Abstract
Background
The purpose of this study was estimation of the cumulative incidence and lifetime prevalence of urolithiasis in Korea.
Methods
We used a National Health Insurance Service (NHIS) sample cohort dataset that included approximately 1 million individuals from Korea. Data from January 2002 to December 2013 were collected. We calculated the annual prevalence, recurrence rate, and estimate lifetime prevalence of urolithiasis. Multivariate logistic regression analysis was used to identify risk factors associated with urolithiasis.
Results
There were 57,921 diagnosed urolithiasis cases in the NHIS database over the 11 years studied. The annual incidence of urolithiasis increased every year (Poisson regression; hazard ratio, 1.025; P < 0.001). Of the patients with urolithiasis, 21.3% experienced disease recurrence within 5 years. The 11-year cumulative incidence was 5.71%, and the incidence in men was higher than that seen in women (7.07% vs. 4.34%, respectively). The 11-year cumulative incidence in the 60- to 69-year-old group (9.08%) was higher than that seen in any other age group. The overall standardized lifetime prevalence rate was estimated to be 11.5%: 12.9% in men and 9.8% in women. Meanwhile, age (> 60 years), income level, diabetes, body mass index, hypertension, and cancer history were identified as contributing factors to urolithiasis.
Conclusion
This study demonstrates that the annual incidence of urolithiasis in Korea is increasing. The overall standardized lifetime prevalence rate was higher than that reported in a previous report. This study is significant in that it is the first retrospective cohort study to estimate the lifetime prevalence of urolithiasis using a large national retrospective cohort.
Keywords: Prevalence of Urolithiasis, Risk Factors, Korea
Graphical Abstract
INTRODUCTION
Urinary tract stone disease is one of the most prevalent diseases encountered in the practice of urology. The lifetime prevalence of urolithiasis is estimated to range from 5%–12% in Europe and the United States, and affects 13% of males and 7% of females in the population.1,2 In Asian countries, there have been limited epidemiological studies regarding urolithiasis. Yasui et al.3 reported that the annual incidence of a first-episode upper urinary tract stone in Japan was 134.0 per 100,000 population, an incidence lower than that reported in western countries. In Korea, Kim et al.4 presented the first systematically assessed epidemiologic survey regarding the incidence of urolithiasis in Korea and found that 6.0% of Korean men and 1.8% of Korean women could be expected to experience urinary stone disease during their lifetime. Previous studies have shown a trend toward an increasing prevalence and incidence of urolithiasis in Korea. However, previous studies have had a limited geographic distribution of the population studied and a dependency on the patients' subjective memory regarding a history of urolithiasis.
To overcome these limitations, we designed a large retrospective national cohort study. The National Health Insurance Service (NHIS) dataset covers the claims of 100% of the Korean population. Accordingly, the NHIS database contains information on almost all insurance claims, including prescribed medications and procedures, of the Korean population of approximately 50 million.5 The NHIS database contains not only individual beneficiary information, but also healthcare service information such as diagnosis, procedures, prescriptions, and diagnostic tests. Because of its tremendous size, researchers have had difficulty in accessing the database to conduct research.6 Since 2013, the Korean NHIS has released a large-scale standardized patient data set called the NHIS sample cohort dataset. The NHIS sample cohort dataset includes medical records from approximately 1,000,000 standardized patients who were selected from the entire Korean population. In this study, we aimed to use the NHIS dataset to calculate the cumulative incidence and lifetime prevalence of urolithiasis and to evaluate risk factors for urolithiasis.
METHODS
Study population selection and design
We used a sample cohort dataset provided by the NHIS.7 These data corresponded to approximately 1 million individuals selected randomly from nearly the entire Korean population, totaling 45 million people, with national claims data for the period from January 1, 2002 to December 31, 2013. The included variables were gender, 10-year age group, socioeconomic status (SES) (subjects divided into 3 categories based on income), diagnosis code, surgery code, drug prescription data (drug name, dosage, and date of prescription), and billing code.
Disease diagnoses held in the database are coded based on the International Codes of Disease 10th Edition Clinical Modification (ICD-10-CM). We searched the NHIS national database to identify patients who had a primary diagnosis of ureteral stone, renal stone, bladder stone, or concomitant ureteral stone with renal stone. Patients were stratified according to gender, age, and region sector.
