Key Points
Altered number, subset composition, and function of bone marrow innate lymphoid cells are early events in monoclonal gammopathies.
Pomalidomide therapy leads to reduction in Ikzf1 and Ikzf3 and enhanced human innate lymphoid cell function in vivo.
Introduction
Innate lymphoid cells (ILCs) have emerged as a new family of innate immune cells implicated in the regulation of diverse processes, including resistance to pathogens, autoimmune inflammation, and tissue homeostasis.1 ILCs lack rearranged antigen receptors and reside predominantly in tissues wherein they express cytokines in response to signals in the tissue microenvironment. Three distinct human ILC subsets have been described on the basis of their cytokine profile and transcriptional regulation which mirror well-characterized T-helper cell subsets: T-bet+ ILC1 secretes interferon-γ (IFN-γ), GATA3+ ILC2 secretes interleukin-4 (IL-4)/IL-5/IL-13, and Rorγt+ ILC3 secretes IL-17 and IL-22.2 Recent studies have examined pro- or antitumor properties of ILC subsets in mouse models.3-5 Changes in circulating ILC subsets in human leukemia have been described,6,7 but data relating to changes in ILC subsets in the tumor microenvironment in human preneoplastic states are lacking.
All cases of multiple myeloma (MM) are preceded by precursor monoclonal gammopathy of undetermined significance (MGUS).8 Prior studies have shown the capacity of the immune system to recognize these earliest lesions, which correlate with reduced risk of progression to clinical malignancy.9-11 Immunomodulatory drugs (IMiDs) such as lenalidomide or pomalidomide form a component of standard MM therapy and have shown promising activity for prevention of malignancy.12 IMiDs are thought to act in part by inducing cereblon-mediated degradation of Ikzf1 and Ikzf3.13 IMiD-mediated T-cell/NK T-cell (T/NKT) activation depends on antigen-mediated stimulation.14,15 However, the spectrum of potential cellular targets of IMiDs still remains to be fully characterized. Recent findings that human ILC1 subsets express high levels of Ikzf316 raised the possibility that ILCs may also be targets of IMiD-mediated immune regulation. Tumor cells in MGUS/MM reside primarily in the bone marrow. However, data relating to functional characterization of human ILC subsets in the normal marrow and in the setting of plasma cell disorders are lacking. These considerations led us to explore changes in ILC subsets in blood and bone marrow of patients with MGUS/MM and after IMiD therapy.
Methods
Patients and samples
Blood and bone marrow samples were obtained from MGUS/asymptomatic multiple myeloma (AMM) patients after informed consent was received and the study was approved by the institutional review board. Mononuclear cells (MNCs) were isolated by density gradient centrifugation.
Immunophenotyping of ILCs
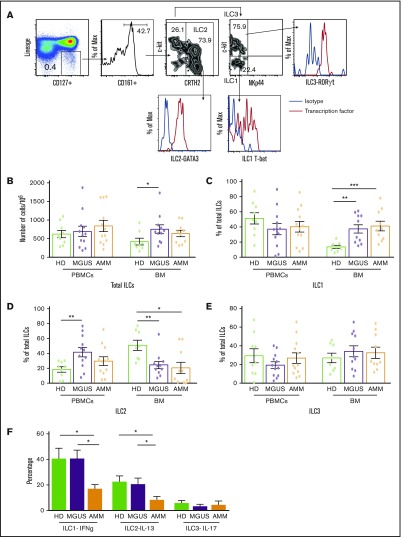
Immunophenotyping of ILCs was performed by flow cytometry as described.2,7 Figure 1A shows the gating strategy for ILCs. An eBioscience transcription factor staining set was used to detect transcription factors in ILCs. For functional assays, cells were stimulated with phorbol myristate acetate (PMA) (100 ng/mL) and ionomycin (500 ng/mL) before staining for the detection of intracellular cytokines. Data were acquired on a BD LSR II flow cytometer and were analyzed by using FlowJo software.
Figure 1.
