Abstract
Objectives:
This study aimed to determine the prevalence of immediate posttransplant hyperglycemia and new onset diabetes after renal transplantation (NODAT). It also aims at answering whether posttransplant hyperglycemia per se is a risk factor for future development of NODAT.
Methods:
A retrospective study was conducted among patients undergoing kidney transplantation under a single surgical unit in a tertiary care hospital in the past 5 years. All known patients with diabetes were excluded from the study. Immediate postoperative hyperglycemia was defined as random blood sugar (RBS) ≥200 mg/dl or requirement of insulin. NODAT was defined as fasting plasma glucose ≥126 mg/dl or RBS ≥200 mg/dl or if the patient is receiving therapy for glycemic control at 6 weeks or 3 months posttransplantation.
Results:
The study population included 191 patients. The overall prevalence of posttransplant hyperglycemia and NODAT was 31.4% and 26.7%, respectively. NODAT developed in 28 patients (46.7%) of those who had posttransplant hyperglycemia. Thus, posttransplant hyperglycemia was associated with a fourfold increased risk of NODAT (P = 0.000). Posttransplant hyperglycemia was associated with increased infections (P = 0.04) and prolonged hospital stay (P = 0.0001). Increased age was a significant risk factor for NODAT (P = 0.000), whereas gender, acute rejection episodes, cadaveric transplant, hepatitis C virus status, human leukocyte antigen mismatch, and high calcineurin levels were not significantly associated with the future development of NODAT.
Conclusion:
The significant risk of NODAT posed by posttransplant hyperglycemia makes it prudent to follow up these patients more diligently in a resource-limited setting wherein routine monitoring in all patients is cumbersome.
Keywords: Immediate postoperative hyperglycemia, new onset diabetes after renal transplant, prevalence, renal transplant, risk factors
INTRODUCTION
Renal transplantation offers respite to patients of end-stage renal disease (ESRD) providing riddance from lifelong dialysis sessions. The potential benefits of renal transplantation have to be weighed against the risk of opportunistic infections, development of new onset diabetes, risk of graft rejection, and failure. Nevertheless, transplantation remains the best way out for ESRD patients not only prolonging life but also improves the quality of life. New onset diabetes after renal transplant (NODAT) is a common metabolic complication in the posttransplant scenario which increases the risk for infections and cardiovascular diseases which are the leading cause of mortality in renal allograft recipients. A number of factors contribute to this risk of developing NODAT some of which may be modifiable. Apart from the traditional risk factors common in the nontransplant setting, the use of diabetogenic immunosuppressants (calcineurin inhibitors [CNIs] and steroids), hepatitis C virus (HCV) infection, cytomegalovirus infection, human leukocyte antigen (HLA) matching characteristics, and perioperative hyperglycemia have been implicated to increase the risk of NODAT.
A significant proportion of patients develops hyperglycemia in the postoperative period. The reasons for this postoperative hyperglycemia are multifactorial and include the stress of the surgery, dextrose containing fluid infusions, and high doses of pulse glucocorticoids used during transplant surgery. Some of these patients require insulin for glycemic control and may even be discharged on insulin. Whether this postoperative hyperglycemia is just transient or persists as NODAT is of concern. Although there are many studies on NODAT, very few studies have looked into this risk factor.
Hence, our study aims at answering whether postoperative hyperglycemia per se is a significant risk factor for NODAT.
METHODS
This is a retrospective observational study conducted in a tertiary care center (All India Institute of Medical Sciences, New Delhi) among patients undergoing renal transplant surgery. All such cases operated under a single surgical unit from July 2011 to July 2016 were included in the study. Patients were excluded if they were known diabetics, had undergone multiple organ transplants (combined liver kidney transplant), or if their records of hospitalization or follow-up were not available. Only patients under a single surgical unit were included so as to avoid interunit discrepancies in fluid administration in the postoperative period. All patients received uniform prescription for their fluid management. Immediate posttransplant hyperglycemia was defined as a random blood sugar (RBS) ≥200 mg/dL[1] or requirement of insulin on >2 days whereas the patient was of dextrose-containing fluid infusions (usually from the 4th postoperative day). NODAT was defined as fasting plasma glucose ≥126 mg/dL or RBS ≥200 mg/dL or if the patient was receiving therapy (oral hypoglycemic drugs or insulin) at 6 weeks or 3 months posttransplant.
All patients undergoing renal transplant were admitted in the kidney transplant ward. They underwent blood sugar monitoring by capillary finger-prick method. The monitoring was done hourly on the 1st postoperative day and then 6 hourly from the 2nd postoperative day. The dose of insulin was decided by the treating doctors. The patients on discharge were followed up on outpatient basis twice a week in the transplant clinic. On follow-up, either venous plasma glucose or capillary blood glucose values were recorded.