The sample cohort dataset included age, sex, and diagnosis-based ICD-10 codes. To investigate the incidence of upper tract urolithiasis, patients who were diagnosed as “N20.0” (renal stone), “N20.1, N20.9, N22.0, N22.8, N23, N13.2” (ureter stone), “N20.2, N13.2” (renal stone with ureteral stone), and “N21.0, N21.1, N21.8, N21.9” (lower urinary tract stone) were included. To discount patients on going treatment, we exclude patients who were diagnosed with urolithiasis in 2002 who had received related treatment in this period.
Definition
The definition of annual prevalence was the proportion of the population diagnosed with urolithiasis in one year. It was expressed as a percentage of the population and can be described by the following formula:
We defined lifetime prevalence as the proportion of the population that, up to the time of assessment, had experienced urolithiasis at some point in their life. The recurrence rate was assessed by calculating the subjects' annual recurrence rate after the initial stone event. We defined recurrence by requiring that the interval between the two urolithiasis episodes was ≥ 180 days. If the interval between a subsequent stone event and the initial diagnosis was < 180 days, the subsequent stone event was arbitrarily defined as residual urolithiasis rather than a recurrence.
Lifetime prevalence and recurrence rate calculations
In the NHIS sample cohort dataset, the first occurrence of urolithiasis in a defined period of time is organized into gender and ten-year age groups (0–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80+ years). The incidence rate of urolithiasis in age group k is denoted by Pk, k = 1, 2, ..., and 9 for 0–9, 10–19, …, and 80+ years, respectively. We defined the relationship between the incidence of the first attack and the incidence rate during the study period as follows:
where Pk is the incidence proportion in age group k, Ik is the incidence of a first attack in age group k, S(k, i) is the survival proportion from age group i to age group k, and R(k, i) is the recurrence proportion in age group k after the last occurrence in age group i.
To calculate the incidence of a first attack in an age group, we used the survival proportion of the lifetime table in 2010 from the Korea National Statistics Organization:
| S(k, i) = Nk/Ni |
where Nk is number of persons in age group k in the lifetime table; and we assumed that the urolithiasis recurrence risk was similar at each time. The recurrence rate of urolithiasis at a particular point was calculated as follows:
| R(t) = 1 − exp(−λt) |
where λ is recurrence risk of urolithiasis. The recurrence proportion in an age group k after a last occurrence in age group i, R(k, i) was calculated as:
| R(k, i) = R(10) × [1 − R(10)]k−i−1 |
by assuming that the age groups have the same recurrence rate and recurrences between the different age groups are independent.
We estimated the lifetime risk (lifetime prevalence) of having urolithiasis using the sum of the age-specific incidence of a first attack weighted by the person number of the age group in a cohort of 100,000 individuals at age 0.8 The lifetime risk (lifetime prevalence) was calculated as:
Statistical analysis
Statistical analysis was conducted using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA). Logistic regression analysis was used for analyzing risk factors for urolithiasis. Statistical significance was considered at a 2-tailed P of ≤ 0.05.
Ethics statement
To protect the patients' information and identity, all patients received an anonymous identification code in the NHIS sample data. The authors could not identify any patients included in the sample data. Therefore, our study did not require the approval of the Institutional Review Board, in accordance with the guidelines of the Institutional Review Board of Seoul National University Hospital.
