Abstract
Background and Aims:
Neutrophil-lymphocyte ratio (NLR) has been suggested to be a predictor of coronary artery disease (CAD), and end-organ damage in type-2 diabetes mellitus (T2DM). Similar data are lacking from Indians with T2DM. Hence, this study aimed to evaluate the role of NLR as a predictor of microvascular complications and CAD in T2DM.
Subjects and Methods:
Consecutive T2DM patients attending the outpatient services of 2 different hospitals, who gave consent, underwent clinical, anthropometric evaluation, and evaluation for the occurrence of retinopathy, nephropathy, neuropathy, and CAD.
Results:
A total of 298 patients were screened of which 265 patients' data were analyzed. Occurrence of hypertension, neuropathy, nephropathy, retinopathy, and CAD was 12.8%, 18.5%, 41.5%, 62.3%, and 3.8%, respectively. Patients in higher NLR quartiles had significantly higher diabetes duration, occurrence of nephropathy, albuminuria, retinopathy, CAD and lpwer glomerular filtration rate. Patients with more microvascular complications had significantly longer diabetes duration, blood pressure, NLR, creatinine, and urine albumin excretion. Binary logistic regression revealed NLR followed by body mass index were best predictors of microvascular complications. NLR had areas under the receiver operating characteristic curve (AUC) of 0.888 (95% CI: 0.848–0.929; P < 0.001), 0.708 (95% CI: 0.646–0.771; P < 0.001), and 0.768 (95% CI: 0.599–938; P = 0.004) in predicting albuminuria, retinopathy, and CAD, respectively. NLR of 2.00 had sensitivity and specificity of 86.4% and 69% in predicting albuminuria; sensitivity and specificity of 64.2% and 63% in predicting retinopathy; sensitivity and specificity of 80% and 47.1% in predicting CAD.
Conclusion:
NLR is inexpensive, easy to use, reliable predictor of nephropathy, retinopathy, and CAD in Indian T2DM.
Keywords: Diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, microvascular complications, microvascular inflammation, neutrophil-lymphocyte ratio, type-2 diabetes
INTRODUCTION
India is the diabetes capital of the world, with diabetes and prediabetes prevalence of 9% and 11-14%, respectively.[1] Type-2 diabetes mellitus (T2DM) has an aggressive clinical phenotype in Indians.[2] T2DM onset in Indians is nearly two decades earlier as compared to Caucasians, along with the highest rates or prediabetes progression to T2DM (18% in Indians as compared to 2% in the USA, 6% in Finland, and 11% in China).[3,4] Increased systemic inflammation, increased insulin resistance along with a more aggressive beta cell loss may explain this phenomenon.[2,3,4] Literature has highlighted the relationship between systemic inflammation, vascular disease and occurrence of microvascular and macrovascular complications in diabetes.[5] This increased burden of diabetes in Indians is a grim precursor of an exponential increase in diabetes-related end-organ damage and associated morbidity in the next few decades. There is an urgent need for cheap and easy to measure predictors of the occurrence of diabetes-related end-organ damage in Indians. This would help in institution preventive therapy targeting these specific individuals to improve long-term clinical outcomes.
Increased white blood count (WBC) is a conventional inflammatory marker, which co-relates well with several cardiovascular disease risk factors, diabetes, and its sequel.[6,7,8,9,10] Apart from WBC count, inflammatory markers such as interleukin (IL)-1, IL6, IL8, transforming growth factor β1, tumor necrosis factor-α have been linked to end organ damage in diabetes.[11,12,13,14,15] Limitations of these markers include lack of their availability in routine clinical practice compounded by the associated increased expenses and assay standardization.[16,17] Among the multiple parameters of complete blood count, the neutrophil-lymphocyte ratio (NLR) has been studied extensively as an inflammatory marker in cardiac and noncardiac diseases. NLR has been suggested to be a prognostic marker in acute myocardial infarction, heart failure, and stroke.[4,5] NLR stands out as a novel marker of chronic inflammation that reflects a counterbalance between two complementary components of the immune system; neutrophils being the active nonspecific mediator of inflammation, whereas lymphocytes acting as the protective or regulatory component of inflammation.[18,19] Data are lacking on the role of NLR as a predictor of end-organ damage in Indians with T2DM. This study, therefore, aimed to evaluate whether NLR has a role in predicting diabetic microvascular complications such as retinopathy, neuropathy, and nephropathy in Indians.