Changes in ILC and ILC subsets in gammopathies. (A) Representative fluorescence-activated cell sorter FACS plots showing the gating strategy to identify ILC1, ILC2, and ILC3 along with their transcription factors T-bet, GATA3, and RORγt, respectively. MNCs were stained with dye to exclude dead cells and a cocktail of antibodies (lineage: CD1a, CD34, CD94, CD123, TCRαβ, TCRγδ, FCεR1, CD303, CD11c, CD14, CD19, CD3, and CD138 in bone marrow from patients with plasma cell disorders) to exclude cells with known lineages. ILCs were then identified in a lineage-negative subset based on the expression of CD127 and CD161, and then classified as ILC1, ILC2, or ILC3 on the basis of the expression of CRTH2, c-kit, and NKp44. ILCs were identified as lineage CD127+CD161+ cells and subclassified as ILC2 (CRTH2+c-kit+/−), ILC1 (NKp44–c-kit–), or ILC3 (Nkp44–c-kit+). (B-E) Total ILCs and ILC subsets in peripheral blood mononuclear cells (PBMCs) or bone marrow (BM) samples from healthy donors (HDs; n = 9) and MGUS (n = 12) or AMM patients (n = 12) were evaluated by using flow cytometry. All graphs show mean ± standard error of the mean (SEM). (B) Total ILCs in peripheral blood and BM of HDs as well as MGUS and AMM patients. Proportion of (C) ILC1, (D) ILC2, and (E) ILC3 in PBMCs and BM samples of HDs and MGUS and AMM patients. (F) Cytokine production by ILC subsets upon stimulation with phorbol myristate acetate (PMA) and ionomycin. Bar graph (mean + SEM) shows the IFN-γ–producing ILC1s, IL-13–producing ILC2s, and IL-17–producing ILC3s in HD BM (n = 4), MGUS BM (n = 6), and AMM BM (n = 6). *P < .05; **P < .01; ***P < .001.
Single-cell RNA sequencing
Marrow MNCs were flow-sorted to isolate NK and ILC1 cells. Barcoded libraries were prepared by using manufacturer’s (10x Genomics) protocol and sequenced with a HiSeq2500 high-throughput sequencing system. Filtered barcode matrices were generated by using Cell-Ranger (10x Genomics) followed by detection and elimination of doublets. Digital expression matrices were imported to Seurat software,17 and cells with >10% mitochondrial scripts were eliminated. Principal component analysis was used to identify clusters and differentially expressed genes.
In vitro and in vivo effect of pomalidomide on human ILCs
To analyze the effect of pomalidomide on cytokine-mediated activation of ILCs, peripheral blood mononuclear cells were treated overnight with pomalidomide (100 ng/mL) and IL-12 (10 ng/mL) or with only IL-12 as a control. To detect changes in transcription factors, MNCs were cultured overnight with pomalidomide and analyzed for changes in the expression of Ikzf1 and Ikzf3 in ILCs as well as CD3+ T cells. To analyze the effect of pomalidomide on ILCs in vivo, samples obtained from MM patients treated with pomalidomide as a single agent in the context of a prior clinical trial18 were analyzed. For these studies, samples obtained before therapy and at 4 hours after the first dose were compared for the expression of Ikzf1 in ILCs as well as cytokine production after in vitro activation.
Statistics
Mann-Whitney U test was used to compare groups, and significance was set at P < .05.
Results and discussion
The proportion of bone marrow ILCs but not circulating ILCs was increased in MGUS patients compared with healthy donors (Figure 1B). MGUS patients also had altered proportions of marrow ILC subsets (Figure 1C-E) with an increase in ILC1 (Figure 1C), but not ILC2 (Figure 1D). The capacity of both ILC1 and ILC2 to secrete lineage-specific cytokines (IFN-γ and IL-13, respectively) was preserved with a net increase in IFN-γ–producing ILC1 in MGUS patients but significantly declined in AMM patients (Figure 1F; supplemental Figure 1). Together these data suggest that altered number, subset composition, and function of ILCs is an early feature of plasma cell neoplasm and can be detected as early as MGUS.
