Abstract
Introduction:
Gestational diabetes mellitus (GDM) is common and is accompanied with other comorbidities. Challenges to treatment exist at our institute as it serves women with low income. This study assessed the burden of comorbidities and the outcome of GDM.
Methods:
This was a prospective, observational study of women with gestational diabetes attending the obstetrics department from September 2012 to April 2014. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria. Medical comorbidities were noted, and lipid profile was done. All the women were followed up till delivery, and the complications were recorded. Age- and parity-matched pregnant women with normal oral glucose tolerance test were recruited as controls.
Results:
One hundred and thirty-nine women were followed up till delivery. The average age was 28 years. Eighteen percent had bad obstetric history. The average body mass index was 28.8. Twenty-five percent had gestational hypertension (HTN), and 6.4% had chronic HTN. Thirty percent had hypothyroidism. 65% women received insulin. The glucose values were within the recommended range in 60% of the women. Maternal hypoglycemia occurred in 7 (5%) women. Forty-four percent of the women required cesarean section and 34% had complications either during pregnancy or labor. Three neonates had macrosomia. Twenty-six neonates (20%) required admission to the Neonatal Intensive Care Unit. Four neonates (3%) died. Newborns of mothers whose GDM optimally treated had less complications.
Conclusion:
Gestational diabetes is associated with HTN, hypothyroidism, obesity, and lipid abnormalities. The majority of women required insulin for treatment and optimal control of blood glucose resulted in lower neonatal complications.
Keywords: Comorbidities, gestational diabetes mellitus, maternal outcome, neonatal outcome
INTRODUCTION
Gestational diabetes mellitus (GDM) is a condition characterized by carbohydrate intolerance with the onset or first recognition during pregnancy.[1] GDM is common in South India, and the prevalence of 17.8% has been found in Chennai city.[2] Mothers with GDM are expected to have other comorbidities such as hypertension (HTN). In fact, they are at an increased risk of the development of gestational HTN or preeclampsia (odds ratio [OR] 1.5).[3] The comorbidities can affect the outcome. Hypertriglyceridemia in gestational diabetes has been found to have a positive correlation with birth weight which in turn is related to neonatal complications.[4] The association of such comorbidities with GDM has not yet been studied at our institute.
GDM is associated with an increased risk of complications for both the mother and the child. The rate of preeclampsia and cesarean section is increased in the mother and the risk of macrosomia is increased in the newborn.[5] The benefit of blood glucose control during pregnancy has primarily been noted in the reduction of certain neonatal complications such as macrosomic babies and shoulder dystocia.[6] The outcome in our hospital has not been assessed. Our hospital serves underprivileged patients and challenges to treatment exist here. This study was undertaken to study the burden of comorbidities and the outcome of GDM.
METHODS
This was a prospective, observational study of women with gestational diabetes attending the Obstetrics Department from September 2012 to April 2014. The study was approved by the Institute ethics committee, and a written informed consent was obtained from the women. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria.[7] Bad obstetric history (BOH) was defined as history of one stillbirth or neonatal death or three or more consecutive abortions. Medical comorbidities when present were noted. Anthropometric measurements (height, weight [prepregnancy body weight if available]) were recorded. HbA1c, fasting lipid profile, 24 h urine protein, and fundus examination were done at admission. All women had received advice relating to physical activity and diet. Women who required insulin were admitted to the hospital. Patient's glucose values were recorded at diagnosis and before delivery. GDM was considered to be optimally controlled if the fasting glucose (FBS) was <95 mg/dl and 2nd h postprandial glucose (PPBS) was <120 mg/dl as defined.[8] All the women were followed up till delivery.
Maternal complications during pregnancy and during delivery were recorded. Shoulder dystocia was defined as a vaginal cephalic delivery that requires additional obstetric maneuvers to deliver the fetus after the head has delivered, and gentle traction has failed.[8] Macrosomia was defined as birth weight >4 kg. Normative birth weight data according to gestational age were compiled using birth weights of 773 consecutively born neonates. This data were used to determine whether the neonate was large for gestational age (LGA) or small for gestational age (SGA). The neonatal complications were recorded. Neonatal hypoglycemia was defined as plasma glucose level <45 mg/dl by point of care glucose testing later confirmed by laboratory testing.[9]
Pregnant women with normal oral glucose tolerance test were recruited as controls. They were matched for age and parity. Written informed consent was obtained. Their body weight and height were recorded. A fasting lipid profile was done. They were followed up till delivery, and the maternal and fetal outcomes were recorded.