SUBJECTS AND METHODS
This was a cross-sectional observational study. Consecutive patients with T2DM attending the outpatient services of two different hospitals in central and northern India were considered for this study. The exclusions were patients with T1DM; patients with infections or recent history of infections in the past 1 month, otitis media, viral hepatitis, pyrexia of unknown origin, parasitic infection, viral infection, tuberculosis, local infection, skin infection, AIDS; patients with known systemic disorder such as cardiovascular disease, chronic kidney disease, chronic liver disease, blood disorders, autoimmune disorders, malignancy, poisoning; patients on anti-inflammatory drugs, systemic or topical steroids, drugs acting on the renin-angiotensin aldosterone system, alcohol; patients with uncontrolled blood pressure; patients having diseases affecting urinary protein excretion as nephrotic syndrome, urolithiasis, renal insufficiency, renal artery stenosis, dehydration state, urinary tract infection, and patients having low glomerular filtration rate (GFR) without microalbuminuria. The study protocol was explained to the patients and those who gave informed written consent were included in the study. The study duration was March 2015 to April 2017. The Institutional Ethics Committee of both the hospitals approved the study.
Information was collected from the patients on their duration of T2DM, treatment history, age and sex. Data were collected on the anthropometric parameters and vitals of the patients (height, weight, waist to hip ratio [WHR], body mass index [BMI], pulse rate, and blood pressure). Blood samples of 5 mL each were collected in plain and ethylenediaminetetraacetic acid vacutainer (Becton Dickinson). Serum was separated from blood collected in plain vacutainer and processed immediately for routine biochemical analysis. Clinical chemistry autoanalyzer based on dry chemistry microslide technology (VITROS 350 chemistry system, Johnson and Johnson, USA) was used for investigations such as kidney function tests, blood glucose parameters, and urine analysis. Ultrasonography abdomen was done for the evaluation of kidney echotexture and size. GFR was calculated using Chronic Kidney Disease Epidemiology Collaboration formula. Albuminuria was tested by MICRAL-II TEST strips by dipstick method. Urinary albumin excretion of 20–200 mg/L was defined as microalbuminuria and >200 mg/L was defined as overt albuminuria. The presence of albuminuria was reconfirmed by testing of urine sample again after 1-week follow-up. Only those patients who had persistent albuminuria on both the testing were defined to have albuminuria in this study. A patient was defined to have diabetic kidney disease if GFR was <60 mL/min/1.73 m2 and/ or presence of albuminuria >20 mg/L.[20] Digital fundus photography was done to assess diabetic retinopathy. Diabetic retinopathy was diagnosed using the Early Treatment Diabetic Retinopathy Study criteria.[21] Nerve conduction velocity studies were done of all limbs to assess for and diagnose diabetic neuropathy.[22] Among the macrovascular complications of T2DM only coronary artery disease (CAD) was screened for in the study cohort. Clinical and/or electrocardiogram (ECG) evidence of CAD was used for diagnosis in this study.[23]
Statistical analysis
Normality of the distribution of variables was checked using the Kolmogorov–Smirnov test. Continuous variables were expressed as mean ± standard deviation nonnormally distributed (skewed) variables were expressed as median [25th–75th percentile]. Analysis of variance (ANOVA) with post hoc analysis and Kruskal–Wallis nonparametric (ANOVA) with Dunn's postcorrection was performed for normally and nonnormally distributed variables, respectively. Chi-squared tests were used for categorical variables. The value of P < 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS) version 16 (Chicago, IL, USA) was used for analyses.
