Abstract
Background
We investigated an association between the levels of plasma microRNA (miRNA)-21, -26a, and -29a-3p and treatment outcomes following transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC).
Methods
A total of 198 patients with TACE-treated HCC were followed up for TACE refractoriness and liver transplantation (LT)-free survival. Pretreatment plasma miRNA-21, -26a, and -29a-3p levels were measured using quantitative real-time polymerase chain reaction.
Results
During the mean follow-up of 22.3 (range, 0.7–79) months, 118 (59.6%) patients exhibited TACE refractoriness. Multivariate analyses showed that expression of a specific combination of miRNAs (miRNA-21 ≥ 2.5, miRNA-26a ≥ 1.5, and miRNA-29a-3p < 0.4) was associated with early TACE refractoriness (within 1 year; hazard ratio [HR], 2.32; 95% confidence interval [CI], 1.08–4.99; P = 0.031) together with tumor size (HR, 4.62; 95% CI, 1.50–14.21; P = 0.008), and macrovascular invasion (HR, 3.80; 95% CI, 1.19–12.20; P = 0.025). However, miRNA-21, -26a, and -29a-3p levels were not significantly associated with overall TACE refractoriness or LT-free survival. Additionally, large tumor size and macrovascular invasion were common predictive factor of overall TACE refractoriness and survival.
Conclusion
Combination of plasma miRNA-21, -26a, and -29a-3p expression could predict early TACE refractoriness in patients with TACE-treated HCC.
Keywords: Chemoembolization, Therapeutic; Hepatitis B, Chronic; Carcinoma, Hepatocellular; MicroRNAs
Graphical Abstract
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most predominant and important malignancies in the world. It is the fifth most common cause of cancer in men and seventh in women, and is the third most common cause of cancer-related death worldwide.1 HCC possesses distinctive pathogenic features and its prognosis is affected by both the effect on the liver function and the overall tumor burden.2
According to the Barcelona Clinic Liver Cancer (BCLC) guideline, transarterial chemoembolization (TACE) is the standard treatment for intermediate HCC.3 However, there is increasing evidence that it may also be used in treatment of patients with early or advanced HCC.4,5,6 Nevertheless, the long-term prognosis of patients with TACE-treated HCC varies substantially because of the heterogeneous pathogenic nature, as well as tumor burden, liver function, and disease etiology. Therefore, identifying and predicting the patients who would benefit most from TACE is important for providers faced with the challenge of determining the most effective therapeutic strategy and assessing the prognosis.7
MicroRNAs (miRNAs) are endogenous, evolutionarily conserved, non-coding RNAs recognized as epigenetic factors regulating gene expression.8 Aberrant miRNA expression is a ubiquitous feature in numerous cancers including HCC.9 miRNAs play important roles in several aspects of carcinogenesis, including cell proliferation, cell cycle regulation, apoptosis, local invasion, and metastasis.10 Therefore, it has been theorized that several tumor-suppressing and oncogenic miRNAs may have potential applications in early diagnosis and prediction of prognosis in HCC.11,12
miRNA-21 is considered a typical “onco-miRNA.” It inhibits the expression of phosphatases that downregulates AKT and mitogen-activated protein kinase (MAPK) signaling pathways.13 miRNA-21 can lead to upregulate cell proliferation. Therefore, miRNA-21 is associated with a wide variety of cancers, including liver cancer.14 A recent meta-analysis evaluated circulating miRNA-21 as a biomarker of various carcinomas and uncovered its potential as an early diagnostic tool.15 Inversely, miRNA-26a induces cell cycle arrest at the G1 phase in human HCC cells, in part through direct downregulation of cyclin D2 and cyclin E2.16 miRNA-26a is consistently downregulated in HCC.17 miRNA-29a-3p is also downregulated in HCC tissues, compared to adjacent non-cancerous liver tissues, and regulates HCC growth stems from modulation of the secreted protein acidic, rich in cysteine-AKT (SPARC-AKT) pathway.18
Even though these pathways are well studied, there is limited research regarding the prognostic role of levels of circulating miRNA-21, -26a, and -29a-3p in patients with HCC, especially with regard to TACE. The present study aimed at evaluating the association between pretreatment plasma levels of miRNA-21, -26a, and -29a-3p, treatment responses, and survival, in patients with TACE-treated HCC.
METHODS
Patients
A total of 1,024 patients underwent initial TACE for unresectable HCC, between February 2005 and December 2013 at Ajou University Hospital, Suwon, Korea. Of them, plasma samples from 198 patients were kept in the Ajou Human Bio-Resource Bank and were analyzed in this study. Patients were diagnosed with HCC if their tumor had a maximum diameter > 1 cm, and typical features of HCC (hypervascularity in the arterial phase and washout in the portal venous or delayed phase) were observed using dynamic computed tomography (CT) and/or magnetic resonance imaging (MRI). For tumors < 1 cm, ultrasonography was performed after 3 months.19 Patients were excluded from the study if 1) they had been previously treated with TACE or chemotherapy, or 2) they had undergone surgical resection or radiofrequency ablation within 6 months of their first TACE.
TACE procedure
TACE procedures were performed by 2 interventional radiologists. Doxorubicin (50 mg, ADM®; Dong-A Pharm. Co. Ltd., Seoul, Korea) and iodized oil (10 mL, Lipiodol Ultrafluide® emulsion; Laboratoire André Guerbet, Aulnay-sous-Bois, France) were injected into the tumor-feeding vessels per the typical TACE protocol followed by injection of gelatin sponge particles (Cutanplast®; Mascia Brunelli Spa, Viale Monza, Italy) until the blood flow was mostly obstructed.