The data were tabulated using Microsoft Excel software and analyzed using StatsDirect software, (version 2.7.9, StatsDirect Ltd, Cheshire, UK). Student's t-test was used to test the difference between the women and the controls with respect to weight, skinfold thickness, and lipid profile. Fisher's exact test was used to test the difference in maternal and fetal outcomes between GDM women with or without optimal blood glucose control.
RESULTS
A total of 148 women with gestational diabetes had been recruited into the study. Nine women were lost to follow-up, 139 women were followed up till delivery, and the maternal and neonatal outcomes were recorded. One hundred and thirty-nine age and parity matched controls were recruited.
Clinical characteristics
The women were from Puducherry (35%) and the surrounding districts of Tamil Nadu (65%). The average age was 28 years. The average duration of pregnancy at diagnosis of GDM was 30 weeks. The median gravidity was two. Fifty-three women (38%) were primigravida. Forty-nine women had prior history of abortion, intrauterine death or both and 25 (18%) of them satisfied the definition of BOH. Twenty women (23%) had a history of GDM, and 60 women (43%) had a first-degree relative having diabetes [Table 1].
Table 1.
Clinical characteristics of the women with gestational diabetes mellitus and controls
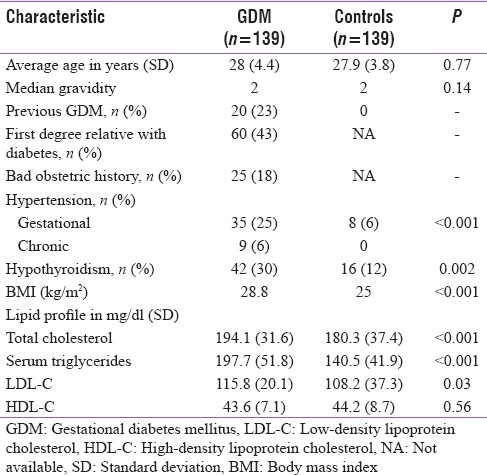
GDM mothers weighed a mean of 9.9 kg more than the controls. Their average body mass index (BMI) was 28.8 when compared to 25 in the controls. The mean FBS, 1 h and 2 h glucose values were 109 mg/dl, 149.5 mg/dl and 132.1 mg/dl, respectively, at diagnosis. Serum triglycerides, serum cholesterol, and low-density lipoprotein levels were significantly higher in GDM mothers compared to controls. High-density lipoprotein cholesterol levels were similar in both the groups. Forty-four women (31%) had HTN of which 35 women had gestational HTN, and 9 women had chronic HTN. Forty-two women (30%) had hypothyroidism. It was subclinical in 11 of them.
Treatment
Twenty-four women (17%) were managed with dietary advice alone. Twenty-five women (18%) were treated with metformin. Eighty women (58%) received subcutaneous insulin, and 10 women (7%) were treated with metformin and subcutaneous insulin. Women who required insulin had been admitted to the hospital for treatment. The median FBS improved from 109 mg/dl at diagnosis to 88 mg/dl before delivery. At 32 weeks, both the fasting and the postprandial blood sugars were within the recommended range in 35 women (25%) and this improved to 83 women (60%) before delivery. The proportion of women with blood glucose in recommended range was similar in women treated with diet alone or diet and metformin or diet and insulin. HbA1c was available for 138 women, and the mean value was 6.3%. It had been done in the third trimester for 87% of the women and hence reflected the glycemic control in the latter part of second trimester and early third trimester. Maternal hypoglycemia had been documented in hospital in seven women.
