatively high relapses rates and low toxicity of auto-HCT compared with allogeneic procedures stimulated investigators to evaluate additional agents in the autologous setting. In addition, significant advances have been made in the development of new therapies for diseases for which auto-HCT is typically performed, such as multiple myeloma and lymphoma. In contrast, until recently, only a limited number of successful interventions were available for the common diseases for which allo-HCT is used (AML, acute lymphoblastic leukemia [ALL], and MDS).
Early efforts at maintenance after auto-HCT for both lymphoma and myeloma did focus on drugs thought to have immunomodulatory properties, such as interferon-α. Most studies demonstrated a marginal benefit with substantial toxicity.20,21 The most successful application of post-HCT intervention has been after auto-HCT for multiple myeloma, specifically with immunomodulatory drugs and proteasome inhibitors. Although trials of thalidomide after transplant have yielded conflicting results, lenalidomide maintenance has been evaluated in 3 prospective, controlled randomized studies, all of which demonstrated a significant improvement in progression-free survival (PFS) and, to some degree, OS.22-24 Lenalidomide maintenance therapy is associated with myelosuppression, which has led to discontinuation in some patients. There has also been an increased risk of both hematologic malignancies and solid tumors in patients receiving lenalidomide compared with placebo. Nonetheless, a clear overall benefit remains, which has led to lenalidomide maintenance therapy after auto-HCT being established as a standard of care in the United States. Bortezomib has also been studied as maintenance therapy after auto-HCT with promising results,25 although a formal prospective randomized study has not yet been reported.
In contrast to myeloma, post-HCT maintenance therapy has not been widely adopted for lymphoma. The most commonly studied agent has been the anti-CD20 monoclonal antibody, rituximab. A prospective randomized study in recurrent large cell lymphoma failed to demonstrate an advantage in relapse-free or OS for rituximab maintenance therapy.26 Although a prospective randomized trial in recurrent follicular lymphoma of post-HCT rituximab suggested an improvement in PFS, no differences were documented in OS.27 Recently, a trial of the anti-CD30 antibody-drug conjugate brentuximab vedotin as maintenance therapy after auto-HCT for adverse risk recurrent classical Hodgkin lymphoma similarly yielded improved PFS, but no increase in OS,28 yet received approval to be administered in this setting. The results of these trials do raise the question of whether an improvement in PFS but not OS is sufficient to warrant adoption of a particular maintenance approach. Supporters might argue that for more indolent malignancies, survival can be prolonged even after post-transplant relapse so that improvement in PFS should carry significant weight. Detractors would argue that if the condition of a patient can be salvaged by additional lines of therapy, the value of maintenance therapy to a patient population is limited, especially if that particular therapy is highly efficacious when given as treatment for relapse. In younger patients with mantle cell lymphoma, recently presented phase 3 results from the LyMa Trial have shown that rituximab after auto-HCT prolongs PFS and OS. The 4-year PFS from randomization was higher in the rituximab arm (82.2% vs 64.6%, P = .0005), as was the 4-year OS (88.7% vs 81.4%, P < .05).29 Thus, the difference in survival advantage from maintenance strategies from these trials may be related to biologic differences of the underlying disease or alternatively to the application of this strategy in transplantation performed after first response rather than in the relapsed disease setting.
Breakpoint cluster region–Abelson tyrosine kinase inhibitors in chronic myeloid leukemia and ALL
The most commonly used maintenance strategy after allo-HCT has been the use of Abelson tyrosine kinase inhibitors (TKIs) in Philadelphia chromosome-positive (Ph+) leukemias including advanced-phase chronic myeloid leukemia and Ph+ ALL. Most of the data supporting this practice come from single-arm or retrospective experiences. Initial studies with imatinib revealed that it could safely be given after HCT without major myelosuppression or unmanageable interactions with calcineurin inhibitors.30 In one trial, imatinib was administered to 22 patients with Ph+ leukemia from the time of engraftment to 1 year after HCT, with 77% maintaining major molecular remission and only 9% with hematologic relapse.31 In a separate study of 22 patients with chronic myeloid leukemia in which imatinib was administered from day 35 to 1 year post-HCT, 95% completed 11 months of therapy and remained without cytogenetic or hematologic relapse, although 15 patients did experience disease relapse once imatinib treatment was stopped.32 Second- and third-generation TKIs also have been studied after allo-HCT. A number of small retrospective series have reported successful maintenance with dasatinib and nilotinib,33-35 and many have adopted this as a standard of care.
