Abstract
Background
Lead (Pb), mercury (Hg), and cadmium (Cd) are well-known environmental pollutants. They are unnecessary in the biological processes of humans. This study was performed to estimate the representative background exposure levels to the metals by measuring concentrations in whole blood of the Korean general population.
Methods
This population-based cross-sectional study included 4,000 subjects (1,886 males and 2,114 females) 0–83 years of age in 2010 and 2011. Adult subjects (≥ 19 years of age) were collected by sex- and age-stratified probability method, and preschool- and school-aged subjects were recruited by a cluster sampling method. Written consent was provided prior to blood sampling. Pb and Cd blood concentrations were determined by a flameless atomic absorption spectrophotometry, and blood Hg was analyzed by a direct Hg analyzer.
Results
The geometric mean, median and 95th percentile of blood Pb was 1.82 µg/dL, 1.83 µg/dL, and 3.78 µg/dL, respectively. The respective values were 2.92 µg/L, 2.87 µg/L, 9.12 µg/L for Hg, and 0.56 µg/L, 0.59 µg/L, 2.20 µg/L for Cd. Blood Pb and Hg were higher in males than in females, but no sex difference was observed, respectively, in subjects 0–4 years of age for Pb and in subjects less than 20 years for Hg. However, blood Cd was higher in females than in males and no sex difference was observed in subjects < 30 years of age.
Conclusion
This study provides representative data of human exposure to Pb, Hg, and Cd covering whole age groups of the general population in Korea.
Keywords: Lead, Mercury, Cadmium, Whole Blood, Korean General Population
Graphical Abstract
INTRODUCTION
Lead (Pb), mercury (Hg), and cadmium (Cd) are toxic to humans and are not necessary in biological processes in the body. However, these metals are widely distributed in the environment as major environmental pollutants and are listed as top toxic substances.1 Toxic metals are emitted from natural and various iatrogenic sources. Human exposure is inevitable, especially since the metals are non-degradable, are environmentally persistent and can accumulate in ecosystems. The adverse health effects are well-known through several episodes including itai-itai disease, Minamata disease, and childhood Pb poisoning, as well as in industrial exposure.2,3,4
Determining the background exposure is necessary for the effective control of environmental pollutants including metals, with polluted regions and small population areas as the controls. Data concerning exposure level and distribution of pollutants exist in a few developed countries according to sex and age. The data include the National Health and Nutrition Examination Survey (NHANES) in the United States, German Environmental Survey (GerES) and Canadian Health Measures Survey (CHMS). Recently, Korea undertook to prepare representative exposure data for heavy metals as part of the Korean National Human Exposure and Bio-monitoring Examination (KNHEBE), Korea National Health and Nutrition Examination Survey (KNHANES), and Korean National Environmental Health Survey (KoNEHS). However, the data were limited to adults.5,6,7
Children are more susceptible and vulnerable to toxic metals compared to adults concerning the nervous system, development and behavioral performance.8,9 Recommended guidelines for metals to prevent the health effects for children and susceptible population have been periodically revised. The established provisional tolerable weekly intakes (PTWIs) have been withdrawn for Pb and strengthened for Hg. Provisional tolerable monthly intake (PTMI) has been established for Cd. The changes were made as the previous standards were not protective for human health.10 Previously, the United States Centers for Disease Control and Prevention (CDC) recommended a blood Pb level of < 10 µg/dL to prevent Pb poisoning for children. The level was changed to < 5 µg/dL.11 The Human Biomonitoring Commission in Germany also suspended the human biomonitoring (HBM) values for blood Pb.12
Similar efforts have been hampered in Korea because of the limited human data concerning toxic metals for children. In addition, human exposure to the metals could be affected by individual lifestyles including diet. Therefore, the exposure level of metals in the body may involve demographic factors like sex and age, which influence individual lifestyle. Blood concentrations of metals including Pb, Hg, and Cd would better represent current human internal exposure than the urinary levels of those metals.
In this study, we present comprehensive background internal exposure level data to environmental pollutants, such as Pb, Hg, and Cd, by measuring blood concentrations from a representative Korean general population covering whole age groups. We also explored sex- or age-dependent differences in human exposure to each metal.
METHODS
Study design and subjects
This population-based cross-sectional study was designed to obtain a representative sample of the Korean population. The study subjects included 4,000 persons (1,886 males and 2,114 females) aged 0 to 83 years old over the period 2010–2011 (Table 1). The study design and sampling method have been detailed previously.13,14 Briefly, the target sampling area was 15 metropolitans and provinces such as Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam of Korea except Jeju. Adult subjects older than 19 years of age were sampled by a stratified probability method. Namely, we first conducted a stratified probability sampling by region, 15 metropolitans and provinces, sex, and age. The number of study subjects in each stratification was allocated by the square root proportional method. Next, we extracted 34 cities and counties (si, gun, and gu) from 15 metropolitans and provinces, and followed sampling of 102 towns and townships (eup, myeon, and dong) from 34 cities and towns. Then we randomly sampled study subjects from the 102 different sampling sites distributed nationwide. Preschool- and school-aged subjects were collected with cluster sampling. Sampling sites for preschool- and school-aged subjects were allocated by region of 15 metropolitans and provinces from the 102 sampling sites. Preschool-aged subjects were recruited from health centers, pediatric clinics, nurseries and kindergartens; school-aged subjects were from elementary, middle, and high schools. Written consent was obtained from every participant or their parents after being informed about the study aim, process and details. Blood sampling was performed by experienced nurses under the observation of a doctor. Whole blood was sampled with a metal-free blood collection tube containing K2 EDTA for trace metals analysis (Becton Dickinson Vacutainer™, Plymouth, UK) and stored at −80°C until analysis.
