Abstract
Context:
Primary hypothyroidism has been thought of as an inflammatory condition characterized by raised levels of cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Depression is also well known to occur in hypothyroidism. Depression is also characterized by elevated inflammatory cytokines. We planned to study whether cytokines play an important part in linking these two conditions.
Objectives:
(1) To know the prevalence of depression in overt hypothyroidism due to autoimmune thyroid disease. (2) To correlate the levels of inflammatory markers with the occurrence of depression. (3) To study the effect of levothyroxine on inflammatory markers and depression.
Materials and Methods:
In this longitudinal, case–controlled study, 33 patients with autoimmune hypothyroidism (thyroid-stimulating hormone >10 uIU/ml) were included with 33 age-, sex-, and body max index-matched healthy controls. Individuals were tested for Serum TNF-α, IL-6, high-sensitivity-CRP (hs-CRP). They were assessed for depression using Montgomery Asberg Depression Rating Scale (MADRS) and World Health Organization Quality of Life (QOL) Scale. Patients received L Thyroxine titrated to achieve euthyroidism and were reassessed for inflammatory markers and cognitive dysfunction.
Results:
Nineteen patients (57%) had mild to moderate depression (MADRS >11). After 6 months of treatment, eight patients (42%) had remission of depression with significant improvement in QOL scores (P < 0.05). TNF-α, IL-6, and hs-CRP were significantly elevated in patients compared with controls and reduced with therapy but did not reach baseline as controls. The change in inflammatory markers correlated with improvement in QOL scores in social and environmental domains (P < 0.01).
Conclusions:
Primary autoimmune hypothyroidism is an inflammatory state characterized by elevated cytokines which decline with LT4 therapy. It is associated with depression and poor quality of life. Treatment of hypothyroidism results in alleviation of depression in the majority of patients. Similarly, patients with mild to moderate depression should be tested for hypothyroidism as simple treatment may ameliorate their depression and improves MADRS score and QOL.
Keywords: Depression, high-sensitivity C-reactive protein, hypothyroidism, interleukin-6, Montgomery Asberg Depression Rating Scale, tumor necrosis factor alpha, World Health Organization Quality of Life Scale
INTRODUCTION
Primary hypothyroidism is a commonly encountered clinical problem. Treatment is indicated if thyroid-stimulating hormone (TSH) is more than 10 uIU/ml.[1] The most common cause of hypothyroidism is autoimmune thyroiditis.
Hypothyroidism has been thought of as an inflammatory condition characterized by raised levels of inflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL 6), and tumor necrosis factor-alpha (TNF-α).[2] These inflammatory markers may have a role in the pathogenesis of many complications associated with hypothyroidism by causing endothelial dysfunction, smooth muscle cell proliferation and migration, and recruiting and activating inflammatory cells.[3] In addition, they induce the production of interferon-gamma and also mediate apoptosis.
Depression and cognitive impairment are also well known to occur in overt hypothyroidism.
According to the review by Boswell et al.[4] the prevalence of depressive symptoms in hypothyroidism was nearly 50%. Clinical depression occurs in more than 40% of people suffering from hypothyroidism.[2]
Interestingly, elevated titers of antithyroid antibodies have been documented in patients with clinical depression. The prevalence of antithyroid antibody positivity in depressed patients is 20% as opposed to 5%–10% in general population.[5,6,7] The finding that depression often co-exists with autoimmune subclinical thyroiditis suggests that depression may also be caused by alterations in the immune system or vice versa or that in fact, it could be an autoimmune disorder itself.[8] Patients with depression have been found to have increased biomarkers of inflammation, including innate immune cytokines, acute-phase proteins, chemokines, and adhesion molecules.[9] It is a possibility that depression could belong to the spectrum of inflammatory and degenerative disorders.[10] Similarly, hypothyroidism is a proinflammatory state characterized by elevated cytokines and multiple depressive symptoms.
This brings in forefront several questions – Could depression and hypothyroidism be linked? Are cytokines the missing link between the two? Can treatment with levothyroxine improve depression and reduce the level of cytokines? There are no Indian studies about the association of both thyroid function and inflammatory markers with depression. To seek an answer to these questions, this study was planned.