Larger registry experiences evaluating the efficacy post–allo-HCT maintenance, TKI therapy for Ph+ ALL have yielded contrasting results. The CIBMTR analyzed 197 patients with Ph+ ALL undergoing allo-HCT in CR1, 43 of whom received post-transplant therapy with TKIs. There was no difference in the 3-year cumulative incidence of relapse.36 However, in an EBMT study of 473 patients with Ph+ ALL in CR1, of which 60 patients received maintenance therapy with TKIs, imatinib administration was independently associated with a lower relapse rate and improvements in both leukemia-free survival and OS.37 The danger in interpreting these registry studies is that patients initiated maintenance at different time points after allo-HCT and likely received differing doses and varying durations of therapy.
Because MRD can easily be monitored in Ph+ leukemias through quantitative breakpoint cluster region–Abelson (BCR-ABL) polymerase chain reactions in peripheral blood, some investigators have advocated for a preemptive, MRD-triggered approach. In one prospective evaluation of 27 patients undergoing allo-HCT for Ph+ ALL, preemptive imatinib therapy at MRD detection post-HCT was associated with prolonged disease-free survival in approximately half of patients, which could be anticipated by rapid achievement of molecular CR in response to therapy.38 A phase 2 study of 55 patients comparing maintenance and preemptive, MRD-triggered imatinib in Ph+ ALL resulted in low rates of hematological relapse in both arms. Although the maintenance strategy reduced molecular recurrence compared with preemptive therapy, there was no significant difference in overall outcomes between the 2 arms.39
TKIs targeting FMS-like tyrosine kinase 3-internal tandem duplication in AML
Internal tandem duplication mutations involving the FMS-like tyrosine kinase 3 (FLT3-ITD) occur in 25% to 40% of patients with de novo AML. Allogeneic HCT in CR1 appears to improve the prognosis of patients with FLT3-ITD AML; however, disease relapse remains significant.40 A number of FLT3 TKIs are being investigated in various phases of therapy including the post-HCT maintenance setting for FLT3-ITD AML, including sorafenib, midostaurin, quizartinib, crenolanib, and gilteritinib. Midostaurin is the first FLT3 inhibitor approved for the upfront treatment of AML in conjunction with chemotherapy. Benefit was particularly noted in patients who went on to undergo HCT.41 A phase 2 study evaluating midostaurin, a multitarget kinase inhibitor, as post-HCT or postconsolidation maintenance has reported an encouragingly low relapse rate at 12 months (9.2%).42
The recognition that sorafenib could induce remissions in patients who had relapsed post-HCT43 led to a phase 1 study of 22 patients with FLT3-ITD AML receiving sorafenib maintenance therapy after allo-HCT. Sorafenib was relatively well tolerated. The PFS rate at 1 year was 85%, and the OS rate was 95%.44 A subsequent analysis compared 26 patients with FLT3-ITD AML treated with sorafenib maintenance after allo-HCT in CR1 to a cohort of 43 contemporary matched controls with FLT3-ITD AML. The use of sorafenib was associated with improved PFS (85% vs 52%, P = .0047) and OS (83% vs 58%, P = .019) with a significant decrease in the rate of disease relapse in patients who received sorafenib (8.2% vs 37.7%, P = .0077).45 A trial of peritransplant sorafenib (given both before and after HCT) in 28 patients with FLT3-ITD AML in CR1 undergoing HCT has preliminarily reported only 5 relapses and 6 deaths.46 Another study of 27 patients reported that 25 of 27 patients received sorafenib post-HCT remained in remission with a 1-year PFS rate of 92%.47 A number of other retrospective studies have also reported encouraging results using sorafenib as maintenance therapy, preemptive therapy, or therapy for relapsed disease post-allo HCT.48-50 Additional FLT3 TKIs being studied as maintenance after HCT include quizartinib51 and crenolanib.52 A large prospective cooperative group international phase 3 placebo-controlled randomized trial is underway to definitely determine the benefit of administering a FLT3 TKI (gilteritinib) as maintenance therapy after allo-HCT for patients with FLT3-ITD AML in CR1 (NCT02997202).
The mechanism of action of TKIs targeting FLT3 may extend beyond direct tumor cell killing. Reports suggest that sorafenib may synergize with allogeneic T cells to improve survival in several murine models. Sorafenib treatment increased interleukin-15 (IL-15) production in leukemic cells, rendering them more immunogenic. Sorafenib exposure reduced ATF4 (activating transcription factor 4) expression in leukemic cells. ATF4 is a negative regulator of IRF-7 (interferon regulatory factor 7) activation, which in its active form enhances IL-15 transcription. pIRF-7 levels increased in sorafenib-treated leukemic cells, suggesting that sorafenib may affect the pIRF-7/IL-15 axis by reducing ATF4 production.53 It is hoped that the completion of existing trials and the analysis of correlative samples will help to elucidate the exact mechanism.