RESULTS
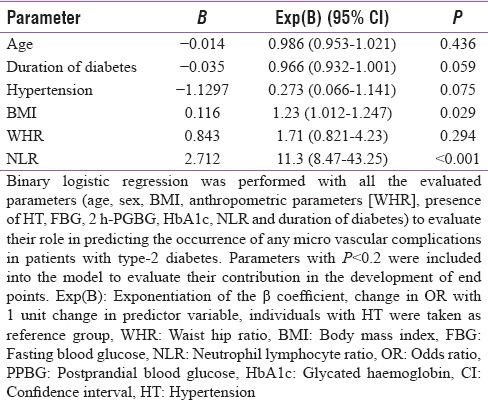
A total of 298 patients were considered for this study (155 from Bhopal and 143 from New Delhi). The study protocol and the associated investigations were explained, and only those who gave informed written consent were included for the study. Fifteen patients refused to consent for the study, and 18 patients did not turn up for the necessary investigations, hence excluded. Data from a total of 265 patients (143 from Bhopal and 122 from New Delhi) who fulfilled all criteria and gave consent were analyzed in this study. Mean age of the patients in this study was 51.12 ± 11.28 years, having disease duration of 3 [1–8 years], BMI 25.93 ± 4.15 kg/m2 and HbA1c of 8.55 ± 1.78%. The occurrence of hypertension, neuropathy, nephropathy, retinopathy, and CAD among patients in this study was 12.8% (n = 34), 18.5% (n = 49), 41.5% (n = 110), 62.3% (n = 165), and 3.8% (n = 10), respectively. Analysis performed on the basis of quartiles of NLR revealed that patients in the higher quartiles of NLR had a significantly higher occurrence of diabetic nephropathy, albuminuria, retinopathy, and CAD [Table 1]. These patients also had significantly longer duration of diabetes and lower GFR [Table 1]. Analysis based on the number of microvascular complications a patient has revealed that patients with a larger number of microvascular complications had a significantly longer duration of diabetes, higher WHR, higher blood pressure, NLR (with a comparable total leukocyte count), creatinine, and urine albumin excretion [Table 2]. NLR had significant correlation with diastolic blood pressure (σ: 0.163; P = 0.008), urine microalbumin excretion (σ: 0.331; P < 0.001), and GFR (σ: −0.144; P = 0.019). Correlation (σ) between NLR and age was 0.113, which approached statistical significance (P = 0.065). Binary logistic regression analysis revealed that NLR followed by BMI were the best independent predictors of the occurrence of microvascular complications in patients with T2DM [Table 3].
Table 1.