Definitions
Liver cirrhosis was defined by histological evidence or ultrasonographic findings of: spleen size > 12 cm, portal vein diameter > 16 mm, or nodules within the hepatic parenchyma.20 Treatment outcomes after TACE were assessed using TACE refractoriness and liver transplantation (LT)-free survival. TACE refractoriness was defined as; 1) 2 or more consecutive insufficient responses of the treated tumor (viable lesion > 50%), even after changing chemotherapeutic agents, and/or revascularization of the feeding artery observed during post procedure evaluation CT/MRI at 1–3 months after selective TACE, 2) appearance of more than 2 consecutive intrahepatic lesions visualized during post procedure-evaluation CT, 3) increased vascular invasion, 4) extrahepatic spread, 5) continuous elevation of tumor markers, including immediately after TACE, and 6) technical failure of TACE.21,22,23 If the patients received LT during the follow-up period, they were included in the analyses until the time of LT. Early TACE refractoriness was arbitrarily defined as TACE refractoriness within 1 year of the first TACE procedure.22,23
Contrast-enhanced CT was performed 4 weeks after TACE, and every 3 months thereafter, to evaluate the tumor response. TACE was repeated every 6 to 8 weeks if viable tumors were detected on the follow-up imaging and if the liver function was adequate. Tumor response was estimated according to the modified Response Evaluation Criteria in Solid Tumors.24
miRNAs measurements
Pre-TACE plasma samples were collected and stored at −70°C until processing for miRNA analyses. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to identify and quantify the miRNA levels in plasma. Magnetic beads were utilized for miRNA extraction, using the TaqMan® miRNA ABC Purification Kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer's instructions. TaqMan® miRNA hsa-miRNA-specific primers (miRNA-29a-3p #002112; Applied Biosystems) and TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) were used to identify complementary DNA strands from the isolated miRNAs. An ABI PRISM 7300 instrument (Life Technologies, Foster City, CA, USA) and TaqMan® Universal Master Mix (Applied Biosystems) were used for polymerase chain reaction (PCR) amplification. The expression levels of miRNA were detected as Ct values, and the relative level of miRNA expression (fold change) was calculated by the 2(−ΔΔCt) method. miRNA-16 was selected as the internal control to normalize miRNA data. Samples from patients with HCC were compared with 5 reference samples obtained from patients with liver cirrhosis. The calculations used were as follows:
| ΔCt = CtmiRNA-122 − CtmiRNA-16 |
| ΔΔCt = ΔCtHCC patients − ΔCtliver cirrhosis patients |
The mean was calculated after the qRT-PCR assays were performed in duplicate, using the same ABI PRISM 7300 instrument.
Statistical analysis
The results were reported as mean ± standard deviation. Hepatitis B virus (HBV) DNA levels were logarithmically adjusted for analysis. Significant cut-off values for each miRNA were determined with the Kaplan-Meier analysis with a log-rank test. miRNA levels were further analyzed as a panel (all 3 criteria met or not). Continuous variables were compared using independent-samples t-tests. Categorical data were compared using a Pearson χ2 test or Fisher's exact test. The cumulative probabilities of LT-free survival and overall TACE refractoriness were assessed by Kaplan-Meier analysis. The Cox proportional hazard model was used to identify factors associated with LT-free survival and overall TACE refractoriness. Binary logistic regression analysis was performed to identify factors associated with early TACE refractoriness, and variables that yielded P values < 0.05 in the univariate analyses were considered significant. miRNA performance, with respect to LT-free survival and overall TACE refractoriness, was assessed using an integrated area under the receiver operating characteristic curve (IAUC) plot and was compared by the bootstrapping method. IAUC was performed with R 3.1.1 (R Core Team [2014]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; URL: http://www.R-project.org/). Other statistical tests were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Ethics statement
The study design and all experimental procedures were approved by the Institutional Review Board of Ajou University Hospital, Suwon, Korea (AJIRB-BMR-GEN-14-466). Informed consent was waived by the board.
RESULTS
Baseline characteristics of study population and HCC
The mean age of the 198 patients included in the study was 58.6 ± 10.4 years and 75.8% of these patients were men. Most of the patients enrolled in this study had liver cirrhosis (94.9%) and HBV-associated HCC (99.0%). Among the patients with HBV-associated HCC, 67 (33.8%) patients were receiving oral antiviral therapy at the time of HCC diagnosis. Approximately one-half of all patients (44.4%) had a single tumor with a mean tumor size of 4.7 ± 3.2 cm. The percentage of patients with stage A, B, C, and D per the BCLC classification criteria was 44.9%, 28.3%, 25.3%, and 1.5%, respectively.3 The median follow-up duration was 22.3 months (range, 0.7–79.0 months). The baseline characteristics of the study population are summarized in Table 1.
Table 1. Baseline characteristics of study population.
| Characteristics | Values (n = 198) | |
|---|---|---|
| Gender (male) | 150 (75.8) | |
| Age, yr | 58.6 ± 10.4 | |
| Diabetes mellitus | 35 (17.7) | |
| HBsAg positivity | 196 (99.0) | |
| HBeAg positivity | 90 (45.9) | |
| Antiviral treatment | 67 (33.8) | |
| Cirrhosis | 188 (94.9) | |
| Child-Pugh class | ||
| A | 163 (82.4) | |
| B | 30 (15.2) | |
| C | 4 (2.0) | |
| Platelet, ×109/L | 133.0 ± 78.5 | |
| Creatinine, mg/dL | 1.0 ± 0.9 | |
| Albumin, g/L | 3.7 ± 0.6 | |
| Bilirubin, mg/dL | 1.2 ± 0.9 | |
| ALT, IU/L | 52.0 ± 57.1 | |
| INR | 1.16 ± 0.20 | |
| AFP, ng/mL | 2,895.2 ± 10,405.0 | |
| HBV DNA, log10 IU/mL | 4.18 ± 2.12 | |
| Tumor number | ||
| 1 | 88 (44.4) | |
| 2 | 50 (25.3) | |
| 3 | 18 (9.1) | |
| 4 | 6 (3.0) | |
| ≥ 5 | 36 (18.2) | |
| Tumor size, cm | 4.7 ± 3.2 | |
| Macrovascular invasion | 42 (21.2) | |
| BCLC stage | ||
| A | 89 (44.9) | |
| B | 56 (28.3) | |
| C | 50 (25.3) | |
| D | 3 (1.5) | |
| Modified UICC stage | ||
| I | 18 (9.1) | |
| II | 63 (31.8) | |
| III | 87 (43.9) | |
| IV | 30 (15.2) | |
| Follow-up duration, mon | 22.3 (0.7–79.0) | |
Values are presented as number (%), mean ± standard deviation, or median (range).