DNA hypomethylating agents
The most common nontargeted pharmacologic approach to treatment and prevention of relapse after HCT for AML and MDS has been DNA hypomethylating agents (HMAs). The mechanism of action of HMAs is uncertain, but they appear to silence tumor-suppressing genes through epigenetic modification. HMAs may also induce the GVL response through increased expression of tumor antigens. The EBMT examined the tolerability and activity of azacitidine in 181 patients with MDS or AML who relapsed after undergoing HCT in which the complete response rate was 15% with an overall response rate of 25%. The 2-year OS rate was 12% for the whole population. OS was influenced by a time to relapse post-transplant of >6 months and the percentage of blasts in the bone marrow at the time of relapse. The concurrent administration of DLI did not improve either response rates or OS in patients treated with azacitidine.54
A phase 1 study of 45 patients established an optimal dosing schedule for azacitidine maintenance to be 32 mg/m2 IV × 4 cycles and resulted in a 1-year OS rate of 77%.55 Reversible thrombocytopenia was the dose-limiting toxicity. A phase 1 study of 27 patients with AML post-HCT found that azacitidine both augmented the expansion of regulatory T cells and induced cytotoxic CD8+ T-cell response to several tumor antigens, and led to hopes that it might help lead to successful cultivation of the GVL response without inducing significant GVHD.56 The RICAZA trial assessed maintenance therapy with azacitidine in 51 patients with AML of whom only 37 patients initiated therapy at a median of 54 days post-HCT. Treatment was well tolerated and did not induce excessive chronic GVHD (cGVHD). Sixteen patients relapsed. Induction of a CD8+ T-cell response was associated with a reduced risk of disease relapse and improved relapse-free survival. It is uncertain whether the azacitidine is responsible for the induction of CD8 responses.57
A phase 2 National Cancer Institute/Alliance trial (CALGB 100801) tempered enthusiasm a bit. Of 66 enrolled patients, only 42 were able to initiate maintenance therapy with azacitidine, with 17 patients completing all 6 planned cycles.58 A randomized trial to assess the impact of maintenance azacitidine therapy is near completion (NCT00887068). Decitabine has also been studied as maintenance therapy following myeloablative allo-HCT; as expected, many patients experienced hematologic toxicities during treatment. In one study, the 2-year OS rate was 56% and the cumulative incidence of relapse was 28%.59 Just as with targeted TKI therapy, azacitidine is also being explored as preemptive therapy once MRD can be detected but before frank relapse. The RELAZA trial initiated therapy in patients with CD34+ AML whose post-transplant CD34+ donor chimerism dropped to <80%, and found that although this delayed relapse to a median of 231 days after the decrease in chimerism, most patients ultimately proceeded to morphological disease relapse.60
Histone deacetylase inhibition
Panobinostat is a potent inhibitor of class 1, 2, and 4 deacetylases and has shown some antileukemic as well as immunomodulatory activity. The PANOBEST trial enrolled 42 patients who had undergone allo-HCT for high-risk AML or MDS. Panobinostat was initiated a median of 98 days post-HCT. Of 42 patients, 22 (54%) completed 1 year of therapy with side effects accounting for most study withdrawals. Still, at 2 years, the cumulative incidence of relapse was 21% with probabilities for 2-year OS and disease-free survival rates of 88% and 74%.61 The results are certainly provocative and likely merit further evaluation.
Immunomodulation with lenalidomide
Lenalidomide, which has an established role in maintenance therapy after auto-HCT for multiple myeloma, is also being investigated in the post allo-HCT setting given its immunomodulatory mechanism of action. In one prospective study of 30 patients given lenalidomide maintenance at a dose of 10 mg daily after nonmyeloablative HCT for myeloma as part of first-line treatment, grade 2 or greater acute GVHD or extensive cGVHD developed in 16 patients (53%) at a median of 18 days (range, 4 to 217 days) after the start of lenalidomide.62 The investigators concluded that this approach was not feasible due to the development of GVHD. In another trial investigating maintenance therapy following myeloablative allo-HCT for patients with relapsed/refractory disease after previous auto-HCT, the major toxicity observed with lenalidomide maintenance therapy were grade 2 to 4 acute GVHD (28%).63 In a separate multi-institutional trial that enrolled 30 patients with high-risk myeloma, 12 months of lenalidomide maintenance therapy following RIC HCT was completed in 37% of patients, with the majority (91%) at a dose of ≤10 mg/d.64 The most common reasons for discontinuation of lenalidomide were acute GVHD (37%) and disease progression (37%). When lenalidomide was studied as maintenance therapy post-HCT in 10 patients with del(5q) MDS or AML, grade 3 to 4 acute GVHD was observed in 6 patients, further dampening enthusiasm for its use early after allo-HCT.65
Proteasome inhibition
Like with auto-HCT, there is interest in proteasome inhibition after allo-HCT treatment for myeloma. A phase 2 trial of bortezomib maintenance therapy in 16 patients with high-risk myeloma undergoing RIC HCT found the approach to be feasible with acceptable rates of GVHD.66 Proteosome inhibition is now formally being evaluated in BMT Clinical Trials Network trial 1302 in a double-blind randomized trial of maintenance therapy with the oral proteasome inhibitor ixazomib or placebo (NCT02440464).