Maternal outcome
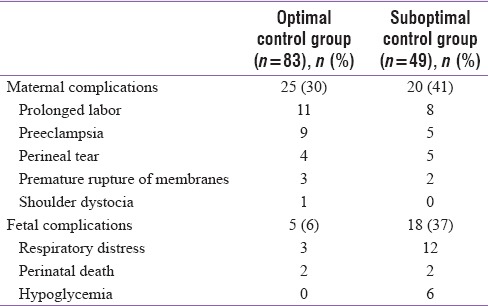
The outcome is being reported for 132 mothers. Seven mothers with twin pregnancy have been excluded as it could have independently affected outcomes. The mean gestational age at delivery was 37 weeks (38.7 in the control group). Seventy-four (56%) mothers delivered vaginally (7 required forceps assistance) and 58 (44%) required cesarean section. In comparison, 22% of controls underwent cesarean section. Thirty-four cesarean sections were elective. The indications were previous cesarean section (19), BOH (10), in vitro fertilization (4), and macrosomia (1). Twenty-four women underwent emergency cesarean section, and the most common indication was unsatisfactory progress of labor. Thirty-four percent of the women had complications either during pregnancy or labor and all complications were significantly less in the control group [Table 2]. The maternal complications were more common in mothers with less than optimal control (41% vs. 30%), but this was not statistically significant (P = 0.26) [Table 3].
Table 2.
Maternal and neonatal complications among cases and controls
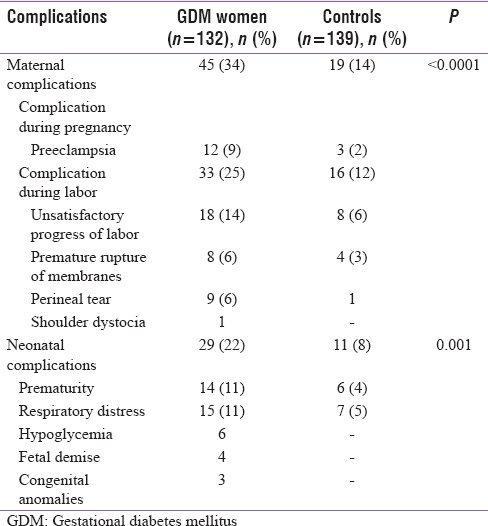
Table 3.
Blood sugar control and complications
Neonatal outcome
A total of 146 neonates were born. Fourteen neonates born as twins have been excluded from the analysis. The average birth weight was 2.85 kg (standard deviation 0.48). The average birth weight among controls was 2.75 kg (P = 0.04). Three neonates had macrosomia, 24 neonates were LGA, and one neonate was SGA.
Twenty-nine neonates (22%) had suffered complications, and they had been admitted to the Neonatal Intensive Care Unit (NICU) [Table 2]. In comparison, 11 (8%) in the control group required NICU admission. There were 14 (11%) premature births. Fifteen (11%) developed respiratory distress (seven had transient tachypnea of newborn). Two of them died. Six neonates (4.5%) had developed hypoglycemia which could be managed with oral feeds alone. Three neonates had congenital anomalies which were turner syndrome, sacral agenesis, and ventricular septal defect. Four neonates (3%) died. Two as mentioned above had respiratory distress. The third was a stillbirth. The fourth had developed hypoxic ischemic encephalopathy due to prolonged labor.
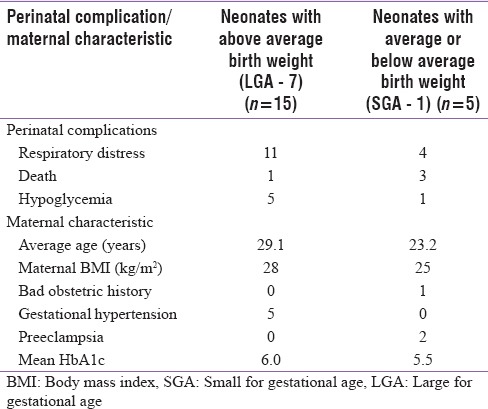
BOH did not seem to affect outcome. There were no neonatal deaths in this subgroup, and average birth weight was similar to mothers without it. The presence of associated gestational HTN did not affect fetal growth. The average birth weight did not differ among those who had or did not have HTN. Neonates born to mothers who had optimal control of blood sugar had fewer complications (6%) than those born to mothers with suboptimal control (37%) (P < 0.001) [Table 3]. The perinatal complications appeared to be related to birth weight [Table 4]. Deaths more commonly occurred in neonates with average or below average birth weight. Respiratory distress in the neonates was more common if they were of above average birth weight. The mothers in this group had higher HbA1c.