HBsAg = hepatitis B surface antigen, HBeAg = hepatitis B e antigen, ALT = alanine aminotransferase, INR = international normalized ratio, AFP = alpha-fetoprotein, HBV = hepatitis B virus, BCLC = Barcelona Clinic Liver Cancer, UICC = Union for International Cancer Control.
LT-free overall survival following TACE treatment and TACE outcomes
The 1-, 3-, and 5-year LT-free survival rates (95% confidence interval [CI]) were 65.9% (59.2–72.6), 40.6% (33.2–48.0), and 19.9% (11.5–28.3), respectively. During the follow-up period, 10 patients underwent LT. TACE refractoriness was evaluable in 7 of these transplant patients, of whom 2 exhibited TACE refractoriness before LT and 5 did not. Three of the 10 patients received LT prior to demonstrating TACE refractoriness and were categorized with the no-TACE refractoriness group. The cumulative LT-free survival rate is presented in the Supplementary Fig. 1A.
During the follow-up period, 59.6% of all patients demonstrated a complete response with repetitive TACE treatments. The mean number of TACE procedures needed to achieve a complete response was 1.49 (range, 1–6). Of these cases, 96 patients (81.4%) eventually showed tumor recurrence. The median time of recurrence was 10.7 (range, 1–37) months.
Among the 198 patients, TACE refractoriness was evaluated in 177 patients, as worsening liver function in 21 patients prevented evaluation. Of patients with TACE refractoriness, 118 patients (66.3%) became refractory during the follow-up period (Supplementary Fig. 1B). The 1-, 3-, and 5-year TACE refractoriness rates (95% CI) were 47.2% (39.8–54.6), 71.3% (63.5–79.1), and 80.4% (71.8–89.0), respectively. The median time of TACE refractoriness was 14.0 (0.77–73) months. Etiology of TACE refractoriness was as follows: incomplete necrosis of the intrahepatic lesion or new intrahepatic lesion development (45.6%), metastatic spread (42.2%), further vascular invasion (20.3%), and TACE failure due to technical problems (3.3%).
Predictive factors for LT-free survival
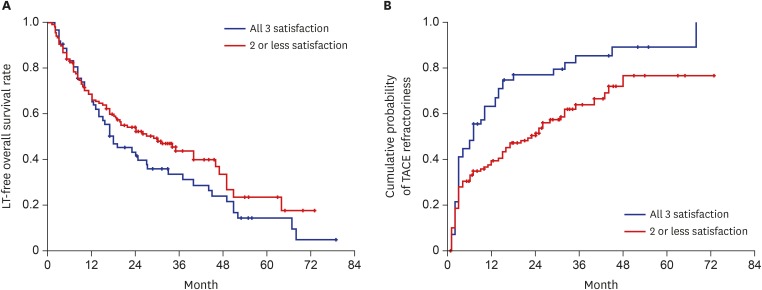
Predictive factors for LT-free survival were evaluated in all the 198 patients included in this study. In the univariate analysis, absence of liver cirrhosis, high Child-Pugh class (B/C vs. A), low albumin level, high bilirubin level, low international normalized ratio, low alpha-fetoprotein (AFP) level (< 400 ng/mL), low HBV DNA level, small tumor size (≤ 2 cm), and absence of macrovascular invasion represented positive predictive factors for LT-free survival. Multivariate Cox regression analyses revealed that large tumor size (hazard ratio [HR], 3.04; 95% CI, 1.51–6.11; P = 0.002) and macrovascular invasion (HR, 3.57; 95% CI, 2.32–5.48; P < 0.001) were the only independent risk factors for poor LT-free survival. Individual or combined miRNA-21, -26a, and -29a-3p levels could not statistically predict LT-free survival after a Cox regression analysis (Table 2 and Fig. 1A).
Table 2. Predictive factors of overall liver transplantation-free survival in HCC patients treated with TACE.