Monoclonal antibodies
As noted above, the use of monoclonal antibodies and antibody-drug conjugates has been studied extensively after auto-HCT, however, there is much less experience after allo-HCT. Rituximab has been evaluated extensively for autoimmune cytopenia and for cGVHD after allo-HCT with promising results.67,68 A recently completed Alliance study incorporated rituximab after allo-HCT for treatment of chronic lymphocytic leukemia (CLL) to both target residual neoplastic cells and reduce cGVHD (NCT01027000). Target specificity may also be achieved with the use of antibody-drug conjugates. Gemtuzumab ozogamicin, an antibody against CD33 conjugated to the chemotherapy calicheamicin, has been evaluated as maintenance therapy in conjunction with azacitidine in a small series with treatment being limited by hematologic toxicities.69 In addition, there is concern that this and other conjugates (ie, SGN33a, which is an anti-CD33 antibody conjugated to a pyrrolobenzodiazepine dimer) that target CD33 could contribute to hepatic veno-occlusive disease. Inotuzumab ozagamicin targets CD22 and has been approved for relapsed refractory ALL with activity in patients who relapsed after HCT.70 It too has been has been associated with veno-occlusive disease in 11% of patients who did not go on to HCT and up to 22% of patients who did.71 Blinatumomab, a novel bispecific CD19-directed CD3 T-cell engager, active in relapsed and refractory ALL, has been used alone72 or in conjunction with DLI73 after allo-HCT for treatment of relapsed disease, although no large-scale formal assessment of its efficacy has been reported.
Checkpoint inhibition
Immune escape (ie, tumor evasion of the donor immune system) likely plays a fundamental role in the pathogenesis of relapse after allo-HCT. Immune escape can be mediated by high levels of checkpoint receptors such as CTLA-4 and PD-1 on donor-derived lymphocytes and high expression of cognate ligands on residual tumor cells. It has been postulated that checkpoint inhibitors, so effective in many solid tumors and certain lymphomas, might be ideal agents to test for post-HCT relapse. One study of the PD-1 blocker pidiluzumab after auto-HCT for diffuse large B-cell lymphoma suggested excellent tolerability with promising long-term relapse-free survival, especially in patients who had residual disease.74 A study using the PD-L1 antibody pembrolizumab in this setting is underway for patients with diffuse large B-cell lymphoma, classical Hodgkin lymphoma (HL) and T-cell non-HL (NCT02362997). For patients who relapse after allo-HCT, there has been understandable concern that checkpoint inhibition might lead to uncontrollable immune breakthrough events, specifically significant GVHD. In murine models, PD-1 blockade has led to an increase in GVHD,75 whereas selective blockade of CTLA-4 to treat relapse after allo-HCT demonstrated graft-versus-tumor effects without inducing GVHD.76
A phase 1 study of a single low dose of ipilimumab (0.1 to 3.0 mg/kg) for patients who relapsed after allo-HCT did not seem to cause clinically significant GVHD and achieved responses in 3 patients with lymphoid malignancies.77 A subsequent phase 1/2 study was conducted in which patients were to receive 4 doses of ipilimumab every 3 weeks. Patients with stable disease or better could then receive maintenance doses every 3 months for a year. Dosing started at 3 mg/kg but could escalate to 10 mg/kg. Although no objective responses were seen at 3 mg/kg, 7 of 22 patients at the 10 mg/kg dose responded including 6 CRs and 1 partial remission. Interestingly, CRs were achieved in 3 of 3 patients with leukemia cutis. Responders had decreased CD4+ regulatory T cells with increased conventional T cells in peripheral blood as well as an increase in CD62L− effector memory T cells. Immunohistochemical and gene expression profiling revealed an influx of CD8+ T cells expressing perforin in responding leukemia cutis patients. GVHD responsive to corticosteroids developed in 4 of 28 patients. Immune-related adverse events typical of ipilimumab were observed in 6 patients, one of which was fatal. The median time from transplant to ipilumuab treatment was 675 days, so it is uncertain what the safety profile would be if administered early post-transplant as a maintenance strategy.78
Given the success of PD-1 blockade in patients with HL, anti–PD-1 monoclonal antibodies are beginning to be used following allo-HCT.79 A multicenter retrospective analysis of 31 lymphoma patients revealed a high response rate (77%). However, GVHD developed in 17 of 31 patients after a median of 1-2 doses. The response of GVHD to steroids alone was low, and 8 patients died of complications due to GVHD.80 A prospective trial of PD1 blockade for post–allo-HCT with lower doses of nivolumab is underway (NCT01822509). It would be difficult to recommend the use of PD1 inhibitors outside of the context of a clinical trial.