Clinical, anthropometric and biochemical parameters of patients with type-2 diabetes as per the quartiles of neutrophil-lymphocyte ratio
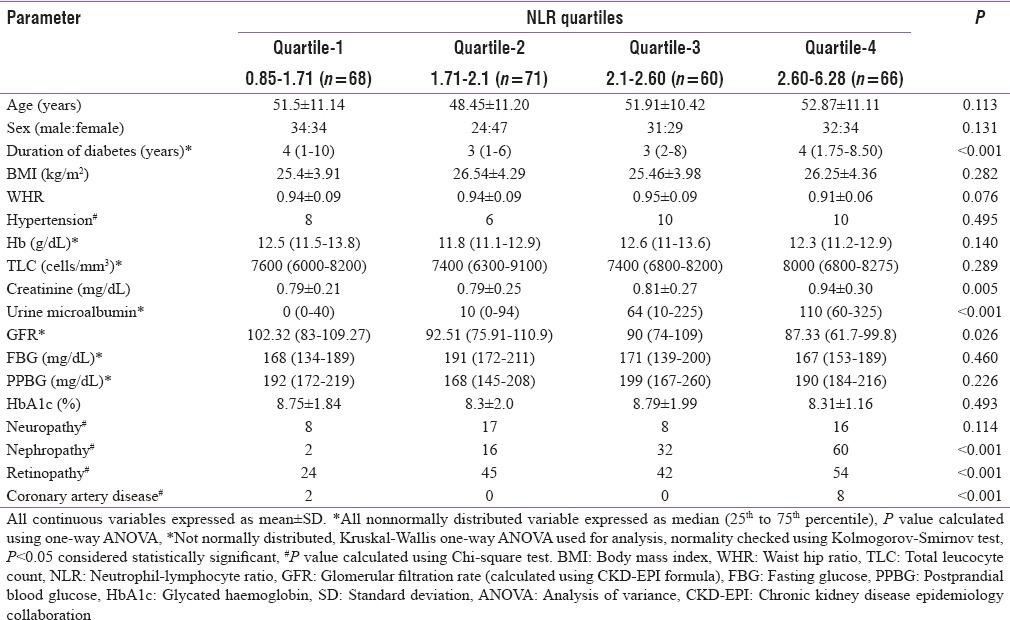
Table 2.
Clinical, anthropometric and biochemical parameters of patients with type-2 diabetes as per the occurrence of microvascular complications
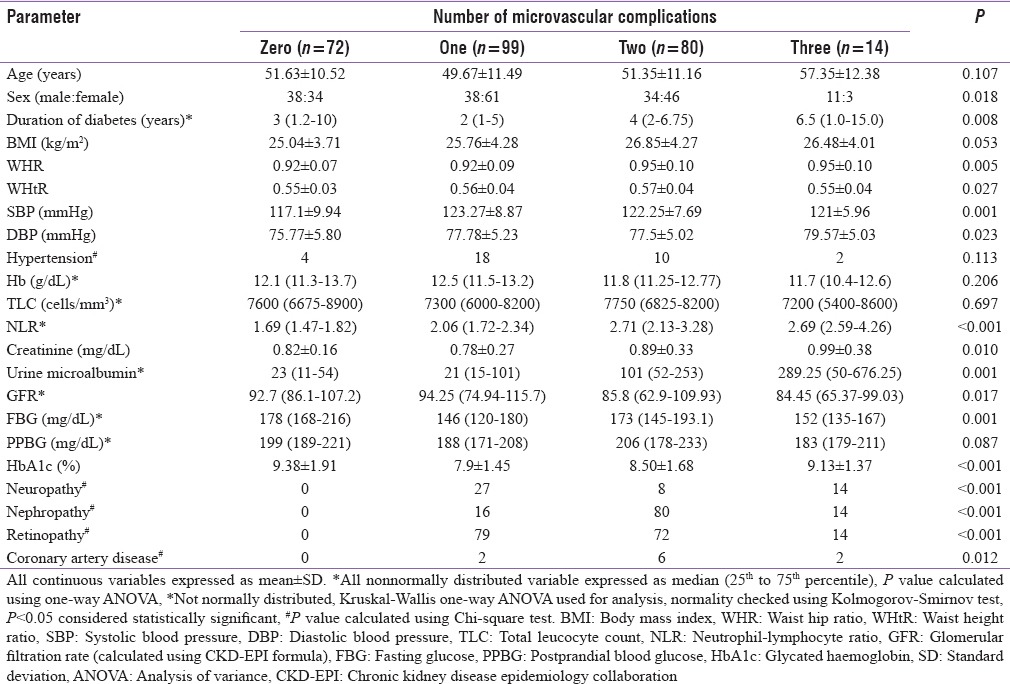
Table 3.