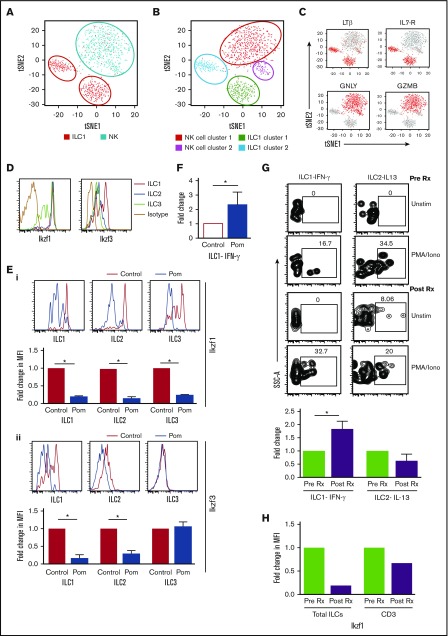
Recent studies have debated differences between human NK cells and ILC1.19,20 To address this in the setting of human marrow, we analyzed transcriptional profiles of sorted CD3–CD56+ NK cells and ILC1 from healthy bone marrow using single-cell RNA sequencing (GEO submission, GSE104600). NK cells and ILC1s each form 2 distinct clusters, with ILC1 vs NK distinguished by lymphotoxin-β (LT-β), IL-7R, granulysin, and granzyme B as in human tonsil tissue.16 ILC1 clusters (ILC cluster 1: TRAC+CCL5+GZMA+SELL–; ILC cluster 2: SELL+CCL5–GZMA–TRAC–) were distinct from NK clusters (NK cluster 1: GNLY+GZMB+FGFBP2+; NK cluster 2: XCL1+XCL2+GZMBlo) (Figure 2A-C; supplemental Figure 2). These data demonstrate that ILC1 identified in our studies is distinct from NK cells.
Figure 2.
ILC subsets and changes in ILCs after pomalidomide. (A-C) Student t distribution analysis of the single-cell RNA sequencing data showing the NK and (A-B) ILC1 cluster and the (C) key genes (LTβ, IL7-R, GNLY, GZMB) that distinguish ILC1 and NK cells in human marrow. (D) Expression of Ikaros (Ikzf1) and Aiolos (Ikzf3) in ILC subsets (ILC1, ILC2, and ILC3). (E) Depletion of Ikzf1 and Ikzf3 in ILCs following exposure to pomalidomide in vitro. (i) Depletion of IKZF1: representative plot showing the level of Ikaros (Ikzf1) in ILC1, ILC2, and ILC3 in control cells and cells treated with pomalidomide (100 ng/mL). Bar graph shows data from 3 experiments. (ii) Depletion of IKZF3: representative FACS plots showing the level of Aiolos (Ikzf3) in ILC1, ILC2, and ILC3 in control cells and cells treated with pomalidomide (100 ng/mL). Bar graph shows data from 3 experiments. (F) Fold change in IFN-γ–producing ILC1 after culture with pomalidomide. Bar graph shows the IFN-γ production by ILC1 in HD PBMCs (n = 5) after overnight culture with IL-12 (10 ng/mL) alone (control) or with pomalidomide (100 ng/mL). (G) Changes in cytokine production by ILCs after a single dose of pomalidomide. PBMCs from MM patients (n = 3) were isolated before therapy and 4 hours after a single dose of pomalidomide and analyzed for cytokine production by ILCs after stimulation with PMA and ionomycin. Representative FACS plot showing the cytokine production by ILCs. Bar graph shows cumulative data from 3 patients. (H) Change in Ikaros levels in ILCs as well as CD3 in vivo after pomalidomide therapy.
The finding that human ILC1 differentially express Ikzf316 (Figure 2D), a known target for IMiD-mediated degradation, encouraged us to directly test the impact of pomalidomide on ILCs. ILCs cultured with pomalidomide exhibited reduction in Ikzf1 (Figure 2Ei) and Ikzf3 (Figure 2Eii) levels and greater cytokine secretion upon in vitro stimulation (Figure 2F). Circulating ILCs from pomalidomide-treated MM patients isolated just 4 hours after first dose had increased capacity for IFN-γ production (Figure 2G) and reduced levels of Ikzf1 (Figure 2H). Together these data indicate that pomalidomide leads to reduction in Ikzf1 and Ikzf3 and enhanced function of ILC1 in vitro and in vivo.