Novel agents
The recent approval of several agents for blood cancers has led to interest in testing them in the transplant setting. Ibrutinib recently been approved for the treatment of cGVHD has demonstrated activity for the relapse of CLL in the post-HCT setting.81 This has prompted the initiation of studies to assess the role of ibrutinib as maintenance therapy after both autologous and allogeneic HCT for non-HL and CLL, respectively. Enasidenib, an oral selective inhibitor of isocitrate dehydrogenase 2 (IDH2), was recently approved for the treatment of relapsed, refractory patients with IDH2 mutation, which occur in ∼12% of patients with AML. In a phase 1/2 study including 176 relapsed refractory patients, 24 of whom had relapsed after allo-HCT, the overall response rate was 40.3%. Responses were associated with blast differentiation without evidence of aplasia. Nearly 20% of patients attained a CR.82 Further data are needed to assess the safety and efficacy in patients who have relapsed after HCT. No data are currently available for enasidinb as a maintenance strategy, but data will likely be forthcoming in the next few years. Venetoclax, a highly selective, oral small-molecule BCL2 inhibitor approved for CLL has been studied in AML with a reported response rate of 19%, including patients with high-risk molecular features.83 Anecdotal data exist as to its activity post-HCT. Again, formal safety assessments are necessary before these can be incorporated into treatment or maintenance strategies for post-HCT relapse.
Personalized approach to post-HCT relapse
No simple algorithm or single approach can be adopted to address relapse after transplantation. Unfortunately, there are no mature randomized trials to provide guidance, and indeed evidence from phase 2 trials is not clear cut. Consideration must be given to disease histology, the presence of targetable mutations, proliferative thrust of the malignancy, the post-transplant interval, the presence of GVHD, concurrent immune suppression medications, and patient performance status. In general, a reasonable first step would be withdrawal of immune suppression, particularly if the relapse is relatively indolent. Responses to immune suppression withdrawal are almost always associated with the development of GVHD, which can be severe so patients must be monitored carefully. If there is no response or patients are not receiving immune suppression therapy at relapse or if there is aggressive recurrence, then disease-specific, hopefully mutation-directed, therapy should be initiated such as with BCR-ABL, FLT3-ITD, or now even IDH2 inhibitors. Alternatively, antigen-directed therapy with blinatumumab for CD19+ ALL, inotuzumab for CD22 ALL, or gemtuzumab for CD33 AML all are reasonable options, though they carry the potential of substantial toxicity.
Immune stimulation with agents such as lenolidamide or immune checkpoint inhibitors must be considered investigational at this juncture, and their use should ideally be confined to clinical trials. It is important for the transplant community to expeditiously design, conduct, and report results on trials of checkpoint inhibitors so that their use can be safely incorporated.
Lacking a specific mutational target, it is not unreasonable to return to agents with demonstrated disease activity such as those in lymphoma, CLL, and myeloma. For diseases like AML and MDS, HMAs hold the promise of upregulating neoantigens, exposing blasts to immune attack. Standard chemotherapy can also be used here, though response rates are <50% and toxicity can be high. If indeed there is success in inducing complete or near CRs with any of the above maneuvers, the next logical question is: What’s next: ongoing maintenance, observation, DLI, or second transplant? Again, there is no clear answer. In general, if remission after chemotherapy is associated with the development of GVHD, then there may be limited benefit (and possibly harm) in consolidating with some form of cellular therapy. However, for patients in whom remission has been achieved without GVHD, either DLI or second transplant can be recommended.
Perhaps more important than treating relapse is preventing it. Here again, we are limited by a lack of definitive data other than anecdotal or phase 2 experience with TKIs for BCR-ABL and FLT3-ITD–related diseases. Although conventional interventions like histone deacetylase inhibitors or HMAs have gained popularity, there remains no convincing evidence that they by themselves are helpful and certainly can induce some morbidity. Particularly for maintenance strategies, prospective randomized studies need to be conducted.