The hospital records of all the patients were reviewed from the day of admission till discharge. Posttransplant hyperglycemia was defined according to the criteria detailed above. Patient details such as demographic data, native kidney disease, comorbidities, immunosuppressants used, blood sugar values, oral hypoglycaemic agents, or insulin used if any were obtained from the hospital records. From the transplant clinic records, blood glucose values and requirement of insulin or oral hypoglycemic drugs at 6 weeks or 3 months posttransplant were obtained to detect patients with NODAT.
Statistical analysis
The data were analyzed on Stata version 14. Prevalence of NODAT among patients with posttransplant hyperglycemia was calculated as the fraction of patients who had persistently deranged blood glucose values at 6 weeks or 3 months posttransplant among those who had posttransplant hyperglycemia. The association between categorical variables and the risk of NODAT was calculated using Pearson Chi-square method, whereas that between continuous variables was done using a two-sample t-test for skewed data such as age and using Wilcoxon rank-sum (Mann–Whitney) test for nonskewed data. Univariate analysis was used to determine the risk of NODAT in these patients.
Ethical considerations
The study was approved by the Institutional Ethics Committee of the All India Institute of Medical Sciences, New Delhi. All the patients' details were kept confidential, and participant's identity was coded for further analysis.
RESULTS
A total of 191 cases were enrolled into the study with the mean age of 30.13 ± 11.09 years (median age 29 years). Males constituted 80.9% (154 cases) of the study population. The native kidney disease was presumed chronic tubulointerstitial disease in 55 (28.8%), presumed chronic glomerulonephritis 67 (35.1%), and unclassified in 69 (36.1%). Underlying comorbidities were hypertension in 156 cases (81.7%), past history of tuberculosis in 24 (12.6%), and HCV in 17 (8.9%) patients. Living donor transplantation was more common and constituted 88.48% (169 cases) of all transplantation. All patients received CNIs, of which majority were on tacrolimus (89.5%, n = 171), whereas the remaining were on cyclosporine. The mean duration of hospital stay of the study cohort was 16 days.
Of the 191 included cases, sixty patients (31.4%) had posttransplant hyperglycemia (confidence interval [CI]: 24.77–38.05). All these patients had RBS ≥200 mg/dL and received insulin during hospital stay in 49 cases. Among these 60 patients, 55 patients had deranged fasting glucose of ≥126 mg/dL during hospitalization. On follow-up, NODAT was diagnosed in 51 cases among the study population. The prevalence of NODAT in our study was 26.7% (CI 20.37–33.03). At 6 weeks, the prevalence of NODAT was 18.8% (CI 13.25–24.44), whereas at 3 months, it was 24.1% (CI 17.96–30.20).
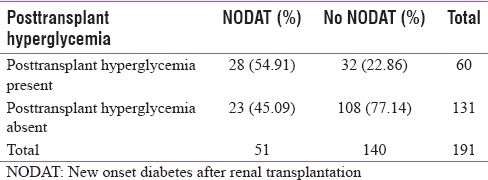
Out of the sixty patients with posttransplant hyperglycemia, 28 patients (46.7%) developed NODAT on follow-up, whereas 23 patients out of 131 patients (17.6%) without posttransplant hyperglycemia developed NODAT [Table 1]. The presence of posttransplant hyperglycemia was associated with a fourfold increase in the risk of NODAT (P < 0.001). At 6 weeks, 27 patients (45%) with posttransplant hyperglycemia developed NODAT, whereas 9 patients (6.8%) without posttransplant hyperglycemia had NODAT. At this time period, the odds ratio of developing NODAT among patients with posttransplant hyperglycemia is 11.09 (P < 0.001). At 3 months, only 24 cases with posttransplant hyperglycemia had NODAT, whereas 22 cases with NODAT had no antecedent history of hyperglycemia. Even at this time period, history of posttransplant hyperglycemia increased the risk of developing NODAT by 3.3 fold (P = 0.001).
Table 1.
Relationship of posttransplant hyperglycemia to new onset diabetes after renal transplantation at 6 weeks or 3 months
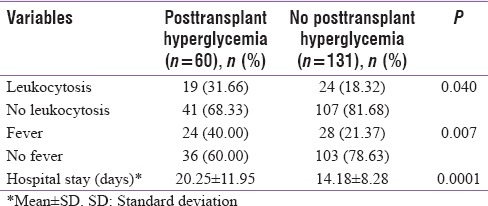
Patients with posttransplant hyperglycemia had a prolonged hospital stay [Table 2] with a mean duration of 20.25 ± 11.95 days as compared to 14.18 ± 8.28 days in cases without posttransplant hyperglycemia, and the difference was statistically significant (P < 0.001). Similarly, these patients had a higher prevalence of leukocytosis (P - 0.04) and fever (P - 0.007), probably reflecting the increased risk of infections in this subgroup of patients.