Binary logistic regression showing variables that independently predict the occurrence of any microvascular complication in patients with diabetes
The areas under the receiver operating characteristic curves (AUCs) were constructed to evaluate the predictive values of NLR for predicting the microvascular complications of T2DM. NLR had an AUC of 0.888 (95% CI: 0.848–0.929; P < 0.001) in predicting the occurrence of albuminuria (micro/overt albuminuria) in T2DM [Figure 1]. An NLR of 2.00 had a sensitivity and specificity of 86.4% and 69%, respectively, in predicting albuminuria in T2DM [Figure 1]. NLR had an AUC of 0.708 (95% CI: 0.646–0.771; P < 0.001) in predicting the occurrence of retinopathy (nonproliferative/proliferative/maculopathy) in T2DM [Figure 2]. An NLR of 2.00 had a sensitivity and specificity of 64.2% and 63%, respectively, in predicting retinopathy in T2DM. NLR was not a significant predictor of occurrence of diabetic neuropathy (AUC: 0.572 [0.487–0.657; P = 0.116). NLR was a significant predictor of CAD in this study (AUC: 0.768 [0.599–938]; P = 0.004). NLR of 2.00 had a sensitivity and specificity of 80% and 47.1%, respectively, in predicting CAD.
Figure 1.
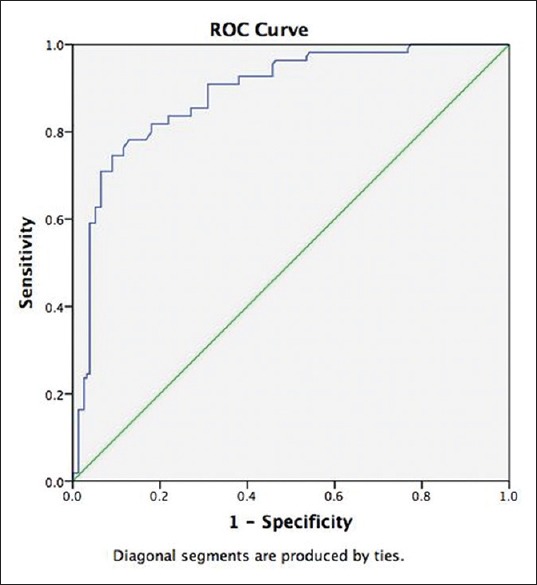
The areas under the receiver operating characteristic curve were constructed to evaluate the predictive values of neutrophil-lymphocyte ratio for predicting albuminuria in type-2 diabetes. Neutrophil-lymphocyte ratio had an areas under the receiver operating characteristic curve of 0.888 (95% CI: 0.848–0.929; P < 0.001) in predicting the occurrence of albuminuria (micro/overt albuminuria) in type-2 diabetes mellitus
Figure 2.
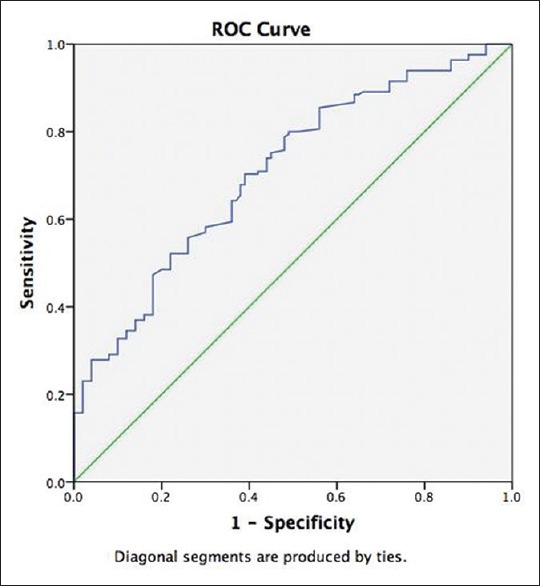
The areas under the receiver operating characteristic curve were constructed to evaluate the predictive values of neutrophil-lymphocyte ratio for predicting retinopathy in type-2 diabetes. Neutrophil-lymphocyte ratio had an areas under the receiver operating characteristic curve of 0.708 (95% CI: 0.646–0.771; P < 0.001) in predicting the occurrence of retinopathy (nonproliferative/proliferative/maculopathy) in type-2 diabetes mellitus
DISCUSSION
Inflammatory molecules and endothelial dysfunction have known to play an important role in the development of insulin resistance, diabetes and associated microvascular and macrovascular complications.[3,23,24,25,26,27] Neutrophilia and relative lymphocytopenia have been shown to be independent markers such as nephropathy, neuropathy, and retinopathy in previous studies from Caucasians.[5,26,27,28,29,30] However, similar data on the utility of NLR among Indians with diabetes are not available. The study highlighted that NLR can be a cheap and reliable predictor of the occurrence of microvascular complications in Indians with T2DM. Patients in the highest NLR quartile had a significantly higher occurrence of nephropathy and retinopathy. Among the microvascular complications, based on the receiver operating characteristic curve analysis, NLR was the best predictor of nephropathy followed by retinopathy.