Last, a critical treatment choice for patients who relapse post-HCT is that of supportive care alone without disease-directed therapy. Providers must have frank discussions with patients and their families about the prospects for success so that they can make informed decisions. These decisions may include earlier referral to hospice programs when appropriate.84
Conclusions
The transplant community needs to continue its commitment to discover new approaches to preventing and treating relapse post-HCT. We must discard the notion that allo-HCT by itself is definitive therapy and must work with colleagues development of disease-specific therapy to take advantage of advances in drug development to design synergistic or additive strategies. We must understand better through tumor genomic profiling who is at the greatest risk to relapse and must work to determine how to select donors with heightened reactivity against a tumor in a specific host. We must also convince pharmaceutical companies to engage with the transplant community to test novel agents earlier in the development cycle to best understand how they might be successfully applied to improve the outcome of the transplant recipient.
Footnotes
This article was selected by the Blood Advances and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2017. It is reprinted in Hematology Am Soc Hematol Educ Program. 2017;2017:699-707.
Authorship
Contribution: R.J.S. and Y.-B.C. wrote the article.
Conflict-of-interest disclosure: R.J.S. has consulted for Sandoz, Juno, and Kiadis. Y.-B.C. has consulted for Takeda, Magenta, Incyte, and Jazz. Off-label drug use: Multiple drugs for the prevention or treatment of relapse post-transplant including tyrosine kinase inhibitors directed at BCR-ABL and FLT3, hypomethylating agents, checkpoint inhibitors, and lenalidomide are discussed. There are no approved agents for such use.
Correspondence: Robert J. Soiffer, Dana Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: robert_soiffer@dfci.harvard.edu.
References
- 1.Wingard JR, Majhail NS, Brazauskas R, et al. . Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanarajasingam G, Kim HT, Cutler C, et al. . Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(12):1713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kekre N, Kim HT, Thanarajasingam G, et al. . Efficacy of immune suppression tapering in treating relapse after reduced intensity allogeneic stem cell transplantation. Haematologica. 2015;100(9):1222-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armand P, Kim HT, Zhang MJ, et al. . Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18(2):280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armand P, Kim HT, Logan BR, et al. . Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsley RC, Saber W, Mar BG, et al. . Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlenk RF, Döhner K, Krauter J, et al. ; German-Austrian Acute Myeloid Leukemia Study Group. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909-1918. [DOI] [PubMed] [Google Scholar]
- 8.DeFilipp Z, Chen YB. Strategies and challenges for pharmacological maintenance therapies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(12):2134-2140. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Appelbaum FR, et al. . Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867-1871. [PubMed] [Google Scholar]
- 10.Arai Y, Kondo T, Shigematsu A, et al. ; Japan Society for Haematopoietic Cell Transplantation. Increased non-relapse mortality due to high-dose cytarabine plus CY/TBI in BMT/PBSCT for acute lymphoblastic leukaemia in adults. Br J Haematol. 2017;178(1):106-111. [DOI] [PubMed] [Google Scholar]
- 11.Alyea EP, Kim HT, Ho V, et al. . Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10):1047-1055. [DOI] [PubMed] [Google Scholar]
- 12.Aoudjhane M, Labopin M, Gorin NC, et al. ; Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304-2312. [DOI] [PubMed] [Google Scholar]
- 13.Passweg JR, Labopin M, Cornelissen J, et al. ; Acute Leukemia Working Party of the European Blood and Marrow Transplant Group (EBMT). Conditioning intensity in middle-aged patients with AML in first CR: no advantage for myeloablative regimens irrespective of the risk group-an observational analysis by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2015;50(8):1063-1068. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24(5):1050-1052. [DOI] [PubMed] [Google Scholar]
- 15.Rambaldi A, Grassi A, Masciulli A, et al. . Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16(15):1525-1536. [DOI] [PubMed] [Google Scholar]
- 16.Lucchini G, Labopin M, Beohou E, et al. . Impact of conditioning regimen on outcomes for children with acute myeloid leukemia undergoing transplantation in first complete remission. An analysis on behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(3):467-474. [DOI] [PubMed] [Google Scholar]
- 17.Pagel JM, Gooley TA, Rajendran J, et al. . Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009;114(27):5444-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mawad R, Gooley TA, Rajendran JG, et al. . Radiolabeled anti-CD45 antibody with reduced-intensity conditioning and allogeneic transplantation for younger patients with advanced acute myeloid leukemia or myelodysplastic syndrome. Biol Blood Marrow Transplant 2014;20(9):1363-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott BL, Pasquini MC, Logan BR, et al. . Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björkstrand B, Svensson H, Goldschmidt H, et al. . Alpha-interferon maintenance treatment is associated with improved survival after high-dose treatment and autologous stem cell transplantation in patients with multiple myeloma: a retrospective registry study from the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2001;27(5):511-515. [DOI] [PubMed] [Google Scholar]