RESULTS
Incidence of urolithiasis
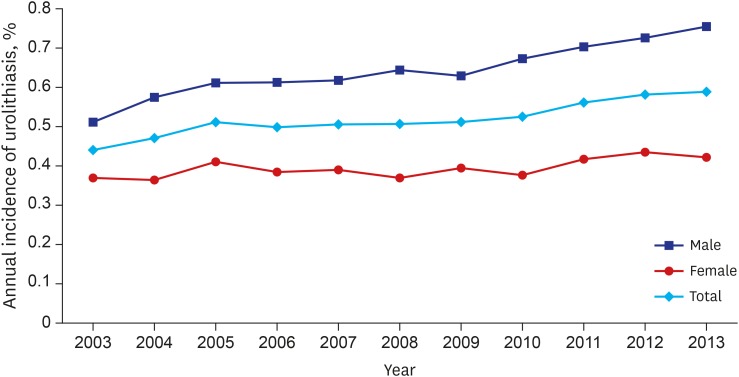
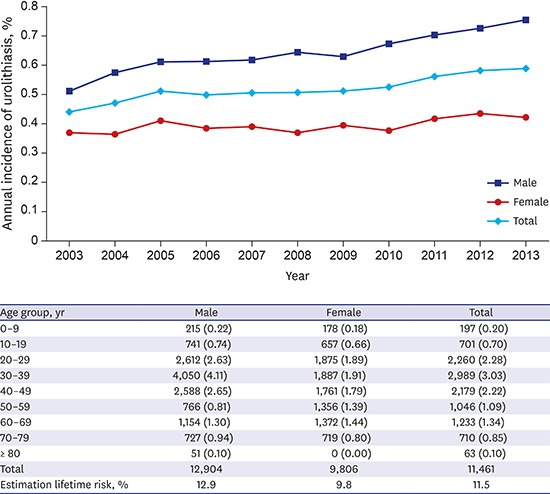
The age and gender distribution of the study subjects and lifetime table from 2010 are shown in Supplementary Tables 1 and 2. In total, there were 57,933 diagnosed urolithiasis cases in the NHIS-National Sample Cohort data for the 11 years studied. The annual incidence of urolithiasis increased every year (Poisson regression; hazard ratio [HR], 1.025; P < 0.001) (Fig. 1). The risk of urolithiasis in men was greater than that seen in women (Poisson regression; HR, 1.627; P < 0.001).
Fig. 1.
Annual incidence of urolithiasis. Annual incidence of urolithiasis is increasing every year (Poisson regression; HR, 1.025; 95% CI, 1.023–1.028; P < 0.001). And risk of urolithiasis in men is higher than women (Poisson regression; HR, 1.627; 95% CI, 1.600–1.654; P < 0.001).
HR = hazard ratio, CI = confidence interval.
The 11-year cumulative incidence was 5.71%, and the incidence was higher in men than that seen in women (7.07% vs. 4.34%, respectively) (Table 1). The 11-year cumulative incidence in the 60- to 69-year-old age group was higher than that seen in any other age group (9.08%). The 11-year cumulative incidence increased as each gender aged and peaked in the 60- to 69-year-old age group. The cumulative incidence decreased after age 70 years.
Table 1. Eleven-year cumulative incidence of urolithiasis in each age and gender group.
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 0–9 | 133 (0.29) | 91 (0.21) | 224 (0.25) |
| 10–19 | 891 (1.36) | 617 (1.05) | 1,508 (1.21) |
| 20–29 | 3,535 (5.14) | 1,987 (3.14) | 5,522 (4.19) |
| 30–39 | 7,903 (9.93) | 3,434 (4.50) | 11,337 (7.63) |
| 40–49 | 9,248 (10.30) | 5,082 (5.84) | 14,330 (8.11) |
| 50–59 | 7,465 (9.16) | 5,271 (6.67) | 12,636 (7.92) |
| 60–69 | 4,557 (10.53) | 3,555 (7.71) | 8,112 (9.08) |
| 70–79 | 1,883 (4.24) | 1,656 (3.04) | 3,539 (3.55) |
| ≥ 80 | 370 (3.36) | 355 (1.60) | 725 (2.12) |
| Total | 35,885 (7.07) | 22,048 (4.34) | 57,933 (5.71) |
Values are presented as number of person (%).
Lifetime urolithiasis prevalence and recurrence rate
We followed 23,809 urolithiasis patients who were initially diagnosed during the 5-year period from 2003 to 2008. Of these patients, 5,068 patients experienced a recurrence, and the 5-year recurrence rate was 21.3%. Assuming that the risk of recurrence is the same at each point, the recurrence risk (λ) was calculated according to the formula:
| R(5) = 0.213 = 1 − exp(−5λ), λ = 0.0479 |
| R(10) = 1 − exp(−0.0479*10) = 38.1% |
The recurrence rate within 10 years was estimated to be 38.1%.
The estimated 10-year cumulative incidence, according to the above formula, of a first-episode of urolithiasis is shown in Table 2. Calibrated after adjustment for life expectancy, the overall standardized lifetime prevalence rate can be estimated to be 11.5%: 12.9% in men and 9.8% in women (Table 2).
Table 2. Estimation of 10-year cumulative incidence of a first-episode urolithiasis in each age group and lifetime prevalence adjust 2010 lifetime table of Korea.