WBC count and its components are classic inflammatory markers that are commonly available and inexpensive to measure.[31] However, there are multiple biases associated in establishing a diagnosis individually with either WBC, neutrophil, or lymphocyte counts. NLR, on the other hand being a dynamic parameter has a higher predictive value.[28,29,32] NLR has been previously demonstrated to have a prognostic marker role in various malignancies.[33,34,35] Recent studies have also highlighted the importance of NLR as a prognostic marker for vascular diseases and cardiovascular events.[36,37] NLR has been linked with hypertension, the severity of glucose intolerance, and insulin resistance.[38,39,40]
Our results are in concordance with Huang et al. who also found that NLR was significantly higher in diabetic patients with evidence of nephropathy as compared to those without nephropathy.[41] Similarly in another study, Akbas et al.[42] have shown that NLR was significantly increased in patients with increased albuminuria indicating an association between inflammation and endothelial dysfunction in diabetics with nephropathy. Afsar has shown that NLR could be related to diabetic nephropathy and is also correlated as a predictor of end-stage renal disease.[43] Another 3 years follow-up study of diabetic patients, found NLR to be a prognostic indicator for a decline in renal function.[44] Moursy et al. have also shown that NLR values to be significantly higher in diabetic patients with nephropathy (P < 0.001) than those of diabetic patients without any microvascular complications and healthy control subjects.[45] A recently published study in Turkish patients has also shown that NLR significantly correlated with albuminuria.[46] In this study, NLR did not correlate with the occurrence of neuropathy. This is in sharp contrast with a study conducted in Egyptian patients, which has shown that NLR was significantly higher among patients with diabetic neuropathy.[45] The ethnic difference, heterogeneity in a cohort of patients evaluated may explain this difference. Similar to our findings, Yue et al. have also shown that patients with diabetic retinopathy had increased NLR values as compared to diabetics who did not have retinopathy.[47] Ulu et al. in another study demonstrated NLR to be a quick and reliable prognostic marker for diabetic retinopathy and its severity.[48] However in contrast to our results, Ciray et al. found NLR not to be significantly different between patients with or without diabetic retinopathy.[49] Use of ECG for diagnosis of CAD is a limitation of this study, as ECG alone has poor sensitivity and specificity in the diagnosis of CAD.[23]
CONCLUSION
It may me said that NLR may be considered as a predictor and a prognostic risk marker of diabetic nephropathy and retinopathy in Indians. Further research is recommended to shed light on the lack of association of NLR with diabetic neuropathy in Indians. NLR is a simple and easy to calculate. This test is inexpensive and done routinely. In a setup with limited laboratory facilities, NLR can be a cheap effective alternative marker as predictor of diabetes end-organ damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dutta D, Mukhopadhyay S. Intervening at prediabetes stage is critical to controlling the diabetes epidemic among Asian Indians. Indian J Med Res. 2016;143:401–4. doi: 10.4103/0971-5916.184281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta D, Maisnam I, Shrivastava A, Sinha A, Ghosh S, Mukhopadhyay P, et al. Serum Vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138:853–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta D, Mondal SA, Kumar M, Hasanoor Reza AH, Biswas D, Singh P, et al. Serum fetuin-A concentration predicts glycaemic outcomes in people with prediabetes: A prospective study from Eastern India. Diabet Med. 2014;31:1594–9. doi: 10.1111/dme.12539. [DOI] [PubMed] [Google Scholar]