- 21.Remenyi P, Varga G, Mikala G, et al. . Early versus delayed autologous stem cell transplantation and interferon maintenance in multiple myeloma: single-center experience of 18 years. Transplant Proc. 2016;48(1):177-184. [DOI] [PubMed] [Google Scholar]
- 22.Attal M, Lauwers-Cances V, Marit G, et al. ; IFM Investigators. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy PL, Owzar K, Hofmeister CC, et al. . Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbo A, Cavallo F, Gay F, et al. . Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905. [DOI] [PubMed] [Google Scholar]
- 25.Sivaraj D, Green MM, Li Z, et al. . Outcomes of maintenance therapy with bortezomib after autologous stem cell transplantation for patients with multiple myeloma. Biol Blood Marrow Transplant. 2017;23(2):262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gisselbrecht C, Schmitz N, Mounier N, et al. . Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36):4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettengell R, Schmitz N, Gisselbrecht C, et al. . Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31(13):1624-1630. [DOI] [PubMed] [Google Scholar]
- 28.Moskowitz CH, Nademanee A, Masszi T, et al. ; AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853-1862. [DOI] [PubMed] [Google Scholar]
- 29.Le Gouill S, Thieblemont C, Oberic L, et al. . Rituximab maintenance after autologous stem cell transplantation prolongs survival in younger patients with mantle cell lymphoma: final results of the randomized phase 3 LyMa trial of the Lysa/Goelams Group [abstract]. Blood. 2016;128(22). Abstract 145. [Google Scholar]
- 30.Ribera JM, Oriol A, González M, et al. ; Programa Español de Tratamiento en Hematología; Grupo Español de Trasplante Hemopoyético Groups. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter PA, Snyder DS, Flowers ME, et al. . Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olavarria E, Siddique S, Griffiths MJ, et al. . Posttransplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients undergoing reduced-intensity allografts for chronic myeloid leukemia. Blood. 2007;110(13):4614-4617. [DOI] [PubMed] [Google Scholar]
- 33.Caocci G, Vacca A, Ledda A, et al. . Prophylactic and preemptive therapy with dasatinib after hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2012;18(4):652-654. [DOI] [PubMed] [Google Scholar]
- 34.Teng CL, Yu JT, Chen HC, Hwang WL. Maintenance therapy with dasatinib after allogeneic hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Hematol. 2013;92(8):1137-1139. [DOI] [PubMed] [Google Scholar]
- 35.Nishimoto M, Nakamae H, Koh KR, et al. . Dasatinib maintenance therapy after allogeneic hematopoietic stem cell transplantation for an isolated central nervous system blast crisis in chronic myelogenous leukemia. Acta Haematol. 2013;130(2):111-114. [DOI] [PubMed] [Google Scholar]
- 36.Bachanova V, Marks DI, Zhang MJ, et al. . Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brissot E, Labopin M, Beckers MM, et al. . Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassmann B, Pfeifer H, Stadler M, et al. . Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2005;106(2):458-463. [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer H, Wassmann B, Bethge W, et al. ; GMALL Study Group. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-1262. [DOI] [PubMed] [Google Scholar]
- 40.Brunet S, Labopin M, Esteve J, et al. . Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735-741. [DOI] [PubMed] [Google Scholar]
- 41.Stone RM, Mandrekar SJ, Sanford BL, et al. . Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlenk R, Doehner K, Salih H, et al. . Midostaurin in combination with intensive induction and as single agent maintenance therapy after consolidation therapy with allogeneic hematopoietic stem cell transplantation or high-dise cytarabine (NCT01477606) [abstract]. Blood. 2015;126(23). Abstract 322. [Google Scholar]
- 43.Metzelder SK, Schroeder T, Finck A, et al. . High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353-2359. [DOI] [PubMed] [Google Scholar]
- 44.Chen YB, Li S, Lane AA, et al. . Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for FMS-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):2042-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunner AM, Li S, Fathi AT, et al. . Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175(3):496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratz KW, Gojo I, Karp JE, et al. . Prospective study of peri-transplant use of sorafenib as remission maintenance for FLT3-ITD patients undergoing allogeneic transplantation [abstract]. Blood. 2015;126(23). Abstract 3164. [Google Scholar]
- 47.Battipaglia G, Ruggeri A, Massoud R, et al. . Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3-mutated acute myeloid leukemia. Cancer 2017;123(15):2867-2874. [DOI] [PubMed] [Google Scholar]
- 48.Sammons SL, Pratz KW, Smith BD, Karp JE, Emadi A. Sorafenib is tolerable and improves clinical outcomes in patients with FLT3-ITD acute myeloid leukemia prior to stem cell transplant and after relapse post-transplant. Am J Hematol. 2014;89(9):936-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antar A, Kharfan-Dabaja MA, Mahfouz R, Bazarbachi A. Sorafenib Maintenance Appears Safe and Improves Clinical Outcomes in FLT3-ITD Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2015;15(5):298-302. [DOI] [PubMed] [Google Scholar]