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 0–9 | 215 (0.22) | 178 (0.18) | 197 (0.20) |
| 10–19 | 741 (0.74) | 657 (0.66) | 701 (0.70) |
| 20–29 | 2,612 (2.63) | 1,875 (1.89) | 2,260 (2.28) |
| 30–39 | 4,050 (4.11) | 1,887 (1.91) | 2,989 (3.03) |
| 40–49 | 2,588 (2.65) | 1,761 (1.79) | 2,179 (2.22) |
| 50–59 | 766 (0.81) | 1,356 (1.39) | 1,046 (1.09) |
| 60–69 | 1,154 (1.30) | 1,372 (1.44) | 1,233 (1.34) |
| 70–79 | 727 (0.94) | 719 (0.80) | 710 (0.85) |
| ≥ 80 | 51 (0.10) | 0 (0.00) | 63 (0.10) |
| Total | 12,904 | 9,806 | 11,461 |
| Estimation lifetime risk, % | 12.9 | 9.8 | 11.5 |
Values are presented as number of person (10-year cumulative incidence, %).
Risk factors for urolithiasis
Logistic regression was used to assess risk factors for urolithiasis (Table 3). Female gender was related with a significantly low risk for urolithiasis (odds ratio [OR], 0.693; 95% confidence interval [CI], 0.650–0.739, P < 0.001). Age > 60 years and body mass index (BMI) were found to be risk factors for urolithiasis. A history of hypertension, cancer, and diabetes were associated with a risk for urolithiasis, but cerebrovascular diseases were not. Interestingly, frequent alcohol consumption (> 3 times weekly) was associated with a lower risk of urolithiasis (OR, 0.824; 95% CI, 0.747–0.909; P = 0.001). In addition, a high SES level was identified as a contributing factor to urolithiasis.
Table 3. Multivariate logistic regression analysis of risk factors of urolithiasis.
| Characteristics | OR (95% CI) | P value | |
|---|---|---|---|
| Gender | |||
| Male | Ref. | ||
| Female | 0.693 (0.650–0.739) | < 0.001 | |
| Age, yr | |||
| < 60 | Ref. | ||
| ≥ 60 | 1.292 (1.208–1.382) | < 0.001 | |
| BMI | |||
| ≤ 25 | Ref. | ||
| > 25 | 1.361 (1.275–1.453) | < 0.001 | |
| Alcohol consumption | |||
| Less than twice a week | Ref. | ||
| More than twice a week | 0.824 (0.747–0.909) | 0.001 | |
| Medical history | |||
| Hypertension | 1.339 (1.212–1.480) | < 0.001 | |
| Diabetes | 1.242 (1.070–1.441) | 0.004 | |
| Cerebrovascular disease history | 0.936 (0.660–1.327) | 0.711 | |
| Cancer history | 1.288 (1.056–1.570) | 0.012 | |
| SES | |||
| Low income | Ref. | ||
| Middle income | 1.154 (1.065–1.251) | < 0.001 | |
| High income | 1.229 (1.121–1.347) | < 0.001 | |
OR = odd ratio, CI = confidence interval, BMI = body mass index, SES = socioeconomic status.
DISCUSSION
Urolithiasis is a common and significant health problem that appears to be increasing in frequency in recent decades in both men and women. Previous studies have shown that the prevalence of urolithiasis varies greatly with patient age, gender, race, and geographic region.9,10,11,12 And the annual incidence of urolithiasis has been reported to range from 0.5% to 1.5% in western countries.13,14 The prevalence of urolithiasis has been reported to be 4.7% to 8.8% in western countries. Many other studies have demonstrated a yearly increase in the prevalence of urolithiasis.9,15 However, previous studies have been limited by their local areas and a dependency on the patients' subjective memory regarding the urolithiasis episode. To overcome these limitations, Bae et al.16 recently conducted a study with a larger national sample size and estimated that the annual incidence of upper tract urolithiasis was 457 cases per 100,000 population. However, that study did not evaluate patient follow-up or recurrence.
Our study is the first retrospective cohort study to estimate the lifetime prevalence of urolithiasis using a large national sample. Our sample size was over 1,000,000, and patient data was collected in a standardized manner. The sample size was sufficient to represent the national incidence of urolithiasis.7 Moreover, the diagnosis of urolithiasis was made by a clinician and was not based on patient recall. Typically, X-ray, intravenous pyelogram (IVP), and non-enhanced computed tomography (CT) imaging studies are covered by insurance, and all physicians must provide evidence documenting the presence of a stone, such as imaging study results, to the NHIS.