Table 1. Distribution of study subjects by sex and age.
| Age, yr | Male | Female | Total |
|---|---|---|---|
| 0–4 | 232 (12.3) | 225 (10.6) | 457 (11.4) |
| 5–9 | 326 (17.3) | 313 (14.8) | 639 (16.0) |
| 10–19 | 443 (23.5) | 416 (19.7) | 859 (21.5) |
| 20–29 | 146 (7.7) | 154 (7.3) | 300 (7.5) |
| 30–39 | 147 (7.8) | 227 (10.7) | 374 (9.4) |
| 40–49 | 200 (10.6) | 269 (12.7) | 469 (11.7) |
| 50–59 | 194 (10.3) | 291 (13.8) | 485 (12.1) |
| Above 60 | 198 (10.5) | 219 (10.4) | 417 (10.4) |
| Mean ± SD | 26.9 ± 21.6 | 29.8 ± 21.6 | 28.4 ± 21.6 |
| Total | 1,886 (100.0) | 2,114 (100.0) | 4,000 (100.0) |
Values are presented as number (%).
SD = standard deviation.
Pb, Hg, and Cd analyses in whole blood
Concentrations of Pb and Cd in whole blood were determined using a flameless atomic absorption spectrophotometer equipped with a model GF-AAS Zeeman graphite furnace (Thermo Inc., Cambridge, UK). Briefly, whole blood was diluted to 0.2% in Triton X-100 with 1% nitric acid and 0.2% diammonium hydrogen phosphate. Fifteen microliter aliquots were injected into a graphite boat after mixing vigorously. Determination of total Hg in whole blood was performed by using a model DMA-80 direct Hg analyzer (Milestone, Sorisole, Italy) with a gold-amalgam method. Whole blood (100 mg) was weighed and put into a nickel boat. Pb, Hg, and Cd analyses were validated using standards and a standard reference material (SRM, 955c level 2; National Institute of Standards and Technology, Gaithersburg, MD, USA). The respective limit of detection of Pb, Hg, and Cd was 0.20 µg/dL, 0.20 µg/L, and 0.10 µg/L, respectively. Fourteen samples for Pb, 104 samples for Hg, and 7 samples for Cd were measured below the detection limits. The levels of metals below the detection limits were assigned to values of detection limits divided by a square root of two.
Statistical analysis
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). The concentrations of Pb, Hg, and Cd in whole blood were log-transformed for the statistical analyses, because blood concentrations of those metals were more closely distributed to the log-normally than the normal distribution. The central tendency of metal concentration in whole blood is presented as arithmetic mean, geometric mean and median. The proportion of study subjects according to sex was compared by a χ2 test. The comparison of means between males and females was performed by analysis of two-tailed Student's t-test. Relationships among the metals were evaluated by Spearman rank correlation analysis. The level of statistical significance was set at P < 0.05.
Ethics statement
The present study protocol was reviewed and approved by the Chung-Ang University Ethical Committee for Medical Research and Other Studies Involving Human Subjects (2010-06-01). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Human exposure levels to Pb, Hg, and Cd in the Korean general population are evaluated by measuring the concentrations of Pb, total Hg, and Cd in whole blood.
Blood Pb level in the Korean general population
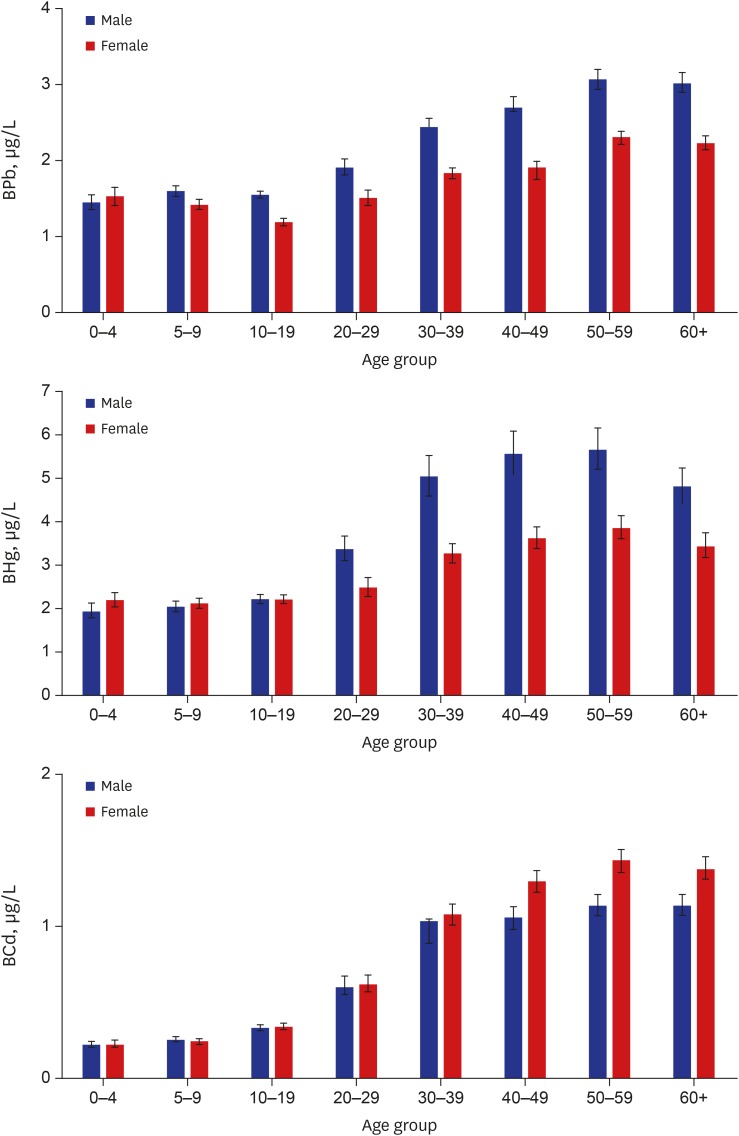
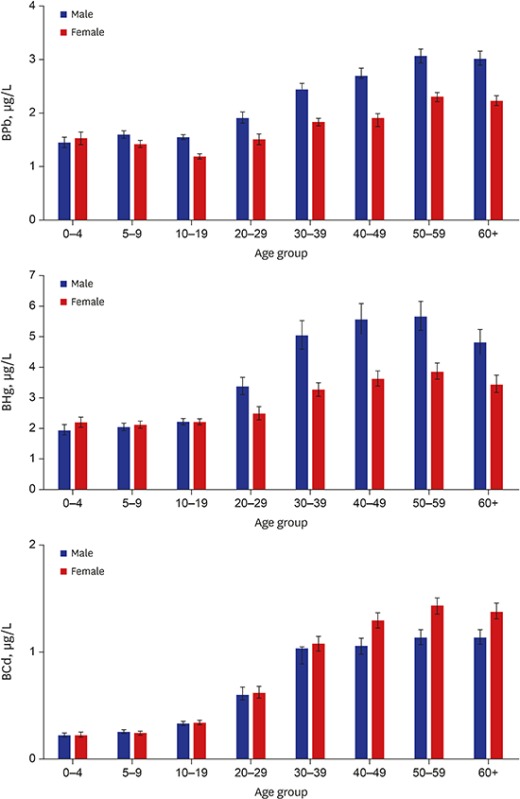
The geometric mean concentration of Pb in whole blood of total study subjects was 1.82 µg/dL, which was significantly higher in males (2.00 µg/dL) than in females (1.67 µg/dL). The arithmetic mean concentration of blood Pb was 2.01 µg/dL (2.22 µg/dL in males and 1.83 µg/dL in females), and median was 1.83 µg/dL (1.98 µg/dL in males and 1.70 µg/dL in females). The 95th percentile of blood Pb in all study subjects was 3.78 µg/dL, and was 4.24 µg/dL and 3.25 µg/dL in males and in females, respectively (Table 2). Blood Pb showed an increasing tendency with age, however, concentrations of blood Pb in those < 10 years of age were similar or a little bit higher compared to those 10–19 years of age. No significant difference of blood Pb levels between males and females was observed in the age group of 0–4 years (Table 3, Fig. 1).