| Factors | Patients with better survival (n = 72) | Patients with worse survival (n = 126) | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Gender (male) | 50 (86.1) | 101 (80.2) | 1.39 (0.91–2.15) | 0.132 | ||
| Age, yr (≥ 55) | 60.1 (10.4) | 57.8 (10.3) | 0.69 (0.48–0.98) | 0.040 | 0.81 (0.55–1.19) | 0.285 |
| Diabetes mellitus | 10 (13.9) | 25 (19.8) | 1.19 (0.77–1.85) | 0.436 | ||
| HBeAg positivity | 33 (46.5) | 57 (45.6) | 0.88 (0.62–1.25) | 0.460 | ||
| Cirrhosis | 71 (98.6) | 117 (92.9) | 0.50 (0.25–0.99)a | 0.046a | ||
| Child-Pugh class (B/C) | 5.4 (0.9) | 5.8 (1.5) | 1.80 (1.16–2.80)a | 0.009a | ||
| Platelet, ×109/L (< 100) | 126.7 (78.8) | 136.7 (78.4) | 0.94 (0.66–1.36) | 0.755 | ||
| Creatinine, mg/dL | 1.1 (1.2) | 1.0 (0.6) | 0.89 (0.56–1.40) | 0.602 | ||
| Albumin, g/L | 3.9 (0.6) | 3.6 (0.6) | 0.60 (0.46–0.80)a | 0.000a | 0.73 (0.51–1.05) | 0.091 |
| Bilirubin, mg/dL | 1.0 (0.7) | 1.2 (1.0) | 1.40 (1.16–1.69)a | 0.000a | 0.93 (0.69–1.27) | 0.665 |
| ALT, U/L | 44.3 (35.4) | 56.4 (66.1) | 1.00 (1.00–1.00) | 0.423 | ||
| INR | 1.1 (0.2) | 1.2 (0.2) | 4.21 (1.89–9.40)a | 0.000a | 3.52 (0.98–12.67) | 0.054 |
| AFP (> 400 ng/mL) | 603.5 (1,889.5) | 4,204.8 (12,800.1) | 2.06 (1.42–2.98)a | 0.000a | 1.38 (0.91–2.07) | 0.126 |
| HBV DNA, log10 IU/mL | 3.6 (2.2) | 4.5 (2.0) | 1.13 (1.04–1.23)a | 0.004a | 1.01 (0.92–1.11) | 0.786 |
| Tumor number (≥ 2) | 1.7 (0.8) | 2.6 (1.7) | 1.32 (0.93–1.88) | 0.126 | ||
| Tumor size (≥ 2 cm) | 3.3 (2.3) | 5.5 (3.4) | 3.86 (2.02–7.38)a | 0.000a | 3.04 (1.51–6.11)a | 0.002a |
| Macrovascular invasion | 4 (5.6) | 338 (30.2) | 4.05 (2.73–6.02)a | 0.000a | 3.57 (2.32–5.48)a | 0.000a |
| miRNA-21 (≥ 2.5) | 4.9 (8.8) | 6.7 (11.2) | 1.28 (0.90–1.81) | 0.174 | ||
| miRNA-26a (≥ 1.5) | 6.5 (36.4) | 3.1 (3.6) | 1.23 (0.86–1.75) | 0.250 | ||
| miRNA-29a (< 0.4) | 0.38 (0.30) | 0.35 (0.31) | 1.20 (0.84–1.71) | 0.328 | ||
| miRNA-panel (all 3 met or not) | 1.30 (0.91–1.87) | 0.155 | ||||
Values are presented as number (%) or HR (95% CI).
HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization, HR = hazard ratio, CI = confidence interval, HBeAg = hepatitis B e antigen, ALT = alanine aminotransferase, INR = international normalized ratio, AFP = alpha-fetoprotein, HBV = hepatitis B virus, miRNA = microRNA.
aP < 0.05.
Fig. 1.
Cumulative probabilities of LT-free survival and TACE refractoriness according to plasma miRNA-21, 26a, and 29a-3p level. (A) Patients with high miRNA-21 (≥ 2.5), high miRNA-26a (≥ 1.5), and low miRNA-29a-3p (< 0.4) levels (all 3 criteria met) showed a shorter LT-free survival than those who did not; however, this was not statistically significant. (B) Patients with high miRNA-21 (≥ 2.5), high miRNA-26a (≥ 1.5), and low miRNA-29a-3p (< 0.4) levels (all 3 criteria met) had a higher probability of overall TACE refractoriness than those who did not.
LT = liver transplantation, TACE = transarterial chemoembolization, miRNA = microRNA.
Predictive factors for overall TACE refractoriness
Univariate and multivariate Cox regression analyses were used to investigate predictive factors for overall TACE refractoriness. In the univariate analysis, advanced age, hepatitis B e antigen positivity, cirrhosis, and a high platelet count were protective factors for overall TACE refractoriness. In patients with HCC, large tumor size (≥ 2 cm), macrovascular invasion, high AFP levels (> 400 ng/mL), high miRNA-21 levels (≥ 2.5), high miRNA-26a levels (≥ 1.5), and low miRNA-29a-3p levels (< 0.4) were found to be risk factors for overall TACE refractoriness (Table 3 and Fig. 1B). However, individual or combined miRNA-21, -26a, and -29a-3p levels did not demonstrate significant predictive power for overall TACE refractoriness. In multivariate analysis, large tumor size (HR, 2.43; 95% CI, 1.27–4.67; P = 0.007), macrovascular invasion (HR, 2.18; 95% CI, 1.28–3.72; P = 0.004), and high AFP levels (HR, 1.88; 95% CI, 1.22–2.90; P = 0.004) were the only independent predictive factors for overall TACE refractoriness.
Table 3. Predictive factors of overall TACE refractoriness in 177 HCC patientsa treated with TACE.