It was difficult to estimate the lifetime prevalence of urolithiasis because we could not determine the number of subjects who had an episode of urolithiasis prior to the study period. However, we were able to calculate the recurrence rate from this retrospective cohort data. We were able to estimate the risk of recurrence based on the assumption that the recurrence risk is similar at each time point. In our study, we were able to calculate the overall 5-year recurrence rate from the NHIS sample cohort dataset. The overall 10-year recurrence rate was estimated to be 38.1%, lower than that of a previous study from Taiwan.17 While few studies have provided reliable information on the recurrence rate, a previous case series indicated that stones form in 30% to 40% of untreated patients within 5 years after the initial episode.18
Our study revealed that the lifetime prevalence was higher than that reported in previous studies. Kim et al.4 conducted a retrospective study in 2002 and found that 6.0% of Korean men and 1.8% of Korean women could expect to experience urinary stone disease during their lifetimes. In 1990, Yoshida and Okada19 reported that 5.4% of the Japanese population could expected to develop at least one urinary calculus in their lifetime.
There are several possible explanations for the increased frequency of urolithiasis in Korea. First, the increasing use and sensitivity of radiological studies may partly explain the increased prevalence of urolithiasis.15,20 Unenhanced CT scanning is increasingly used for the evaluation of urolithiasis and is preferred because of its speed, high sensitivity, and accuracy for detecting renal and ureteral stones, as well as its ability to diagnose alternative pathologies.21 Conventional CT scanning has been reported to have a sensitivity and specificity of up to 98%–100% for ureteral stones. The increased use of non-enhanced CT scanning for the diagnosis of urinary stones may be contributing to an increase in the detection of urinary stones.
Second, differences in the lifestyle of the Korean population may be contributing to the increased prevalence of urolithiasis. Several previous studies have shown that metabolic syndrome, lifestyle, and dietary factors are associated with urolithiasis.22,23,24 Semins et al.25 evaluated data from a national private insurance database from 2002–2006 to identify over 95,000 subjects diagnosed with kidney stones. Of those patients, over 33,000 had a BMI > 30. Moreover, Taylor et al.26 reported a relative risk of urolithiasis of 1.67 in young diabetic women, 1.38 in older diabetic women, and 1.31 in diabetic men. Kim et al.24 also demonstrated that high blood pressure was significantly related to the presence of nephrolithiasis in Korea. Another previous study found that the prevalence rate of metabolic syndrome increased from 24.9% in 1998 to 31.3% in 2007 in Korea. The authors suggested that this increase was likely due to an increase in Western dietary habits and a decreased level of physical activity.27,28 In our study, the association of these factors (diabetes, BMI, and hypertension) was confirmed, and an increase in the prevalence of these diseases may be contributing to an increase in the incidence of urolithiasis in Korea.
Interestingly, habitual alcohol consumption was associated with a decreased risk of urolithiasis in this study. This may be because alcohol intake dilutes stone-forming metabolites in the blood and urine, and the diuretic effect of alcohol increases the frequency of voiding, thus decreasing the risk of urolithiasis. A similar decreased risk of urolithiasis with alcohol intake has been reported in a previous meta-analysis.29 However, the consumption of alcohol is also related to severe oxidative stress in renal tissues, hypercalciuria, and hyperoxaluria, all of which could contribute to urinary stone formation.30,31 Therefore, the advisability of alcohol consumption for current urinary stone patients or as a prophylaxis for urolithiasis remains unclear.
Saint-Elie et al.32 suggested that SES impacts dietary habits and that this may strongly influence stone formation and recurrence. The authors found that higher dietary intakes, with the exception of total fat, were observed in those with a lower SES. Urolithiasis patients with a higher SES consumed less dietary constituents than did their lower SES counterparts. However, our study results are contrary to those of this previous study. We suspect that our findings may be related to a higher consumption of animal protein in Koreans with a higher SES. Kim et al.24 studied the relationship between dietary habits and urolithiasis in Korea and reported that the increased incidence of urinary stones can be attributed to a rapid change in the nutritional environment in Korea. The incidence of urolithiasis has consistently increased as the intake of animal protein and milk has increased. Another possible explanation is that higher SES individuals have easier access to accurate and high-quality health care services, but further study of this possibility is needed.