Table 2. Mean concentrations of Pb, Hg, and Cd in whole blood of study subjects.
| Metals | No. | AM ± SD | GM (95% CI) | Min | Max | Selected percentiles (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | 95th | |||||||
| Pb, µg/dL | Male | 1,824 | 2.22 ± 1.04a | 2.00 (1.96–2.05)a | 0.14 | 8.72 | 1.49 (1.45–1.52) | 1.98 (1.92–2.02) | 2.72 (2.65–2.81) | 3.64 (3.51–3.80) | 4.24 (4.04–4.44) |
| Female | 2,046 | 1.83 ± 0.79 | 1.67 (1.63–1.70) | 0.14 | 10.64 | 1.29 (1.26–1.32) | 1.70 (1.66–1.74) | 2.22 (2.18–2.29) | 2.84 (2.77–2.93) | 3.25 (3.13–3.38) | |
| Total | 3,870 | 2.01 ± 0.94 | 1.82 (1.79–1.85) | 0.14 | 10.64 | 1.38 (1.36–1.40) | 1.83 (1.80–1.86) | 2.46 (2.41–2.52) | 3.22 (3.14–3.30) | 3.78 (3.68–3.89) | |
| Hg, µg/L | Male | 1,874 | 4.09 ± 3.65a | 3.11 (3.01–3.21)a | 0.14 | 41.95 | 1.96 (1.87–2.05) | 3.02 (2.92–3.16) | 4.92 (4.68–5.17) | 7.91 (7.31–8.46) | 10.59 (9.63–11.74) |
| Female | 2,104 | 3.36 ± 2.49 | 2.77 (2.70–2.84) | 0.14 | 25.54 | 1.83 (1.78–1.91) | 2.75 (2.68–2.84) | 3.99 (3.84–4.14) | 5.96 (5.67–6.43) | 7.99 (7.43–8.72) | |
| Total | 3,978 | 3.70 ± 3.12 | 2.92 (2.86–2.98) | 0.14 | 41.95 | 1.89 (1.83–1.95) | 2.87 (2.80–2.93) | 4.38 (4.27–4.49) | 6.91 (6.55–7.15) | 9.12 (8.79–9.54) | |
| Cd, µg/L | Male | 1,844 | 0.72 ± 0.62 | 0.50 (0.48–0.52) | 0.07 | 4.79 | 0.26 (0.25–0.27) | 0.51 (0.47–0.54) | 0.99 (0.95–1.05) | 1.56 (1.47–1.65) | 1.94 (1.82–2.08) |
| Female | 2,056 | 0.91 ± 0.76a | 0.62 (0.60–0.65)a | 0.07 | 5.72 | 0.30 (0.29–0.32) | 0.72 (0.67–0.77) | 1.32 (1.27–1.36) | 1.86 (1.80–1.99) | 2.34 (2.24–2.48) | |
| Total | 3,900 | 0.82 ± 0.70 | 0.56 (0.55–0.58) | 0.07 | 5.72 | 0.28 (0.27–0.29) | 0.59 (0.56–0.63) | 1.17 (1.13–1.21) | 1.74 (1.69–1.79) | 2.20 (2.10–2.26) | |
Pb = lead, Hg = mercury, Cd = cadmium, AM = arithmetic mean, SD = standard deviation, GM = geometric mean, CI = confidence interval.
aP < 0.01, statistically highly significant between males and females.
Table 3. Mean concentrations of Pb, Hg, and Cd in whole blood by age groups in study subjects.