Table 2.
Posttransplant hyperglycemia and in-hospital outcomes
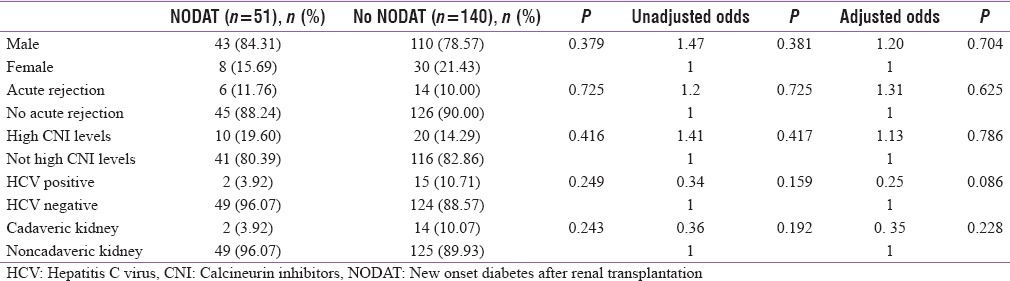
The median age of population with NODAT was 35 years, whereas that without NODAT was 26 years. Thus, increased age was a significant risk factor for NODAT (P = 0.000) [Table 3] with every 1 year increase in age increasing the risk of NDAT by 1.06 times. When a cutoff of 45 years was considered, patients older than 45 years had 3.55 times increased risk of NODAT (P - 0.010). However, gender, history of acute rejection episodes, cadaveric kidney transplantation, HCV status, high CNI levels, degree of HLA mismatch, and tacrolimus dose were not significant risk factors for developing NODAT in our study population [Table 4].
Table 3.
Analysis of risk factors of new onset diabetes after renal transplantation
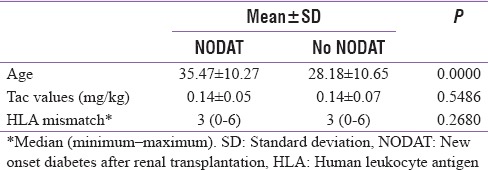
Table 4.
Risk factors of new onset diabetes after renal transplantation
DISCUSSION
Our study aimed at finding out the prevalence of hyperglycemia in postoperative period and whether it is a significant risk factor for NODAT. The prevalence of immediate posttransplant hyperglycemia in our study was 31.4%. Discrepancies in the reported prevalence of posttransplant hyperglycemia are due to different definitions used to define it in literature and the absence of diagnostic criteria for the same. One study[1] reported the incidence of immediate posttransplant hyperglycemia as high as 87%. However, they included even patients with a single reading of high blood sugars. Another study[2] reported the prevalence as 43% when hyperglycemia was diagnosed based on continuous glucose monitoring recordings. Yet, another study[3] had reported the prevalence as 59.78% when defining early hyperglycemia as blood glucose levels as >126 mg/dl in the 1st week after transplantation. Our study defined hyperglycemia using routine finger-prick whole blood glucose method, which is the most commonly used method in practice rather than CGM[2] as used only in research settings.
The prevalence of NODAT in our study was 26.7% taking into account the deranged glycemic values at either 6 weeks or 3 months. The prevalence of NODAT at 6 weeks and 3 months posttransplant was 18.8% and 24.1%, respectively. The prevalence of NODAT varies from 2% to 50% according to the definition used,[4] and the time frame at which it is diagnosed. Here again, varying definitions for NODAT and difference in the time of screening are responsible for the wide variation in the reported prevalence of NODAT. After introduction of the consensus definition and the WHO–ADA guidelines,[5,6] the reported prevalence has been <20%[2,7,8] at 1 year of follow-up. In an Indian study,[9] the overall prevalence of NODAT has been reported as 33.89%. The higher prevalence of NODAT probably represents the predisposition of patients from the Indian subcontinent to develop diabetes in general.
Patients with posttransplant hyperglycemia in our study had a fourfold higher risk of developing NODAT. Similar results were seen in the study[1] by Chakkera et al. in 200 posttransplant patients in Arizona. Moreover, a study from Chile[3] reported 5.4 times greater chance of developing diabetes in patients with early hyperglycemia. Another study from France reported that the first posttransplantation capillary blood glucose measurement and fasting blood glucose on 1st day tended to be higher in patients who developed diabetes 3 months later.[2] A Belgian study[10] carried out in 64 patients demonstrated that a normal OGTT on the 5th postoperative day was associated with a significantly decreased risk of NODAT at 3 months. These studies reaffirm the fact that postoperative hyperglycemia is a significant risk factor for NODAT. The shortcomings of the earlier studies include the use of impractical methods to detect posttransplant hyperglycemia (OGTT or CGM) and the use of a single blood glucose value to diagnose posttransplant hyperglycemia.