In this study, advanced age (age > 60 years) was revealed to be a risk factor for urolithiasis in multivariate analysis. Similar to our results, Bae et al.16 recently reported that the incidence of urolithiasis was the highest in the 60–69 years old age group in Korea. This is thought to be due to the increased morbidity of accompanying diseases with increasing age; these comorbidities are thought to increase the incidence of urinary stones.
Our study had several limitations. Patients with asymptomatic urolithiasis who did not visit the hospital or clinic were not included in this study. Also, asymptomatic patients with urolithiasis who were followed up at 6-month or 1-year intervals were potentially defined as having recurrence in this study. To reduce this possibility, we excluded all patients diagnosed with urolithiasis in 2002 in our retrospective cohort. Nevertheless, this still is a possible limitation of our study.
In addition, there were differences between the diagnostic methods of each physician, and therefore urolithiasis may have been underestimated by physicians who were not as familiar with urolithiasis. Also, this study did not include the characteristics of urolithiasis such as size, history of previous treatment, stone composition, and patient treatment outcomes. In addition, we could not determine if the first diagnosis of urolithiasis in this cohort study was the first episode in the subject's life. Therefore, we calculated the recurrence rate based on the number of first diagnosis patients in each cohort within each age group. Based on this method, we were able to estimate the incidence of a first attack of urolithiasis. However, the incidence of a first attack was nearly 0% in women ≥ 80 years old because of the relatively low incidence of urolithiasis and the smaller number of subjects. To overcome this limitation, a study with a larger number of women ≥ 80 years old is needed. However, the authors believe that this limitation had little impact on the remainder of our results.
These limitations notwithstanding, our findings have important implications for the treatment of patients with urolithiasis in Korea. This is the first study to evaluate the lifetime prevalence of urolithiasis using national sample size. Our study confirmed the trend toward an increased incidence and prevalence of urolithiasis and supports the recent increases in health care expenditures for the treatment of patients with urolithiasis. The rapidly changing prevalence of urolithiasis suggests that further preventive efforts and additional studies of the etiology, frequency, and treatment of urolithiasis in Korea are indicated.
In this study, we estimated the lifetime prevalence of urolithiasis based on the NHIS dataset. The lifetime prevalence of urolithiasis in Koreans was approximately 11.5%. The annual incidence of urolithiasis is increasing, possibly due to increased comorbidities along with lifestyle changes.
ACKNOWLEDGMENTS
This study used National Health Insurance Service-National Sample Cohort (NHIS-NSC) data (NHIS-2014-2-030) collected by the National Health Insurance Service (NHIS). We thank Prof. Myung Jin Jang, Medical Research Collaboration Center, Seoul National University Hospital, for helping us with statistical data analysis.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Tae BS, Jeong CW. Data curation: Tae BS, Jeong CW. Formal analysis: Tae BS, Cho SY. Investigation: Tae BS, Balpukov U, Cho SY, Jeong CW. Writing - original draft: Tae BS. Writing - review & editing: Balpukov U, Cho SY, Jeong CW.
Supplementary Materials
Distribution of age and gender of study sample
Lifetime table of Korea in 2010
References
- 1.Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368(9542):1171–1179. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- 2.Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck C, Gallucci M, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418–2434. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 3.Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology. 2008;71(2):209–213. doi: 10.1016/j.urology.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Jo MK, Kwak C, Park SK, Yoo KY, Kang D, et al. Prevalence and epidemiologic characteristics of urolithiasis in Seoul, Korea. Urology. 2002;59(4):517–521. doi: 10.1016/s0090-4295(01)01606-5. [DOI] [PubMed] [Google Scholar]
- 5.Choi NK, Chang Y, Choi YK, Hahn S, Park BJ. Signal detection of rosuvastatin compared to other statins: data-mining study using national health insurance claims database. Pharmacoepidemiol Drug Saf. 2010;19(3):238–246. doi: 10.1002/pds.1902. [DOI] [PubMed] [Google Scholar]
- 6.Kim L, Sakong J, Kim Y, Kim S, Kim S, Tchoe B, et al. Developing the inpatient sample for the National Health Insurance claims data. Health Policy Manag. 2013;23(2):152–161. [Google Scholar]
- 7.National Sample Cohort (NHIS-NSC) Database. [Updated 2015]. [Accessed April 6, 2015]. http://nhiss.nhis.or.kr/bd/ab/bdaba022eng.do.