| Metals | Age, yr | Male | Female | Total | |||
|---|---|---|---|---|---|---|---|
| No. | GM (95% CI) | No. | GM (95% CI) | No. | GM (95% CI) | ||
| Pb, µg/dL | 0–4 | 193 | 1.45 (1.36–1.55) | 179 | 1.53 (1.41–1.65) | 372 | 1.49 (1.42–1.57) |
| 5–9 | 312 | 1.60 (1.53–1.67)a | 301 | 1.42 (1.36–1.49) | 613 | 1.51 (1.46–1.56) | |
| 10–19 | 438 | 1.55 (1.51–1.60)a | 407 | 1.19 (1.14–1.24) | 845 | 1.36 (1.33–1.40) | |
| 20–29 | 145 | 1.91 (1.81–2.02)a | 154 | 1.51 (1.41–1.61) | 299 | 1.69 (1.62–1.77) | |
| 30–39 | 147 | 2.44 (2.32–2.56)a | 227 | 1.84 (1.76–1.91) | 374 | 2.05 (1.98–2.13) | |
| 40–49 | 198 | 2.70 (2.57–2.84)a | 269 | 1.91 (1.83–1.99) | 467 | 2.21 (2.14–2.29) | |
| 50–59 | 193 | 3.07 (2.93–3.21)a | 291 | 2.31 (2.22–2.39) | 484 | 2.58 (2.50–2.67) | |
| Above 60 | 198 | 3.02 (2.89–3.16)a | 218 | 2.23 (2.14–2.33) | 416 | 2.58 (2.49–2.67) | |
| Hg, µg/L | 0–4 | 224 | 1.91 (1.76–2.09) | 219 | 2.18 (2.02–2.35)b | 443 | 2.04 (1.93–2.16) |
| 5–9 | 323 | 2.02 (1.90–2.15) | 310 | 2.10 (1.98–2.22) | 633 | 2.06 (1.97–2.15) | |
| 10–19 | 443 | 2.20 (2.10–2.31) | 416 | 2.19 (2.09–2.30) | 859 | 2.20 (2.12–2.27) | |
| 20–29 | 145 | 3.36 (3.09–3.65)a | 154 | 2.47 (2.26–2.70) | 299 | 2.87 (2.69–3.05) | |
| 30–39 | 147 | 5.03 (4.58–5.52)a | 227 | 3.26 (3.04–3.49) | 374 | 3.86 (3.64–4.10) | |
| 40–49 | 200 | 5.55 (5.08–6.07)a | 269 | 3.62 (3.38–3.88) | 469 | 4.34 (4.10–4.60) | |
| 50–59 | 194 | 5.66 (5.20–6.16)a | 291 | 3.85 (3.59–4.13) | 485 | 4.49 (4.25–4.75) | |
| Above 60 | 198 | 4.81 (4.42–5.23)a | 218 | 3.43 (3.16–3.73) | 416 | 4.03 (3.79–4.28) | |
| Cd, µg/L | 0–4 | 211 | 0.22 (0.20–0.24) | 199 | 0.22 (0.20–0.25) | 410 | 0.22 (0.21–0.23) |
| 5–9 | 318 | 0.25 (0.24–0.27) | 302 | 0.24 (0.22–0.26) | 620 | 0.25 (0.23–0.26) | |
| 10–19 | 435 | 0.33 (0.31–0.35) | 403 | 0.34 (0.32–0.36) | 838 | 0.33 (0.32–0.35) | |
| 20–29 | 145 | 0.60 (0.55–0.67) | 154 | 0.62 (0.57–0.68) | 299 | 0.61 (0.58–0.66) | |
| 30–39 | 147 | 0.97 (0.89–1.05) | 223 | 1.08 (1.01–1.15)b | 370 | 1.03 (0.98–1.09) | |
| 40–49 | 197 | 1.06 (0.98–1.13) | 266 | 1.30 (1.23–1.37)a | 463 | 1.19 (1.14–1.24) | |
| 50–59 | 194 | 1.14 (1.07–1.21) | 291 | 1.44 (1.36–1.51)a | 485 | 1.31 (1.26–1.36) | |
| Above 60 | 197 | 1.14 (1.07–1.21) | 218 | 1.38 (1.31–1.46)a | 415 | 1.26 (1.21–1.31) | |
Pb = lead, Hg = mercury, Cd = cadmium, GM = geometric mean, CI = confidence interval.
aP < 0.01, statistically highly significant between males and females; bP < 0.05, statistically significant between males and females.
Fig. 1.
Distribution of the concentration of Pb, Hg, and Cd in whole blood according to age. Data are presented as geometric mean values and 95% confidence intervals.
Pb = lead, Hg = mercury, Cd = cadmium, BPb = lead in whole blood, BHg = mercury in whole blood, BCd = cadmium in whole blood.
Blood total Hg level in the Korean general population
The geometric mean concentration of total Hg in whole blood of all subjects was 2.92 µg/L, which was also significantly higher in males (3.11 µg/L) than in females (2.77 µg/L). The arithmetic mean concentration of blood total Hg was 3.70 µg/L (4.09 µg/L in males and 3.36 µg/L in females), and the median was 2.87 µg/L (3.02 µg/L in males and 2.75 µg/L in females). The 95th percentile total Hg level in whole blood corresponded to 9.12 µg/L in all subjects, and was 10.59 µg/L in males and 7.99 µg/L in females (Table 2). The blood total Hg level increased in an age-dependent pattern to 50–59 years of age, after which it tended to decrease slightly. No significant differences of blood total Hg levels between males and females were observed in subjects < 20 years of age (Table 3, Fig. 1).
Blood Cd level in the Korean general population
The geometric mean concentration of Cd in whole blood of all subjects was 0.56 µg/L. In contrast to Pb and Hg, the geometric mean concentration of blood Cd was higher in females (0.62 µg/L) than in males (0.50 µg/L). Arithmetic mean concentration of blood Cd was 0.82 µg/L (0.72 µg/L in males and 0.91 µg/L in females), and median was 0.59 µg/L (0.51 µg/L in males and 0.72 µg/L in females). The 95th percentile of blood Cd was 2.20 µg/L in all subjects, and was 1.94 µg/L in males and 2.34 µg/L in females (Table 2). Blood Cd was increased in an age-dependent pattern to 50–59 years of age, after which it tended to decrease slightly. No significant differences of blood Cd levels between males and females were observed in subjects < 30 years of age (Table 3, Fig. 1).
Relations among blood metals concentrations and age
Significantly positive relationships were observed among the blood concentrations of Pb, Hg, and Cd. The metals correlated with increasing age of the study subjects (Table 4).
Table 4. Spearman rank correlation coefficients among age and blood metals concentrations.
| Variables | Age | BPb | BHg | BCd |
|---|---|---|---|---|
| Age | 1 | 0.5025a | 0.4672a | 0.7795a |
| BPb | - | 1 | 0.3800a | 0.4961a |
| BHg | - | - | 1 | 0.4384a |
| BCd | - | - | - | 1 |
BPb = lead in whole blood, BHg = mercury in whole blood, BCd = cadmium in whole blood.
aP < 0.01, statistically highly significant.