Table 4.
Perinatal complications classified according to birth weight
DISCUSSION
The clinical characteristics, treatment, and outcome in one hundred and thirty-nine women with gestational diabetes have been described in this study. GDM had been diagnosed at a mean of 30 weeks of gestation. This reflects the referral and screening practices seen in this region. Universal screening as is advised, if practiced will allow early diagnosis. This scenario may be changing as demonstrated by a recent survey conducted by Mahalakshmi et al., wherein 85% of the respondents (obstetricians, diabetologists and physicians) said that they were practicing universal screening.[10]
The women with GDM had higher BMI as expected. Eighteen percent of the women had a BOH. It has been reported that mothers with a BOH have a slightly higher incidence of gestational diabetes.[11] However, the presence of BOH did not appear to affect neonatal outcome. Thirty-one percent women had associated HTN. Nine percent had chronic HTN. The presence of chronic HTN increases the risk of developing GDM (OR 1.8).[12] However, the outcome is unaffected in women with chronic HTN and GDM.[13] In the current study too, the presence of any form of HTN did not appear to affect neonatal outcome.
Sixty-five percent of the women received insulin for blood sugar control. Optimal glycemic control was achieved in 60% of women before delivery. Maternal hypoglycemia had been noted in 7 (5%) women. This could not be compared with the previous studies as they had not reported on maternal hypoglycemia. Outpatient management of these women is difficult as self-monitoring of glucose is near impossible due to the socioeconomic and educational constraints. Hence, there are risks involved, and a balanced approach is required. The women are being admitted to the hospital for better control of blood sugars at 32 weeks. This appears to be a reasonable approach as most of the fetal weight gain occurs between 30 and 34 weeks of pregnancy and better sugar control during this period may mitigate it.[14]
Overall, 56% of women with GDM had vaginal delivery, 34% women suffered complications, the birth weight was above average, 20% of the neonates required NICU admission, and the perinatal mortality was 3%. Preeclampsia occurred in 9% of the women. In the trial done by Crowther et al., preeclampsia occurred in 12% of the women in the intervention arm.[15] However, Bhat et al. have reported a much higher (29%) rate of preeclampsia in GDM from Thiruvananthapuram.[16] Gestational diabetes is known to be associated with increased risk of preeclampsia.[17] The reasons for the increased incidence are not known, and the current treatment for GDM does not reduce the risk of preeclampsia.[17] The rate of cesarean section is in general increased in GDM.[18] The rate of cesarean section noted in this study was 45%. In studies by Bhat et al. and Sreelakshmi et al., it has been reported to be 40% and 33%, respectively. Shoulder dystocia occurred in 0.7% of women. It has been previously reported to be 1.4% in treated women with GDM by Wahi et al.[19] The composite outcome of cesarean delivery, antenatal preeclampsia, and shoulder dystocia did not differ significantly among the groups with optimal control of blood sugars before delivery and those without. It is important to note that trials have shown benefit with respect to shoulder dystocia alone, and the rates of preeclampsia and cesarean section are not altered by the treatment of gestational diabetes.[6]
Neonatal complications occurred in 20% of neonates. The perinatal mortality was 3%. Lower mortality has been reported from another tertiary care center in South India.[20] Serious perinatal complications specifically attributable to gestational diabetes are in general rare.[21] Most of the deaths here occurred in neonates with birth weight which was less than average for gestational age. It appears that addressing the causes of low birth weight rather than intensively treating diabetes will reduce mortality in this subgroup of women. A more calibrated approach to treatment like ultrasound measurements of fetal abdominal circumference guided, targeted therapy may be beneficial.[14] Respiratory distress was the most common complication (11%) noted here. A similar proportion has been reported in the study by Sreelakshmi et al.[22] Respiratory distress was more common in neonates with above average birth weight. It was less in neonates born to mothers with optimal BS control. However, this was not an expected finding. Respiratory distress was noted more commonly in the intervention arm of the trial done by Crowther et al.[15] It is interesting to note that a recent retrospective review has found that neonatal respiratory distress syndrome was more common in insulin-treated mothers with any form of diabetes. Neonatal hypoglycemia occurred in six newborns (4%) in this study. All episodes occurred in newborns whose mothers had suboptimal blood sugar control before delivery. This outcome can be improved by adherence to current blood sugar control recommendations.