| Factors | Patients with TACE refractoriness (n = 118) | Patients without TACE refractoriness (n = 59) | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Gender (male) | 92 (78.0) | 40 (67.8) | 1.36 (0.88–2.11) | 0.166 | ||
| Age, yr (≥ 55) | 59.1 (10.8) | 59.7 (9.8) | 0.68 (0.47–0.99)b | 0.042b | 0.82 (0.53–1.26) | 0.363 |
| Diabetes mellitus | 18 (15.3) | 14 (23.7) | 0.76 (0.46–1.25) | 0.276 | ||
| HBeAg positivity | 45 (38.5) | 33 (56.9) | 0.67 (0.46–0.98)b | 0.039b | 0.75 (0.48–1.15) | 0.187 |
| Cirrhosis | 110 (93.2) | 58 (98.3) | 0.27 (0.13–0.56)b | 0.000b | ||
| Child-Pugh class (B/C) | 5.5 (1.2) | 5.7 (1.3) | 0.86 (0.49–1.51) | 0.593 | ||
| Platelet, ×109/L (< 100) | 146.9 (78.4) | 110.3 (71.2) | 0.66 (0.44–0.97)b | 0.036b | 0.98 (0.62–1.53) | 0.918 |
| Creatinine, mg/dL | 1.0 (0.6) | 1.1 (1.4) | 0.95 (0.72–1.27) | 0.733 | ||
| Albumin, g/L | 3.7 (0.6) | 3.7 (0.6) | 0.64 (0.67–1.28) | 0.641 | ||
| Bilirubin, mg/dL | 1.1 (0.7) | 1.1 (1.0) | 1.08 (0.85–1.38) | 0.509 | ||
| ALT, U/L | 55.0 (68.0) | 48.1 (37.9) | 1.00 (1.00–1.00) | 0.711 | ||
| INR | 1.1 (0.2) | 1.1 (0.2) | 1.18 (0.41–3.44) | 0.760 | ||
| AFP (> 400 ng/mL) | 4,631.6 (13,200.2) | 103.3 (267.8) | 2.68 (1.83–3.94)b | 0.000b | 1.88 (1.22–2.90)b | 0.004b |
| HBV DNA, log10 IU/mL | 4.3 (2.0) | 3.7 (2.2) | 1.10 (1.01–1.20) | 0.032 | 1.01 (0.91–1.13) | 0.821 |
| Tumor number (≥ 2) | 2.5 (1.6) | 1.5 (0.6) | 1.45 (1.00–2.09) | 0.051 | 1.35 (0.93–1.97) | 0.116 |
| Tumor size (≥ 2 cm) | 5.5 (3.2) | 2.5 (1.4) | 3.59 (1.97–6.54)b | 0.000b | 2.43 (1.27–4.67)b | 0.007b |
| Macrovascular invasion | 27 (22.9) | 1 (1.7) | 3.51 (2.25–5.49)b | 0.000b | 2.18 (1.28–3.72)b | 0.004b |
| miRNA-21 (≥ 2.5) | 6.4 (10.9) | 4.4 (8.3) | 1.61 (1.12–2.32)b | 0.010b | 1.18 (0.78–1.80) | 0.437 |
| miRNA-26a (≥ 1.5) | 5.9 (28.5) | 2.2 (2.3) | 1.52 (1.05–2.19)b | 0.027b | 1.14 (0.40–3.21) | 0.811 |
| miRNA-29a (< 0.4) | 0.34 (0.32) | 0.37 (0.26) | 1.54 (1.06–2.23)b | 0.024b | 1.02 (0.35–2.95) | 0.974 |
| miRNA-panel (all 3 met or not) | 1.75 (1.21–2.55)b | 0.003b | 1.34 (0.90–2.00) | 0.150 | ||
Values are presented as number (%) or HR (95% CI).
TACE = transarterial chemoembolization, HCC = hepatocellular carcinoma, HR = hazard ratio, CI = confidence interval, HBeAg = hepatitis B e antigen, ALT = alanine aminotransferase, INR = international normalized ratio, AFP = alpha-fetoprotein, HBV = hepatitis B virus, miRNA = microRNA.
aTACE refractoriness was assessable in 161 patients; bP < 0.05.
Predictive factors for early TACE refractoriness in patients with HCC
As the evaluation of predictive factors for overall TACE refractoriness showed an association with plasma miRNA in patients with HCC, further evaluation was performed to determine predictive factors for early TACE refractoriness in the same population.22,23 Univariate analyses revealed male sex, high AFP levels (> 400 ng/mL), high HBV DNA levels, multiple tumors (≥ 2), large tumor size (≥ 2 cm), macrovascular invasion, high miRNA-26a levels (≥ 1.5), low miRNA-29a-3p levels (< 0.4), and the combination of miRNAs were found to be risk factors for early TACE refractoriness. Conversely, advanced age (≥ 55 year) and cirrhosis were found to be protective factors for early TACE refractoriness. A multivariate binary logistic regression analysis of these factors further demonstrated that large tumor size (HR, 4.62; 95% CI, 1.50–14.21; P = 0.008), macrovascular invasion (HR, 3.80; 95% CI, 1.19–12.20; P = 0.025), and a distinct miRNA combination panel (miRNA-21 ≥ 2.5, miRNA-26a ≥ 1.5, and miRNA-29a-3p < 0.4, all 3 met vs. 2 or less conditions being satisfied; HR, 2.32; 95% CI, 1.08–4.99; P = 0.031) were independent predictive factors for early TACE refractoriness (Table 4).
Table 4. Predictive factors of early TACE refractoriness in 177 HCC patientsa treated with TACE.