Blood levels of Pb, total Hg, and Cd by individual lifestyles in adults
The levels of Pb, total Hg, and Cd in whole blood were presented by individual lifestyles such as smoking, alcoholic drinking, and diet habits, in the study subjects over 20 years old (Table 5). Namely, the geometric mean concentrations of Pb, total Hg, and Cd in whole blood were significantly higher in current or ex-smokers than in non-smokers in males, but not significant in females. Blood Pb and total Hg were higher in alcoholic drinkers than in non-drinkers in males, while blood Cd was higher in female non-alcoholic drinkers than in drinkers. Seafood intake during the last 3 days before blood sampling was affected on the level of blood total Hg only, in both males and females.
Table 5. Geometric mean and 95% confidence intervals of Pb, Hg, and Cd in whole blood by individual lifestyles in the study subjects over 20 years-old.
| Variables | BPb, µg/dL | BHg, µg/L | BCd, µg/L | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | ||
| Smoking status | ||||||||||
| Non-smokers | 2.41 (2.29–2.54) | 1.99 (1.95–2.03) | 2.06 (2.02–2.10) | 4.28 (3.96–4.62) | 3.37 (3.25–3.49) | 3.52 (3.41–3.64) | 0.71 (0.66–0.76) | 1.18 (1.14–1.22) | 1.07 (1.04–1.11) | |
| Current- or ex-smokers | 2.74 (2.67–2.81) | 1.92 (1.76–2.09) | 2.62 (2.55–2.69) | 5.15 (4.91–5.40) | 3.65 (3.22–4.13) | 4.93 (4.71–5.15) | 1.11 (1.07–1.16) | 1.18 (1.04–1.34) | 1.12 (1.08–1.16) | |
| P value | < 0.001 | 0.437 | < 0.001 | < 0.001 | 0.219 | < 0.001 | < 0.001 | 0.982 | 0.077 | |
| Alcoholic drinking status | ||||||||||
| Non-drinkers | 2.38 (2.22–2.55) | 2.00 (1.93–2.07) | 2.07 (2.01–2.14) | 4.14 (3.68–4.67) | 3.31 (3.12–3.51) | 3.47 (3.29–3.66) | 0.90 (0.82–0.99) | 1.27 (1.22–1.33) | 1.18 (1.13–1.24) | |
| Drinkers | 2.69 (2.62–2.76) | 1.97 (1.92–2.03) | 2.31 (2.26–2.35) | 5.01 (4.80–5.23) | 3.43 (3.29–3.58) | 4.16 (4.03–4.29) | 0.99 (0.95–1.03) | 1.13 (1.09–1.18) | 1.06 (1.03–1.09) | |
| P value | < 0.001 | 0.599 | < 0.001 | 0.004 | 0.309 | < 0.001 | 0.072 | < 0.001 | < 0.001 | |
| Seafood intake during the last 3 days | ||||||||||
| No | 2.66 (2.56–2.77) | 1.95 (1.87–2.02) | 2.22 (2.16–2.29) | 4.35 (4.09–4.63) | 3.01 (2.84–3.18) | 3.52 (3.37–3.68) | 0.99 (0.93–1.05) | 1.14 (1.08–1.20) | 1.07 (1.03–1.11) | |
| Yes | 2.64 (2.56–2.72) | 2.00 (1.95–2.05) | 2.26 (2.21–2.30) | 5.23 (4.96–5.51) | 3.64 (3.48–3.80) | 4.26 (4.11–4.41) | 0.98 (0.94–1.03) | 1.21 (1.16–1.25) | 1.10 (1.07–1.14) | |
| P value | 0.719 | 0.220 | 0.438 | < 0.001 | < 0.001 | < 0.001 | 0.839 | 0.071 | 0.244 | |
BPb = lead in whole blood, BHg = mercury in whole blood, BCd = cadmium in whole blood.
DISCUSSION
In this study, we provide a representative and descriptive statistics of background exposure to Pb, Hg, and Cd as blood concentrations according to sex covering all ages from 0 to 83 years in the Korean general population.
The geometric mean concentration of Pb in whole blood was 1.82 µg/dL in all subjects and 2.22 µg/dL in adult subjects (older than 19 years of age), which was not too much different from previous national surveys in Korean such as KNHEBE (1.72 µg/dL for > 18 years, survey year 2007–2008), KNHANES (2.289 µg/dL for > 19 years, survey year 2008–2010), and KoNEHS (1.94 µg/dL for > 18 years, survey year 2012–2014).5,6,7 The level of blood Pb in Koreans was higher than Americans (0.97 µg/dL for 1–74 years of age in NHANES, survey year 2011–2012) and Canadians (1.10 µg/dL for 3–79 years of age in CHMS, survey year 2012–2013), but was similar or a little bit lower than for Germans (3.07 µg/dL for 18–69 years of age in GerES III, survey year 1998; 1.9 µg/dL for 18–70 years of age, survey year 2005).15,16,17,18 Blood Pb in German children (1.63 µg/dL for 3–14 years of age in GerES IV, survey year 2003–2006) was similar level with Korean children of this study (1.49 µg/dL and 1.51 µg/dL in the age groups of 0–4 and 5–9 years of age, respectively).19 Presently, the 95th percentile of blood Pb was at 3.78 µg/dL and just one person, an adult woman, exceeded the CDC reference guideline for adults of 10 µg/dL. The level of blood Pb in the Korean general population was markedly decreased after a ban of Pb containing gasoline, which is a similar pattern shown earlier in developed countries. In addition, several Pb reducing policies, such as removal of Pb from paint, solder, water-supply system and children's goods would have contributed to the reduction of human exposure to Pb in the last decades. The differences of blood lead levels among countries could be affected by various factors such as lifestyles, diet habit, culture, and customs, however, the timing of phasedown and complete phase-off for leaded gasoline could have partially contributed to the blood lead levels in each country.20,21 Because children are more vulnerable to Pb compared to adults, the CDC recommended < 5 µg/dL of blood Pb to prevent children Pb poisoning. There were three observed children with > 5 µg/dL blood Pb among 985 children < 10 years of age in this study. However, several studies reported adverse health effects such as cognitive impairment, neurobehavioral symptom and developmental disorders, at low blood Pb level of < 5 µg/dL.11,22,23 Although a Pb reduction policy might be relatively successful in Korea, concern would remain for protection of children health from environmental Pb exposure.