CONCLUSION
Gestational diabetes is associated with HTN, hypothyroidism, obesity, and lipid abnormalities. Medical management required hospitalization as majority of women required insulin for treatment. Optimal control of blood glucose resulted in lower neonatal complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) – A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 3.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158:1148–53. doi: 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 4.Eslamian L, Akbari S, Marsoosi V, Jamal A. Effect of different maternal metabolic characteristics on fetal growth in women with gestational diabetes mellitus. Iran J Reprod Med. 2013;11:325–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes. 2015;6:1065–72. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, et al. Effects of treatment in women with gestational diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;340:c1395. doi: 10.1136/bmj.c1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118:1379–93. doi: 10.1097/AOG.0b013e31823974e2. [DOI] [PubMed] [Google Scholar]
- 9.Jain A, Agarwal R, Sankar MJ, Deorari A, Paul VK. Hypocalcemia in the newborn. Indian J Pediatr. 2010;77:1123–8. doi: 10.1007/s12098-010-0176-0. [DOI] [PubMed] [Google Scholar]
- 10.Mahalakshmi MM, Bhavadharini B, Maheswari K, Anjana RM, Jebarani S, Ninov L, et al. Current practices in the diagnosis and management of gestational diabetes mellitus in India (WINGS-5) Indian J Endocrinol Metab. 2016;20:364–8. doi: 10.4103/2230-8210.180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh G, Sidhu K. Bad obstetric history: A prospective study. Med J Armed Forces India. 2010;66:117–20. doi: 10.1016/S0377-1237(10)80121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zetterström K, Lindeberg SN, Haglund B, Hanson U. Maternal complications in women with chronic hypertension: A population-based cohort study. Acta Obstet Gynecol Scand. 2005;84:419–24. doi: 10.1111/j.0001-6349.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 13.Leon MG, Moussa HN, Longo M, Pedroza C, Haidar ZA, Mendez-Figueroa H, et al. Rate of gestational diabetes mellitus and pregnancy outcomes in patients with chronic hypertension. Am J Perinatol. 2016;33:745–50. doi: 10.1055/s-0036-1571318. [DOI] [PubMed] [Google Scholar]
- 14.Kjos SL, Schaefer-Graf UM. Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets. Diabetes Care. 2007;30(Suppl 2):S200–5. doi: 10.2337/dc07-s216. [DOI] [PubMed] [Google Scholar]
- 15.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 16.Bhat M, Ramesha KN, Sarma SP, Sangeetha Menon SC, Kumar G. Determinants of gestational diabetes mellitus: A case control study in a district tertiary care hospital in South India. Int J Diabetes Dev Ctries. 2010;30:91–6. doi: 10.4103/0973-3930.62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C. Gestational diabetes: Risks, management, and treatment options. Int J Womens Health. 2010;2:339–51. doi: 10.2147/IJWH.S13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 20.Thomas N, Chinta AJ, Sridhar S, Kumar M, Kuruvilla KA, Jana AK. Perinatal outcome of infants born to diabetic mothers in a developing country – Comparison of insulin and oral hypoglycemic agents. Indian Pediatr. 2013;50:289–93. doi: 10.1007/s13312-013-0096-y. [DOI] [PubMed] [Google Scholar]
- 21.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36(6 Pt 2):617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Sreelakshmi PR, Nair S, Soman B, Alex R, Vijayakumar K, Kutty VR. Maternal and neonatal outcomes of gestational diabetes: A retrospective cohort study from Southern India. J Family Med Prim Care. 2015;4:395–8. doi: 10.4103/2249-4863.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]