| Factors | Patients with early TACE refractoriness (n = 81) | Patients without early TACE refractoriness (n = 96) | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Gender (male) | 67 (82.7) | 65 (67.7) | 2.28 (1.11–4.68)b | 0.024b | 1.39 (0.57–3.36) | 0.468 |
| Age, yr (≥ 55) | 56.8 (10.9) | 61.4 (9.6) | 0.33 (0.17–0.61)b | 0.001b | 0.34 (0.15–0.75) | 0.008 |
| Diabetes mellitus | 11 (13.6) | 21 (21.9) | 0.56 (0.25–1.25) | 0.561 | ||
| HBeAg positivity | 31 (38.8) | 47 (49.5) | 0.64 (0.35–1.18) | 0.156 | ||
| Cirrhosis | 73 (90.1) | 95 (99.0) | 0.10 (0.01–0.79)b | 0.029b | ||
| Child-Pugh class (B/C) | 5.5 (1.3) | 5.6 (1.2) | 0.70 (0.30–1.65) | 0.420 | ||
| Platelet, ×109/L (< 100) | 158.9 (85.8) | 114.2 (65.1) | 0.41 (0.22–0.78)b | 0.007b | 0.68 (0.31–1.51) | 0.340 |
| Creatinine, mg/dL | 1.0 (0.7) | 1.1 (1.1) | 0.91 (0.64–1.31) | 0.625 | ||
| Albumin, g/L | 3.7 (0.6) | 3.7 (0.6) | 0.90 (0.53–1.52) | 0.698 | ||
| Bilirubin, mg/dL | 1.1 (0.8) | 1.1 (0.8) | 0.95 (0.66–1.38) | 0.791 | ||
| ALT, U/L | 54.0 (44.7) | 51.5 (70.1) | 1.00 (1.00–1.01) | 0.785 | ||
| INR | 1.1 (0.2) | 1.1 (0.2) | 1.17 (0.20–6.80) | 0.863 | ||
| AFP (> 400 ng/mL) | 6,310.2 (15,605.4) | 432.2 (1,440.4) | 4.17 (2.00–8.70)b | 0.000b | 2.25 (0.95–5.30) | 0.063 |
| HBV DNA, log10 IU/mL | 4.5 (2.0) | 3.8 (2.1) | 1.20 (1.04–1.39)b | 0.015b | 1.05 (0.87–1.25) | 0.627 |
| Tumor number (≥ 2) | 2.9 (1.8) | 1.6 (0.8) | 1.93 (1.05–3.52)b | 0.033b | 1.45 (0.67–3.13) | 0.340 |
| Tumor size (≥ 2 cm) | 6.2 (3.2) | 3.0 (2.1) | 6.58 (2.41–17.96)b | 0.000b | 4.62 (1.50–14.21)b | 0.008b |
| Macrovascular invasion | 23 (28.4) | 5 (5.2) | 7.22 (2.60–20.05)b | 0.000b | 3.80 (1.19–12.20)b | 0.025b |
| miRNA-21 (≥ 2.5) | 7.4 (12.4) | 4.4 (7.6) | 1.78 (0.97–3.25) | 0.062 | 1.23 (0.57–2.66) | 0.591 |
| miRNA-26a (≥ 1.5) | 7.2 (34.3) | 2.5 (2.8) | 2.12 (1.16–3.89)b | 0.015b | 3.17 (0.34–29.75) | 0.313 |
| miRNA-29a (< 0.4) | 0.31 (0.31) | 0.38 (0.29) | 2.12 (1.14–3.91)b | 0.017b | 0.53 (0.05–5.29) | 0.590 |
| miRNA-panel (all 3 met or not) | 2.72 (1.41–5.23)b | 0.003b | 2.32 (1.08–4.99)b | 0.031b | ||
Values are presented as number (%) or HR (95% CI).
TACE = transarterial chemoembolization, HCC = hepatocellular carcinoma, HR = hazard ratio, CI = confidence interval, HBeAg = hepatitis B e antigen, ALT = alanine aminotransferase, INR = international normalized ratio, AFP = alpha-fetoprotein, HBV = hepatitis B virus, miRNA = microRNA.
aTACE refractoriness was assessable in 161 patients; bP < 0.05.
Discriminatory ability of miRNA-21, -26a, and -29a-3p levels in predicting LT-free survival and overall TACE refractoriness
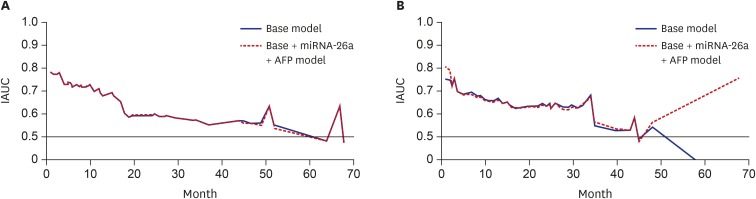
The abilities of plasma miRNA-21, -26a, and -29a-3p levels in predicting LT-free survival and overall TACE refractoriness were assessed using an IAUC plot. The base model included age, sex, tumor number, tumor size, and vascular invasion, alone or in combination. AFP or miRNAs were added and compared to the base model. The discriminatory ability was fair in the base model for LT-free survival (Fig. 2A, Harrell's C-index, 0.728). Adding miRNA-26a and AFP levels to the base model showed a slight improvement (Harrell's C-index, 0.731; 95% CI, −0.02837, −0.0004). With regards to the overall TACE refractoriness, the model that utilized both AFP and miRNA-26a levels showed the best discriminatory ability compared to the base model (Fig. 2B, 0.766 vs. 0.762, respectively; 95% CI, −0.03199, −0.00007).
Fig. 2.
IAUC plots of predictive models, based on a model with or without AFP and miRNAs. (A) IAUC plot of predictive models of LT-free survival. (B) IAUC plot of predictive models of overall TACE refractoriness. The base model consisted of sex, age, tumor number, size, and vascular invasion. Models with AFP and miRNA-26a showed the best discriminatory performance.
IAUC = integrated area under the receiver operating characteristic curve, miRNA = microRNA, AFP = alpha-fetoprotein, LT = liver transplantation, TACE = transarterial chemoembolization.
Correlation between pretreatment miRNA-21, -26a, and -29a-3p levels and patient characteristics
The correlation between miRNA-21, -26a, and -29a-3p levels and baseline characteristics are summarized in Supplementary Table 1. miRNA-21 levels showed a positive correlation with tumor size (r = 0.157; P = 0.027). miRNA-26a levels varied significantly with tumor number, and did not show a linear correlation. Plasma miRNA-29a-3p showed a positive correlation with alanine aminotransferase level (r = 0.152; P = 0.032), depending on the Child-Pugh class, but did not show a linear relationship.