The geometric mean concentration of total Hg in whole blood was 2.92 µg/L in total study subjects and 3.90 µg/L in adults subjects (> 19 years) in this study, which was also similar level with similar age groups in previous studies for Korean subjects (3.80 µg/L for > 18 years of age in KNHEBE surveyed at 2007–2008, 4.303 µg/L for > 19 years of age in KNHANES surveyed at 2008–2010, 3.84 µg/L for > 18 years of age in KoNEHS surveyed at 2012–2014).5,6,7 But, blood Hg in Korean subjects was much higher (3.7–5.0 times) compared to Americans (0.70 µg/L for 1–74 years of age in NHANES, survey year 2011–2012), Canadians (0.79 µg/L for 3–79 years of age in CHMS, survey year 2012–2013), and Germans (0.58 µg/L for 18–69 years of age in GerES III, survey year 1998; 0.9 µg/L for 18–70 years of age, survey year 2005).15,16,17,18 Specifically, blood total Hg in German children was < 0.23 µg/dL in GerES IV (survey year 2003–2006),19 which was approximately one-ninth of Korean children (2.04 µg/L and 2.06 µg/L in the age groups of 0–4 and 5–9 years of age, respectively). The 95th percentile of blood total Hg was at 9.12 µg/L. Approximately 19% of study subjects (761/3,978) had blood Hg > 5 µg/L, which is the level at which no adverse health effects are expected (HBM I). Furthermore, 54 study subjects (1.4%) had blood Hg > 15 µg/L, which is the possible level of risk for adverse health effects (HBM II).24 Accordingly, blood Hg level in Koreans is greatly high compared to western countries. However, the concentrations of blood Hg in Koreans have not changed much during the last several decades.25 These findings suggest that human exposure sources for Hg have remained without effective control in the general population of Korea. Previous studies reported that a diet favoring seafood in Koreans is significantly associated with the high level of blood Hg,13,25 similar to other Asian countries including Japan and Taiwan.26,27 Seafood consumption and also other environmental Hg exposures can explain most of blood Hg in Korean children, even the age group of 0–4 years of age, is markedly higher compared to western countries. Seafood, especially fish and shellfish, contains organic Hg, which is lipid soluble and can easily be transported across cellular membranes.28 When pregnant women ingest a high amount of organic Hg from fish and shellfish, the Hg can be transported to the fetus through the blood-placenta barrier, and could result in neurodevelopmental damage in neonates and children.29,30 This mechanism of methyl-Hg toxicity is infamous historically as Minamata disease.3 Presently, approximately 11.2% of childbearing women of 15–49 years of age (92/821) had a blood Hg level > 5.8 µg/L, which is the recommended blood Hg level for childbearing women by the US Environmental Protection Agency.31 This data could explain why blood Hg levels in Korean children were so high. Therefore, it is necessary to reduce Hg exposure through diet as well as preparing a guideline and effective communication for childbearing aged women in Korea.
The geometric mean concentration of blood Cd was 0.56 µg/L in all study subjects and 1.06 µg/L in adults subjects (> 19 years) of this study, which was similar level with the previous Korean survey (1.02 µg/L for > 18 years of age in KNHEBE surveyed at 2007–2008, 0.967 µg/L for > 19 years of age in KNHANES surveyed at 2008–2010).5,6 The level of blood Cd in Koreans was approximately 2-times higher than Americans (0.28 µg/L for 1–74 years of age in NHANES, survey year 2011–2012) and 1.7-times higher than Canadians (0.33 µg/L for 3–79 years of age in CHMS, survey year 2012–2013).15,16 Blood Cd was also higher in Koreans than in Germans (0.44 µg/L for 18–69 years of age in GerES III surveyed at 1998; 0.38 µg/L for 18–70 years of age, survey year 2005).17,18 Blood Cd in German children was < 0.12 µg/L for 3–14 years of age in GerES IV surveyed at 2003–2006,19 which was less than half of the blood Cd in Korean children (0.22 µg/L and 0.25 µg/L in the age groups of 0–4 and 5–9 years of age, respectively). The 95th percentile of blood Cd was at 2.20 µg/L in this study, and three female adults had blood Cd > 5 µg/L of a biological exposure index (BEI).32 Though, the level of blood Cd in Koreans is higher than in several western countries, it is similar with Asian countries.33,34 Most of the Asian food intake is rice, fish, shellfish and vegetables, which may be a major exposure source to Cd in non-smokers.35,36 In this study, the maximum concentration of metal, such as Pb, Hg, or Cd, respectively, in whole blood was higher level than expected. However, we could not find any specific occupational exposure as well as simultaneous exposure to more than two metals among those subjects. We suppose that further study, such as a longitudinal follow-up study and in-depth research based on individuals, may be necessary to clarify exposure sources.