NODAT was significantly associated with an increase in age[7,11,12] (odd ratio of 3.55 at age >45 years) as is seen in previous studies. A study by Cosio et al.[12] in 2078 patients showed that patients older than 45 years were 2.9 times increased risk of developing diabetes. Every 10-year increase in age leads to 1.5 fold increased risk of diabetes.[13] However, other factors such as CNI levels, acute rejection, cadaveric kidney, and HLA mismatch[3,7,11,13,14,15] were not found significant in our study. The smaller number of HCV-infected patients and cadaveric transplants done may be responsible for this discrepancy in results. Furthermore, as most of the patients were on tacrolimus and there was no comparable group with cyclosporine or mTOR inhibitor, the relative importance of tacrolimus in the causation of NODAT cannot be commented upon from our study. Patients with posttransplant hyperglycemia had increased infections (P - 0.040) and a prolonged hospital stay (0.0001). This would have implications on cost of therapy and also on the renal allograft and patient survival.
The strengths of this study include decent study population size, predominantly younger study population, follow-up at 6 weeks and 3 months, and standardized definition of inpatient hyperglycemia using capillary whole blood glucose after 4th postoperative day to eliminate the contribution of dextrose containing fluids or stress hyperglycemia. The limitations of this study are the inherent problems of a retrospective study and a relatively shorter follow-up of 3 months.
This study highlights that strong association between inpatient hyperglycemia with subsequent development of NODAT. Hence, this cohort of patients with posttransplant hyperglycemia must be followed up more closely, especially in resource-limited settings. All factors which exacerbate hyperglycemia must be tackled more aggressively in this population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the help of staff in Record Section and Transplant Clinic of All India Institute of Medical Sciences for maintaining and providing medical records of the patients. We also acknowledge Ms. Kalaivani and Mr. Ashish, Biostatisticians, for helping in analysis of the data.
REFERENCES
- 1.Chakkera HA, Knowler WC, Devarapalli Y, Weil EJ, Heilman RL, Dueck A, et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol. 2010;5:1669–75. doi: 10.2215/CJN.09481209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wojtusciszyn A, Mourad G, Bringer J, Renard E. Continuous glucose monitoring after kidney transplantation in non-diabetic patients: Early hyperglycaemia is frequent and may herald post-transplantation diabetes mellitus and graft failure. Diabetes Metab. 2013;39:404–10. doi: 10.1016/j.diabet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado F, Tapia G, Ardiles L. Early hyperglycemia: A risk factor for posttransplant diabetes mellitus among renal transplant recipients. Transplant Proc. 2009;41:2664–7. doi: 10.1016/j.transproceed.2009.06.133. [DOI] [PubMed] [Google Scholar]
- 4.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC, et al. Posttransplantation diabetes: A systematic review of the literature. Diabetes Care. 2002;25:583–92. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am J Transplant. 2014;14:1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–85. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Khalili N, Rostami Z, Kalantar E, Einollahi B. Hyperglycemia after renal transplantation: Frequency and risk factors. Nephrourol Mon. 2013;5:753–7. doi: 10.5812/numonthly.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel DD, Modi KP, Patel AK, Chaudhary V. New onset of diabetes mellitus in Indian renal transplant recipient – A retrospective study. Int J Pharm Pharm Sci. 2015;7:228–32. [Google Scholar]
- 10.Kuypers DR, Claes K, Bammens B, Evenepoel P, Vanrenterghem Y. Early clinical assessment of glucose metabolism in renal allograft recipients: Diagnosis and prediction of post-transplant diabetes mellitus (PTDM) Nephrol Dial Transplant. 2008;23:2033–42. doi: 10.1093/ndt/gfm875. [DOI] [PubMed] [Google Scholar]
- 11.Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, et al. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation. 1987;44:376–81. doi: 10.1097/00007890-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM, et al. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440–6. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 13.Gourishankar S, Jhangri GS, Tonelli M, Wales LH, Cockfield SM. Development of diabetes mellitus following kidney transplantation: A Canadian experience. Am J Transplant. 2004;4:1876–82. doi: 10.1111/j.1600-6143.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonato V, Barni R, Cataldo D, Collini A, Ruggieri G, De Bartolomeis C, et al. Analysis of posttransplant diabetes mellitus prevalence in a population of kidney transplant recipients. Transplant Proc. 2008;40:1888–90. doi: 10.1016/j.transproceed.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigo E, Fernández-Fresnedo G, Valero R, Ruiz JC, Piñera C, Palomar R, et al. New-onset diabetes after kidney transplantation: Risk factors. J Am Soc Nephrol. 2006;17:S291–5. doi: 10.1681/ASN.2006080929. [DOI] [PubMed] [Google Scholar]