DISCUSSION
To the best of our knowledge, this is the first study to report the association between plasma miRNA-21, -26a, and -29a-3p levels and outcomes following TACE treatment in patients with unresectable HCC. A combination of pretreatment plasma miRNA-21, -26a, and -29a-3p levels predicted early TACE refractoriness in patients with HCC who were subsequently treated with TACE.
Several studies have reported the diagnostic value of miRNA-21, a typical “onco-miRNA,” and its association with a wide variety of cancers, including HCC.14,15 However, few studies have evaluated the prognostic value of miRNA-21 for HCC, especially after TACE. A recent study reported that higher levels of miRNA-21 were associated with shorter post-operative survival in patients with HCC.25 In contrast, other researchers found serum miRNA-21 level was not significantly associated with survival after TACE.26 In the present study, high pretreatment plasma miRNA-21 levels were associated with early TACE refractoriness, as part of a panel with miRNA-26a and -29a-3p, but not with LT-free overall survival.
The miRNA-26 family genetic markers are emerging as key regulators in carcinogenesis and tumor progression. miRNA-26a induces cell cycle arrest at the G1 phase in human HCC cells, in part through direct downregulation of cyclin D2 and cyclin E2.16 Furthermore, therapeutic miRNA-26a delivery using an adeno-associated virus inhibited liver cancer cell formation while also inducing tumor-specific apoptosis and providing considerable protection from disease progression without toxicity.16 In the present study, a high miRNA-26a level (≥ 1.5) was associated with overall TACE refractoriness in univariate analysis and with early TACE refractoriness, as part of a panel, in multivariate analysis. This result contradicts previous reports that patients with HCC and low miRNA-26a expression had shorter overall survival than those with high expression.17 Subsequent studies by the same researchers also demonstrated that miRNA-26a could suppress tumor growth and metastasis of HCC through interleukin-6-signal transduction and activation of transcription 3 signaling, and exerting an anti-angiogenesis function by inhibiting hepatocyte growth factor-cMet.27,28 The reason for this difference is not clear; however, it might be partly due to the different cellular contexts of the tumors. For example, miRNA-26 is consistently downregulated in HCC, nasopharyngeal carcinoma, lung cancer, and breast cancer,17,29,30,31 but is overexpressed in high-grade glioma and cholangiocarcinoma.32,33 This has ramifications for the present study, as HCC was mainly diagnosed based on imaging studies without pathologic confirmation. Therefore, patients with combined HCC-cholangiocarcinoma may have been accidentally included in the study population. This difference could also be explained by the difference in study populations. The previous study only included patients whose tumors had been resected, using a median value ≈ 0.8 as the cut-off level for differentiating high versus low level of miRNA-26a. This cut-off value is much lower than that of the current study (1.5), in which the median value was 1.655.
miRNA-29a may act as either an oncogene or a tumor suppressor gene. For example, miRNA-29a is known to be under-expressed in lung cancers,34 but upregulated in ovarian and colorectal cancers.35,36 There are conflicting reports about the association between miRNA-29a and HCC pathology. High levels of miRNA-29a-5p in HCC tissue are correlated with early tumor recurrence after HCC surgery, especially in patients with BCLC stage 0/A.37 However, the same study found miRNA-29a-3p levels in HCC tissues were not different depending on early recurrence. A recent study proposed that a high concentration of serum miRNA-29a-3p is associated with poor overall survival and progression-free survival in patients with HCC treated with resection or local ablation.38 However, a contrasting study found miRNA-29 genetic (miRNA-29a-3p/b/c) expression was downregulated in HCC tissue suggesting a potential tumor suppressive role.39 The reason for discrepancy in these results is not clear, but a deeper investigation into different miRNA-29 family members would likely explain the varied reports. miRNA-29a-3p is the counterpart of miRNA-29a-5p in the development and progression of cancers. Consequently, in the present study, pretreatment low miRNA-29a-3p levels tended to be associated with both early and overall TACE refractoriness. This is consistent with a recent study that demonstrated downregulation of miRNA-29a-3p in AFP-positive HCCs. AFP inhibited miRNA-29a expression and induced DNA methyltransferase 3A through c-MYC binding to the transcript of miRNA-29a/b-1. Low levels of miRNA-29a-3p in tumor tissues have been associated with poor overall survival in patients with HCC.40 In the present study, however, miRNA-29a-3p levels did not support a linear correlation with AFP levels (r = −0.081; P = 0.259).
In the present study, individual levels of miRNA-21, -26a, and -29a-3p failed to show a statistically significant predictive power for early TACE refractoriness. However, combining miRNA-21, -26a, and -29a-3p together, the panel proved to be a predictive factor for early TACE refractoriness. This result suggests that multi-biomarker panels may have stronger diagnostic and prognostic power in panels.
The present study has a few limitations. First, the international community lacks a consensus definition of TACE refractoriness. The lack of a precise objective definition has led to conflicting conclusions regarding the benefit of repetitive TACE therapy. The present study borrowed the Japanese definition of TACE refractoriness, which seems to be easily applicable in clinical settings.21 Second, tumor stages of patients with HCC vary, and include all 4 BCLC stages (A, B, C, and D). Because this study was not a planned prospective clinical trial but a retrospective cohort study based on real clinical practice, patients with HCC with heterogeneous tumor stages underwent TACE as the first-line therapy for different reasons. In the present study, BCLC stage A was the most frequently observed.