Blood levels of Pb, Hg, and Cd, which are non-essential and toxic metals, increased according to age, and positively correlated with each other (Table 4). These findings indicate that humans have been exposed continuously to these metals from the environment and from some common exposure sources, with accumulation of the metals in the body. Especially, blood Cd (r = 0.7795) is more closely correlated with age compared to relations of Pb (r = 0.5025) and Hg (r = 0.4672) (Table 4). Therefore, an age-dependent increase of blood level was remarkable in Cd compared to Pb and Hg. The mean blood concentration of Pb or Hg in the age group of the highest metal level (50–59 years of age), was approximately 2-times that of children (0–4 and 5–9 years of age groups), but was 5–6-times that of Cd. These findings could suggest that blood Cd in children may represent an exposure from various external environmental sources after birth that increases with aging, because of the transport of Cd through the blood-placenta barrier is quite limited during pregnancy in contrast to Hg and Pb.29 In addition, a relatively long biological half-life of Cd in the body could contribute to the high correlation coefficient between blood Cd and aging.37 However, the blood concentrations of target metals, such as Pb, Hg, and Cd, were decreased above sixty years of age in this study. This phenomenon was also observed in the previous study,6 which could be understood as a cohort effect due to changes in the exposure patterns of heavy metals in the elderly. In the case of Pb, blood level was higher in subjects 0–4 and 5–9 years of age (1.49 and 1.51 µg/dL, respectively) than in the 10–19 years of age (1.36 µg/dL). This age-dependent U-shaped pattern is similar with the data from NHANES and CHMS, where blood Pb was higher in subjects 1–5 or 3–5 years of age, and 6–11 years of age, than in subjects 12–19 years of age,15,16 because children can be exposed to lead more from hand-to-mouth activities with contaminated soil and lead-containing paint chip compared to teenagers and adults.8 In addition, children under 10 years of age have a lower iron level compared to adolescents and adults, and the highest prevalence of anemia is observed in preschool-age children,38 therefore, this physiological characteristic can increase the lead absorption. More outdoor activities of boys than girls might cause higher blood Pb concentrations in boys compared to girls.
The blood level of Pb and Hg were higher in males than in females, but blood Cd was higher in females than in males. Furthermore, the time when blood levels significantly differed between males and females was different according to the metal (Table 3). Blood Pb and Hg were higher in males than in females beginning at 5–9 and 20–29 years of age, respectively. The significantly high blood level of Cd in females than in males was observed beginning in the 30s. Human exposure to Pb could be affected by individual outdoor physical activities in school-aged subjects and individual behaviors including smoking habits and occupation.20,39 Blood Hg level in the general population could be affected by individual lifestyle aspects like smoking and alcoholic drinking, and by diet, especially seafood consumption. The latter could vary in Korea depending on whether a person lived near the coast or inland.13 Diet is a major exposure source to Cd in the general population and smoking is also an additional important exposure source in smokers.20 However, body iron plays a major role in determining Cd uptake into the body in the gastrointestinal tract. Periodic blood loss and maternity in females may induce an iron deficiency in the body, resulting in increased Cd absorption through the increased expression of metal transporters in the duodenum.20,40 These mechanisms could explain partially why blood Cd is higher in female adults than in male adults. However, no observed significant sex-difference in blood Cd concentrations in children which might be ascribed to the similar levels of exposure to Cd and absorption in the body between genders. This study also presents that blood levels of Pb, Hg, and Cd are affected by individual lifestyles such as smoking, alcoholic drinking and diet habits as well as sex and age.
The health effects of toxic metals, such as Pb, Hg, and Cd, could be an emerging concern during aging as well as during childhood. Metals could be one of the major causes of chronic degenerative diseases including neurodegenerative diseases, cardiovascular disease, diabetes, endocrine disease, kidney disease, and bone damage, which are prominent public health concerns.13,20,36 This study presents a background exposure level to Pb, Hg, and Cd in the Korean general population which could be useful in evaluating the risk of those metals in the polluted region and small population area. Our data also show that human exposure to different metals differs with demographic factors, sex, and age. Therefore, a customized reduction policy is needed to reduce the background metal exposure for health promotion and cost reduction. This entails the understanding of why Korean people are exposed to high levels of certain metals, the source of the exposure and those who are at increased risk. This study will ultimately be used as a basis for public health and environmental policy decisions and will help determine the priority of environmental health research in the future.
However, this study has some limitations. In this study, blood samples were collected over two years throughout the country while the sampling season was not evenly distributed, which limits the reflection of seasonal variations in blood concentrations of metals. Although this study was conducted on a large scale of 4,000 individuals covering the whole age spectrum as a single survey, it might not be enough to provide representative values at once, particularly among children subjects who were sampled using clustering differently from adults. This study has also inherent limitations of a cross-sectional study design, therefore, a more comprehensive cohort study is needed to assess the risk for exposure to heavy metals.
In conclusion, this study provides representative data of background exposure to Pb, Hg, and Cd for a wide age range of the general population in Korea. The concentrations of these metals in whole blood are dependent on sex and age. Overall exposure to Pb, Hg, and Cd is markedly higher in Koreans compared to Americans, Canadians, and Germans, except for Pb, which is similar or slightly lower in Koreans than in Germans.
Footnotes
Funding: This research was supported by a grant (14162MFDS655) from Ministry of Food and Drug Safety, Republic of Korea, in 2014.
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Park JD, Kim H, Kwon HJ, Hwang MS. Data curation: Eom SY, Lee YS, Lee SG, Seo MN, Lim JA, Park KS, Pyo HS. Investigation: Kim YD, Kim YM, Hong YS, Sohn SJ, Choi BS, Kim H, Park JD. Writing - original draft: Park JD, Eom SY.