- 50.Tarlock K, Chang B, Cooper T, et al. . Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. 2015;62(6):1048-1054. [DOI] [PubMed] [Google Scholar]
- 51.Sandmaier BM, Khaled SK, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib (AC220) as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic cell transplantation. Blood. 2014;124(21):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins R, Kantarjian HM, Ravandi F, et al. . Full doses of crenolanib, a type I FLT3 inhibitor, can be safely administered in AML patients post allogeneic stem cell transplant [abstract]. Blood. 2015;126(23). Abstract 4359. [Google Scholar]
- 53.Mathew NR, Baumgartner F, Waterhouse M, et al. . Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in leukemia cells. Biol Blood Marrow Transplant. 2016;22(suppl 3):S90. [Google Scholar]
- 54.Craddock C, Labopin M, Robin M, et al. . Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2016;101(7):879-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lima M, Giralt S, Thall PF, et al. . Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116(23):5420-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodyear OC, Dennis M, Jilani NY, et al. . Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012;119(14):3361-3369. [DOI] [PubMed] [Google Scholar]
- 57.Craddock C, Jilani N, Siddique S, et al. . Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant. 2016;22(2):385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vij R, Hars V, Blum W, et al. . CALGB 100801 (Alliance): a phase II multi-center NCI cooperative group study of the addition of azacitidine (AZA) to reduced-intensity conditioning (RIC) allogeneic transplantation for high risk myelodysplasia (MDS) and older patients with acute myeloid leukemia (AML): results of a “test dose” strategy to target busulfan exposure. Blood. 2014;124(21):543. [Google Scholar]
- 59.Pusic I, Choi J, Fiala MA, et al. . Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Platzbecker U, Wermke M, Radke J, et al. . Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bug G, Burchert A, Wagner EM, et al. . Phase I/II study of the deacetylase inhibitor panobinostat as maintenance therapy after an allogeneic stem cell transplantation in patients with high-risk MDS or AML: the Panobest-Trial. Blood. 2015;126(23):4344. [Google Scholar]
- 62.Kneppers E, van der Holt B, Kersten MJ, et al. . Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118(9):2413-2419. [DOI] [PubMed] [Google Scholar]
- 63.Kröger N, Zabelina T, Klyuchnikov E, et al. . Toxicity-reduced, myeloablative allograft followed by lenalidomide maintenance as salvage therapy for refractory/relapsed myeloma patients. Bone Marrow Transplant. 2013;48(3):403-407. [DOI] [PubMed] [Google Scholar]
- 64.Alsina M, Becker PS, Zhong X, et al. . Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(8):1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. ; German MDS and Cooperative Transplant Study Group (GCTSG). Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97(9):e34-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caballero-Velázquez T, López-Corral L, Encinas C, et al. . Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients. Br J Haematol. 2013;162(4):474-482. [DOI] [PubMed] [Google Scholar]
- 67.Arai S, Sahaf B, Narasimhan B, et al. . Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cutler C, Miklos D, Kim HT, et al. . Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oshikawa G, Kakihana K, Saito M, et al. . Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol. 2015;169(5):756-759. [DOI] [PubMed] [Google Scholar]
- 70.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. . Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4(8):e387-e398. [DOI] [PubMed] [Google Scholar]
- 72.Topp MS, Gökbuget N, Stein AS, et al. . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 73.Ueda M, de Lima M, Caimi P, et al. . Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2016;51(9):1253-1255. [DOI] [PubMed] [Google Scholar]
- 74.Armand P, Nagler A, Weller EA, et al. . Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. . Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171(3):1272-1277. [DOI] [PubMed] [Google Scholar]
- 76.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162(11):6368-6377. [PubMed] [Google Scholar]
- 77.Bashey A, Medina B, Corringham S, et al. . CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davids MS, Kim HT, Bachireddy P, et al. ; Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Cheikh J, Massoud R, Abudalle I, et al. . Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant. 2017;52(7):1074-1077. [DOI] [PubMed] [Google Scholar]
- 80.Haverkos BM, Abbott D, Hamadani M, et al. . PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130(2):221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryan CE, Sahaf B, Logan AC, et al. . Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128(25):2899-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein EM, DiNardo CD, Pollyea DA, et al. . Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konopleva M, Pollyea DA, Potluri J, et al. . Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odejide OO, Cronin AM, Condron NB, et al. . Barriers to quality end-of-life care for patients with blood cancers. J Clin Oncol. 2016;34(26):3126-3132. [DOI] [PubMed] [Google Scholar]