In conclusion, a panel consisting of pretreatment miRNA-21, -26a, and -29a-3p can predict early TACE refractoriness in patients with TACE-treated HCC. Pretreatment circulating miRNA-21, -26a, and -29a-3p levels may also serve as potential candidate biomarkers for the prediction of treatment outcomes in patients with TACE-treated HCC.
ACKNOWLEDGMENTS
The biospecimens and data used for this study were provided by the Biobank of Ajou University Hospital, a member of Korea Biobank Network. All samples derived from the National Biobank of Korea were obtained with informed consent under Institutional Review Board-approved protocols.
Footnotes
Funding: This study was supported by a grant from the Korean Health R & D Project, Ministry of Health and Welfare, Republic of Korea (HI16C2011).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kim SS, Cheong JY. Data curation: Won JH, Kim J, Lee JH, Kim BH. Formal analysis: Kim HJ, Kang DR. Funding acquisition: Cheong JY. Investigation: Cho HJ, Nam JS. Writing - original draft: Kim SS. Writing - review & editing: Kim JK, Cheong JY.
Supplementary Materials
Association of HCC characteristics and miRNA-21, -26a, and -29a
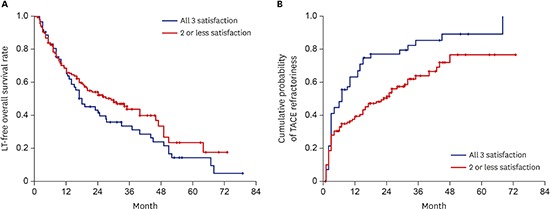
Kaplan-Meier analysis of the cumulative probabilities of LT-free survival and TACE refractoriness. (A) Cumulative probabilities of LT-free survival. (B) Cumulative probabilities of TACE refractoriness.
References
- 1.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15(Suppl 4):42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 4.Hong YM, Yoon KT, Cho M, Kang DH, Kim HW, Choi CW, et al. Trends and patterns of hepatocellular carcinoma treatment in Korea. J Korean Med Sci. 2016;31(3):403–409. doi: 10.3346/jkms.2016.31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo HY, Heo J. New perspectives on the management of hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol. 2015;21(2):115–121. doi: 10.3350/cmh.2015.21.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22(1):7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang XW, Heegaard NH, Orum H. MicroRNAs in liver disease. Gastroenterology. 2012;142(7):1431–1443. doi: 10.1053/j.gastro.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.He S, Zhang DC, Wei C. MicroRNAs as biomarkers for hepatocellular carcinoma diagnosis and prognosis. Clin Res Hepatol Gastroenterol. 2015;39(4):426–434. doi: 10.1016/j.clinre.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Yoon EL, Yeon JE, Ko E, Lee HJ, Je JH, Yoo YJ, et al. An explorative analysis for the role of serum miR-10b-3p levels in predicting response to sorafenib in patients with advanced hepatocellular carcinoma. J Korean Med Sci. 2017;32(2):212–220. doi: 10.3346/jkms.2017.32.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musilova K, Mraz M. MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia. 2015;29(5):1004–1017. doi: 10.1038/leu.2014.351. [DOI] [PubMed] [Google Scholar]
- 14.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Q, Han P, Huang Y, Wu Z, Chen Q, Li S, et al. Potential role of circulating microRNA-21 for hepatocellular carcinoma diagnosis: a meta-analysis. PLoS One. 2015;10(6):e0130677. doi: 10.1371/journal.pone.0130677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361(15):1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL, Ye QH, et al. microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int J Mol Med. 2012;30(6):1321–1326. doi: 10.3892/ijmm.2012.1140. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, Deterding K, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62(5):760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 22.Kim SS, Cho HJ, Won JH, Bae JI, Kang DR, Lee JD, et al. Interleukin-8 level as a prognostic marker in patients with hepatitis B virus-associated hepatocellular carcinoma treated with transarterial chemoembolization. Cytokine. 2015;76(2):449–457. doi: 10.1016/j.cyto.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kim SS, Nam JS, Cho HJ, Won JH, Kim JW, Ji JH, et al. Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(1):199–207. doi: 10.1111/jgh.13448. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhang J, Zhou L, Lu P, Zheng ZG, Sun W, et al. Significance of serum microRNA-21 in diagnosis of hepatocellular carcinoma (HCC): clinical analyses of patients and an HCC rat model. Int J Clin Exp Pathol. 2015;8(2):1466–1478. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H, et al. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS One. 2014;9(10):e109347. doi: 10.1371/journal.pone.0109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, et al. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59(5):1874–1885. doi: 10.1002/hep.26941. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58(1):158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71(1):225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 30.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, et al. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32(1):2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- 32.Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23(11):1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating β-catenin. Gastroenterology. 2012;143(1):246–256.e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127(1):118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 36.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112(1):55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Zhu HT, Dong QZ, Sheng YY, Wei JW, Wang G, Zhou HJ, et al. MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS One. 2012;7(12):e52393. doi: 10.1371/journal.pone.0052393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu HT, Hasan AM, Liu RB, Zhang ZC, Zhang X, Wang J, et al. Serum microRNA profiles as prognostic biomarkers for HBV-positive hepatocellular carcinoma. Oncotarget. 2016;7(29):45637–45648. doi: 10.18632/oncotarget.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51(3):836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- 40.Parpart S, Roessler S, Dong F, Rao V, Takai A, Ji J, et al. Modulation of miR-29 expression by α-fetoprotein is linked to the hepatocellular carcinoma epigenome. Hepatology. 2014;60(3):872–883. doi: 10.1002/hep.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of HCC characteristics and miRNA-21, -26a, and -29a
Kaplan-Meier analysis of the cumulative probabilities of LT-free survival and TACE refractoriness. (A) Cumulative probabilities of LT-free survival. (B) Cumulative probabilities of TACE refractoriness.