- 4.Dutta D, Choudhuri S, Mondal SA, Maisnam I, Reza AH, Ghosh S, et al. Tumor necrosis factor alpha -238G/A (rs 361525) gene polymorphism predicts progression to type-2 diabetes in an Eastern Indian population with prediabetes. Diabetes Res Clin Pract. 2013;99:e37–41. doi: 10.1016/j.diabres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Rudiger A, Burckhardt OA, Harpes P, Müller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451–4. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Twig G, Afek A, Shamiss A, Derazne E, Tzur D, Gordon B, et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care. 2013;36:276–82. doi: 10.2337/dc11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Yan WH, Li CJ, Wang AP, Dou JT, Mu YM. Elevated white blood cell count is associated with higher risk of glucose metabolism disorders in middle-aged and elderly Chinese people. Int J Environ Res Public Health. 2014;11:5497–509. doi: 10.3390/ijerph110505497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–61. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 9.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 10.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: Atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 11.Choudhuri S, Dutta D, Sen A, Chowdhury IH, Mitra B, Mondal LK, et al. Role of N-ε- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol Vis. 2013;19:100–13. [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhuri S, Chowdhury IH, Das S, Dutta D, Saha A, Sarkar R, et al. Role of NF-κB activation and VEGF gene polymorphisms in VEGF up regulation in non-proliferative and proliferative diabetic retinopathy. Mol Cell Biochem. 2015;405:265–79. doi: 10.1007/s11010-015-2417-z. [DOI] [PubMed] [Google Scholar]
- 13.Choudhuri S, Mandal LK, Paine SK, Sen A, Dutta D, Chowdhury IH, et al. Role of hyperglycemia-mediated erythrocyte redox state alteration in the development of diabetic retinopathy. Retina. 2013;33:207–16. doi: 10.1097/IAE.0b013e318256202e. [DOI] [PubMed] [Google Scholar]
- 14.Mandal LK, Choudhuri S, Dutta D, Mitra B, Kundu S, Chowdhury IH, et al. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can J Diabetes. 2013;37:401–7. doi: 10.1016/j.jcjd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Choudhuri S, Dutta D, Chowdhury IH, Mitra B, Sen A, Mandal LK, et al. Association of hyperglycemia mediated increased advanced glycation and erythrocyte antioxidant enzyme activity in different stages of diabetic retinopathy. Diabetes Res Clin Pract. 2013;100:376–84. doi: 10.1016/j.diabres.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne) 2012;3:170. doi: 10.3389/fendo.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajala MW, Scherer PE. Minireview: The adipocyte – At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 18.Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: A cross-sectional study. Vasc Endovascular Surg. 2011;45:227–31. doi: 10.1177/1538574410396590. [DOI] [PubMed] [Google Scholar]
- 19.Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34:155–9. doi: 10.3109/0886022X.2011.641514. [DOI] [PubMed] [Google Scholar]
- 20.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–83. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grading diabetic retinopathy from stereoscopic color fundus photographs – An extension of the modified Airlie House classification. ETDRS report number 10.Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 22.Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve. 2011;44:340–5. doi: 10.1002/mus.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoodzadeh S, Moazenzadeh M, Rashidinejad H, Sheikhvatan M. Diagnostic performance of electrocardiography in the assessment of significant coronary artery disease and its anatomical size in comparison with coronary angiography. J Res Med Sci. 2011;16:750–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Rivero A, Mora C, Muros M, García J, Herrera H, Navarro-González JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 2009;116:479–92. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- 25.Astrup AS, Tarnow L, Pietraszek L, Schalkwijk CG, Stehouwer CD, Parving HH, et al. Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: Association with mortality and decline of glomerular filtration rate. Diabetes Care. 2008;31:1170–6. doi: 10.2337/dc07-1960. [DOI] [PubMed] [Google Scholar]