- 8.Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health. 1994;48(6):596–600. doi: 10.1136/jech.48.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 10.Curhan GC, Rimm EB, Willett WC, Stampfer MJ. Regional variation in nephrolithiasis incidence and prevalence among United States men. J Urol. 1994;151(4):838–841. doi: 10.1016/s0022-5347(17)35101-7. [DOI] [PubMed] [Google Scholar]
- 11.Safarinejad MR. Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urol Res. 2007;35(2):73–82. doi: 10.1007/s00240-007-0084-6. [DOI] [PubMed] [Google Scholar]
- 12.Penniston KL, McLaren ID, Greenlee RT, Nakada SY. Urolithiasis in a rural Wisconsin population from 1992 to 2008: narrowing of the male-to-female ratio. J Urol. 2011;185(5):1731–1736. doi: 10.1016/j.juro.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse A, Brณndle E, Wilbert D, Köhrmann KU, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44(6):709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 14.Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason H, Sigfusson N, Palsson R. Epidemiology of kidney stones in Iceland: a population-based study. Scand J Urol Nephrol. 2006;40(3):215–220. doi: 10.1080/00365590600589898. [DOI] [PubMed] [Google Scholar]
- 15.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae SR, Seong JM, Kim LY, Paick SH, Kim HG, Lho YS, et al. The epidemiology of reno-ureteral stone disease in Koreans: a nationwide population-based study. Urolithiasis. 2014;42(2):109–114. doi: 10.1007/s00240-014-0643-6. [DOI] [PubMed] [Google Scholar]
- 17.Huang WY, Chen YF, Carter S, Chang HC, Lan CF, Huang KH. Epidemiology of upper urinary tract stone disease in a Taiwanese population: a nationwide, population based study. J Urol. 2013;189(6):2158–2163. doi: 10.1016/j.juro.2012.12.105. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CM, Wilson DM, O'Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int. 1979;16(5):624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida O, Okada Y. Epidemiology of urolithiasis in Japan: a chronological and geographical study. Urol Int. 1990;45(2):104–111. doi: 10.1159/000281680. [DOI] [PubMed] [Google Scholar]
- 20.Boyce CJ, Pickhardt PJ, Lawrence EM, Kim DH, Bruce RJ. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017–1021. doi: 10.1016/j.juro.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Katz DS, Scheer M, Lumerman JH, Mellinger BC, Stillman CA, Lane MJ. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: experience with 1000 consecutive examinations. Urology. 2000;56(1):53–57. doi: 10.1016/s0090-4295(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 22.Wong Y, Cook P, Roderick P, Somani BK. Metabolic syndrome and kidney stone disease: a systematic review of literature. J Endourol. 2016;30(3):246–253. doi: 10.1089/end.2015.0567. [DOI] [PubMed] [Google Scholar]
- 23.Besiroglu H, Otunctemur A, Ozbek E. The metabolic syndrome and urolithiasis: a systematic review and meta-analysis. Ren Fail. 2015;37(1):1–6. doi: 10.3109/0886022X.2014.976133. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Kim CH, Sung EJ, Kim SR, Shin HC, Jung WJ. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism. 2013;62(6):808–813. doi: 10.1016/j.metabol.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183(2):571–575. doi: 10.1016/j.juro.2009.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68(3):1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011;34(6):1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JH, Kam S, Shin JY, Kim JY, Lee KE, Kwon GH, et al. Incidence of metabolic syndrome and relative importance of five components as a predictor of metabolic syndrome: 5-year follow-up study in Korea. J Korean Med Sci. 2013;28(12):1768–1773. doi: 10.3346/jkms.2013.28.12.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Xu X, Wu J, Zhu Y, Lin Y, Zheng X, et al. Systematic review and meta-analysis of the effect of alcohol intake on the risk of urolithiasis including dose-response relationship. Urol Int. 2015;94(2):194–204. doi: 10.1159/000365358. [DOI] [PubMed] [Google Scholar]
- 30.Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92(1):137–140. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 31.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157(3):1059–1063. [PubMed] [Google Scholar]
- 32.Saint-Elie DT, Patel PV, Healy KA, Solomon T, Pattaras JG, Qian J, et al. The impact of income and education on dietary habits in stone formers. Urology. 2010;76(2):307–313. doi: 10.1016/j.urology.2009.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of age and gender of study sample
Lifetime table of Korea in 2010