References
- 1.The ATSDR 2015 priority list of hazardous subtances. [Updated 2015]. [Accessed April 13, 2017]. https://www.atsdr.cdc.gov/spl/
- 2.Tsuchiya K. Causation of Ouch-Ouch Disease (Itai-Itai Byõ)--an introductory review. II. Epidemiology and evaluation. Keio J Med. 1969;18(4):195–211. doi: 10.2302/kjm.18.195. [DOI] [PubMed] [Google Scholar]
- 3.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 4.Dooyema CA, Neri A, Lo YC, Durant J, Dargan PI, Swarthout T, et al. Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ Health Perspect. 2012;120(4):601–607. doi: 10.1289/ehp.1103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son JY, Lee J, Paek D, Lee JT. Blood levels of lead, cadmium, and mercury in the Korean population: results from the second Korean National Human Exposure and Bio-monitoring Examination. Environ Res. 2009;109(6):738–744. doi: 10.1016/j.envres.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Lee BK. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the Korean general population: analysis of 2008–2010 Korean National Health and Nutrition Examination Survey data. Environ Res. 2012;118:124–129. doi: 10.1016/j.envres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Choi W, Kim S, Baek YW, Choi K, Lee K, Kim S, et al. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012–2014) Int J Hyg Environ Health. 2017;220(2 Pt A):29–35. doi: 10.1016/j.ijheh.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Au WW. Susceptibility of children to environmental toxic substances. Int J Hyg Environ Health. 2002;205(6):501–503. doi: 10.1078/1438-4639-00179. [DOI] [PubMed] [Google Scholar]
- 9.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Agriculture Organization of the United Nations; World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Blood lead levels in children aged 1–5 years - United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(13):245–248. [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm M, Heinzow B, Angerer J, Schulz C. Reassessment of critical lead effects by the German Human Biomonitoring Commission results in suspension of the human biomonitoring values (HBM I and HBM II) for lead in blood of children and adults. Int J Hyg Environ Health. 2010;213(4):265–269. doi: 10.1016/j.ijheh.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health. 2014;87(5):501–513. doi: 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- 14.Lim JA, Kwon HJ, Ha M, Kim H, Oh SY, Kim JS, et al. Korean research project on the integrated exposure assessment of hazardous substances for food safety. Environ Health Toxicol. 2015;30(1):e2015004. doi: 10.5620/eht.e2015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (US) Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 16.Health Canada. Third Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 3 (2012–2013) Ottawa, Canada: Health Canada; 2015. [Google Scholar]
- 17.Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, et al. German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health. 2002;205(4):297–308. doi: 10.1078/1438-4639-00155. [DOI] [PubMed] [Google Scholar]
- 18.Heitland P, Köster HD. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP-MS. J Trace Elem Med Biol. 2006;20(4):253–262. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Becker K, Müssig-Zufika M, Conrad A, Lüdecke A, Schulz C, Seiwert M, et al. German Environmental Survey for Children 2003/06 (GerES IV): Human Biomonitoring. Dessau-Roßlau, Germany: Umweltbundesamt; 2008. [Google Scholar]
- 20.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68(1):167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 21.Becker K, Schroeter-Kermani C, Seiwert M, Rüther M, Conrad A, Schulz C, et al. German health-related environmental monitoring: assessing time trends of the general population's exposure to heavy metals. Int J Hyg Environ Health. 2013;216(3):250–254. doi: 10.1016/j.ijheh.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Hong SB, Im MH, Kim JW, Park EJ, Shin MS, Kim BN, et al. Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age children. Environ Health Perspect. 2015;123(3):271–276. doi: 10.1289/ehp.1307420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skerfving S, Löfmark L, Lundh T, Mikoczy Z, Strömberg U. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114–120. doi: 10.1016/j.neuro.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Schulz C, Angerer J, Ewers U, Kolossa-Gehring M. The German Human Biomonitoring Commission. Int J Hyg Environ Health. 2007;210(3-4):373–382. doi: 10.1016/j.ijheh.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Kim NY, Ahn SJ, Ryu DY, Choi BS, Kim H, Yu IJ, et al. Effect of lifestyles on the blood mercury level in Korean adults. Hum Exp Toxicol. 2013;32(6):591–599. doi: 10.1177/0960327112467041. [DOI] [PubMed] [Google Scholar]
- 26.Yaginuma-Sakurai K, Shimada M, Ohba T, Nakai K, Suzuki K, Kurokawa N, et al. Assessment of exposure to methylmercury in pregnant Japanese women by FFQ. Public Health Nutr. 2009;12(12):2352–2358. doi: 10.1017/S1368980009005011. [DOI] [PubMed] [Google Scholar]
- 27.Lee CC, Chang JW, Huang HY, Chen HL. Factors influencing blood mercury levels of inhabitants living near fishing areas. Sci Total Environ. 2012;424:316–321. doi: 10.1016/j.scitotenv.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Toxic Substances and Disease Registry (US) Toxicological Profile for Mercury (Update) Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1999. [Google Scholar]
- 29.Sakamoto M, Yasutake A, Domingo JL, Chan HM, Kubota M, Murata K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: potential use as indicators for prenatal exposure. Environ Int. 2013;60:106–111. doi: 10.1016/j.envint.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19(6):417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 31.Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289(13):1667–1674. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 32.American Conference of Governmental Industrial Hygienists. ACGIH Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) Cincinati, OH: American Conference of Governmental Industrial Hygienists; 2014. [Google Scholar]
- 33.Ikeda M, Ohashi F, Fukui Y, Sakuragi S, Moriguchi J. Closer correlation of cadmium in urine than that of cadmium in blood with tubular dysfunction markers in urine among general women populations in Japan. Int Arch Occup Environ Health. 2011;84(2):121–129. doi: 10.1007/s00420-010-0527-1. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Sun H, Wu Y, Zhou Z, Ding Z, Chen X, et al. Tubular and glomerular kidney effects in the Chinese general population with low environmental cadmium exposure. Chemosphere. 2016;147:3–8. doi: 10.1016/j.chemosphere.2015.11.069. [DOI] [PubMed] [Google Scholar]
- 35.Tsukahara T, Ezaki T, Moriguchi J, Furuki K, Shimbo S, Matsuda-Inoguchi N, et al. Rice as the most influential source of cadmium intake among general Japanese population. Sci Total Environ. 2003;305(1-3):41–51. doi: 10.1016/S0048-9697(02)00475-8. [DOI] [PubMed] [Google Scholar]
- 36.Huang M, Choi SJ, Kim DW, Kim NY, Bae HS, Yu SD, et al. Evaluation of factors associated with cadmium exposure and kidney function in the general population. Environ Toxicol. 2013;28(10):563–570. doi: 10.1002/tox.20750. [DOI] [PubMed] [Google Scholar]
- 37.Sugita M, Tsuchiya K. Estimation of variation among individuals of biological half-time of cadmium calculated from accumulation data. Environ Res. 1995;68(1):31–37. doi: 10.1006/enrs.1995.1005. [DOI] [PubMed] [Google Scholar]
- 38.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 39.Rhie J, Lee HE. Physical activity and blood lead concentration in Korea: study using the Korea National Health and Nutrition Examination Survey (2008–2013) J Korean Med Sci. 2016;31(6):852–858. doi: 10.3346/jkms.2016.31.6.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu DY, Lee SJ, Park DW, Choi BS, Klaassen CD, Park JD. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett. 2004;152(1):19–25. doi: 10.1016/j.toxlet.2004.03.015. [DOI] [PubMed] [Google Scholar]