- 26.Pitsavos C, Tampourlou M, Panagiotakos DB, Skoumas Y, Chrysohoou C, Nomikos T, et al. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev Diabet Stud. 2007;4:98–104. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–52. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Ulu S, Bucak A, Ulu MS, Ahsen A, Duran A, Yucedag F, et al. Neutrophil-lymphocyte ratio as a new predictive and prognostic factor at the hearing loss of diabetic patients. Eur Arch Otorhinolaryngol. 2014;271:2681–6. doi: 10.1007/s00405-013-2734-3. [DOI] [PubMed] [Google Scholar]
- 31.Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491–7. doi: 10.1016/j.clinre.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–52. doi: 10.1159/000331494. [DOI] [PubMed] [Google Scholar]
- 33.Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–10. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 34.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil: Lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32:1555–61. [PubMed] [Google Scholar]
- 35.Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–8. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 36.Tsai JC, Sheu SH, Chiu HC, Chung FM, Chang DM, Chen MP, et al. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:111–8. doi: 10.1002/dmrr.647. [DOI] [PubMed] [Google Scholar]
- 37.Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–7. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 38.Buyukkaya E, Karakas MF, Karakas E, Akçay AB, Tanboga IH, Kurt M, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. 2014;20:159–63. doi: 10.1177/1076029612459675. [DOI] [PubMed] [Google Scholar]
- 39.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiny A, Bibin YS, Shanthirani CS, Regin BS, Anjana RM, Balasubramanyam M, et al. Association of neutrophil-lymphocyte ratio with glucose intolerance: An indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16:524–30. doi: 10.1089/dia.2013.0264. [DOI] [PubMed] [Google Scholar]
- 41.Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, et al. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf) 2015;82:229–33. doi: 10.1111/cen.12576. [DOI] [PubMed] [Google Scholar]
- 42.Akbas EM, Demirtas L, Ozcicek A, Timuroglu A, Bakirci EM, Hamur H, et al. Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Exp Med. 2014;7:1794–801. [PMC free article] [PubMed] [Google Scholar]
- 43.Afsar B. The relationship between neutrophil lymphocyte ratio with urinary protein and albumin excretion in newly diagnosed patients with type 2 diabetes. Am J Med Sci. 2014;347:217–20. doi: 10.1097/MAJ.0b013e31828365cc. [DOI] [PubMed] [Google Scholar]
- 44.Azab B, Daoud J, Naeem FB, Nasr R, Ross J, Ghimire P, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study) Ren Fail. 2012;34:571–6. doi: 10.3109/0886022X.2012.668741. [DOI] [PubMed] [Google Scholar]
- 45.Moursy EY, Megallaa MH, Mouftah RF, Ahmed SM. Relationship Between neutrophil lymphocyte ratio and microvascular complications in Egyptian patients with type 2 diabetes. Am J Intern Med. 2015;3:250–5. [Google Scholar]
- 46.Kahraman C, Kahraman NK, Aras B, Cosgun S, Gülcan E. The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: A pilot study. Arch Med Sci. 2016;12:571–5. doi: 10.5114/aoms.2016.59931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health. 2015;12:10009–19. doi: 10.3390/ijerph120810009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15:942–7. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 49.Ciray H, Aksoy AH, Ulu N, Cizmecioglu A, Gaipov A, Solak Y. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2015;123:267–71. doi: 10.1055/s-0035-1547257. [DOI] [PubMed] [Google Scholar]