These data provide evidence that ILCs are among the earliest cell subsets enriched in the tumor microenvironment during the evolution of monoclonal gammopathies. The signals that lead to enrichment of these cells in the MGUS marrow remain to be clarified. In spite of small numbers, ILCs can significantly impact T-cell function by competing for cytokine niches21 and therefore broadly impact immunity in the tumor bed. Alteration in ILC function in AMM patients suggests that changes in ILC function may also occur early in myelomagenesis. ILCs act as innate counterparts of CD4+ T cells and may impact adaptive immunity either directly or indirectly by altering function of tissue-resident dendritic cells.22 Therefore alteration in ILCs observed here has potential implications for immune surveillance in MM. Prospective evaluation in patients and using new humanized models23 may help further dissect the role of ILC dysfunction in MM. Ikzf3 was previously shown to inhibit murine innate cells24 and ikzf3 depletion has been implicated in IMiD-mediated immune activation.14 Therefore, Ikzf3 depletion may account for pomalidomide-mediated enhancement of ILC function observed in this study, although further studies are needed to specifically establish this mechanism. Together, these data illustrate a novel pathway wherein IMiDs may modulate immune microenvironment without the need for antigen-mediated signals. Recent studies have suggested programmed cell death protein 1 (PD-1)–mediated regulation of ILCs.25 These cells may therefore also be underexplored targets of interactions between IMiDs and PD-1 blockade.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
M.V.D. was supported in part by funds from National Institutes of Health, National Cancer Institute grant CA197603, the Leukemia and Lymphoma Society, and the Multiple Myeloma Research Foundation.
Authorship
Contribution: J.K.B. performed experiments, analyzed data, and wrote the article; S.M. and L.Z. performed experiments and analyzed data; N.N., T.P., N.B., T.A., and M.L.X. performed clinical research and analyzed data; K.M.D. analyzed data and wrote the article; and M.V.D. conceived and supervised the project, analyzed data, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, Yale Cancer Center, 333 Cedar St, Box 208021, New Haven, CT 06510; e-mail: madhav.dhodapkar@yale.edu.
References
- 1.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765-774. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145-149. [DOI] [PubMed] [Google Scholar]
- 3.Carrega P, Campana S, Bonaccorsi I, Ferlazzo G. The yin and yang of innate lymphoid cells in cancer. Immunol Lett. 2016;179:29-35. [DOI] [PubMed] [Google Scholar]
- 4.Irshad S, Flores-Borja F, Lawler K, et al. RORγt(+) innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer Res. 2017;77(5):1083-1096. [DOI] [PubMed] [Google Scholar]
- 5.Dadi S, Chhangawala S, Whitlock BM, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell. 2016;164(3):365-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Weerdt I, van Hoeven V, Munneke JM, et al. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica. 2016;101(11):e461-e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trabanelli S, Curti A, Lecciso M, et al. CD127+ innate lymphoid cells are dysregulated in treatment naïve acute myeloid leukemia patients at diagnosis. Haematologica. 2015;100(7):e257-e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhodapkar MV. MGUS to myeloma: a mysterious gammopathy of underexplored significance. Blood. 2016;128(23):2599-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhodapkar MV, Sexton R, Das R, et al. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood. 2015;126(22):2475-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198(11):1753-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spisek R, Kukreja A, Chen LC, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204(4):831-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang DH, Liu N, Klimek V, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108(2):618-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björklund AK, Forkel M, Picelli S, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17(4):451-460. [DOI] [PubMed] [Google Scholar]
- 17.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehgal K, Das R, Zhang L, et al. Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood. 2015;125(26):4042-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernink JH, Mjösberg J, Spits H. Human ILC1: to be or not to be. Immunity. 2017;46(5):756-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni Y, Fehlings M, Kløverpris HN, et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017;46(1):148-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin CE, Spasova DS, Frimpong-Boateng K, et al. Interleukin-7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity. 2017;47(1):171-182. e4. [DOI] [PubMed] [Google Scholar]
- 22.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das R, Strowig T, Verma R, et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med. 2016;22(11):1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes ML, Huntington ND, Thong RP, et al. Peripheral natural killer cell maturation depends on the transcription factor Aiolos. EMBO J. 2014;33(22):2721-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor S, Huang Y, Mallett G, et al. PD-1 regulates KLRG1(+) group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1663-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.