Aims and objectives
The aim of this study was to estimate the prevalence of depression in patients of hypothyroidism, to study the inflammatory markers in hypothyroidism and correlate them with the occurrence of depression and to study the effect of levothyroxine treatment on inflammatory markers and resolution of depression.
MATERIALS AND METHODS
This prospective open label case–control study was carried out at Endocrinology and Psychiatry Departments in a tertiary care hospital in Mumbai, after approval from the Institutional Ethics Committee. Patients visiting the thyroid clinic who were diagnosed to have antithyroid peroxidase (TPO) positive and TSH >10 m IU/ml (newly diagnosed and treatment naïve) qualified for the study if they were 18–45 years old, after informed consent.
The exclusion criteria were:
Patients with any acute or chronic systemic disease
Any autoimmune disease
Any malignant condition
Alcoholism
Tobacco use
Hypertension
Diabetes mellitus
Renal failure
Underlying known cardiac disorder
Pregnancy
History of previous thyroxin therapy or antidepressant medication.
These stringent exclusion criteria were used to avoid the source of confounding factors which could influence the clinical or laboratory data.
We included 33 consecutive patients with overt hypothyroidism, and 33 age-, sex-, and body max index (BMI)-matched volunteers as controls. The patients underwent a detailed clinical evaluation, followed by a collection of a venous blood sample obtained after an overnight fast of 10 h. Samples were taken at baseline and after 6 months of levothyroxine treatment.
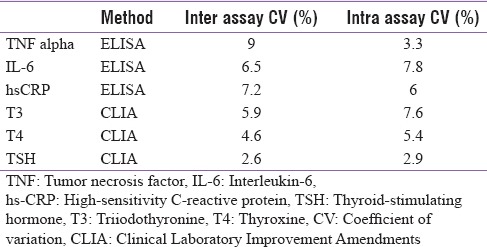
The laboratory evaluation included measurements of thyroid function tests, anti-TPO antibody, serum TNF-α, IL-6, and high-sensitivity-CRP (hs-CRP). The hormonal evaluation was performed by chemiluminometric assay on the Advia Centaur platform manufactured by Siemens and the inflammatory markers by enzyme-linked immunosorbent assay kit from Krishgen Biosystems. The details of the kits and inter- and intra-assay coefficient of variations are given in Table 1.
Table 1.
Assays used and their coefficient of variation
The blood samples from controls were taken at baseline to get normative values of inflammatory markers. They did not receive any treatment.
Psychiatric evaluation
Patients were assessed for depression clinically using a semi-structured pro forma. This part of the study was carried out by a qualified and experienced psychiatrist who was unaware of the patient's thyroid status. Montgomery Asberg Depression Rating Scale (MADRS) was used to rate the severity of depression. This scale was validated in the local language. Participants were also asked to fill a questionnaire in the language that the participant best understood to assess the quality of life, i.e., the World Health Organization Quality of Life scale (WHO QOL).
MADRS is a very simple scale for even nonpsychiatric physicians to use. This scale assigns a score from 0 to 60. Score <10 is considered normal. A score of 10–35 is suggestive of mild to moderate depression, whereas a score more than 35 denotes severe depression.[11] This scale consistently has the highest Cronbach's alpha levels reaching 0.92.[12]
The quality of life was assessed by the WHO quality of life scale.[13] This scale has a very broad focus beyond traditional health indicators to measures the actual impact of disease on impairment of daily activities and behavior in relation to the social background, thus actually giving the “the missing measurement in health.”
This scale has 4 domains, physical, psychological, social, and environmental. Each domain has a raw score which is then expressed as a transformed score ranging from 0 to 100.
Treatment
Thirty-three patients with hypothyroidism received treatment with L-thyroxin with the standard dose of 1.6 μ/kg bodyweight. Titration of the dose was done at 6 weeks and 3 months to achieve euthyroid status. After 6 months, patients were also reassessed and scores on depression scale (MADRS) and WHO QOL were recorded.
Statistical analysis
Data were entered into Microsoft Excel and analyzed using SPSS statistical software18.0 (SPSS Inc., Chicago, IL, USA). Demographic data were analyzed using descriptive statistics. Means were calculated for inflammatory markers. The mean values of pre- and post-treatment inflammatory markers were compared using paired t-test after evaluating assumption of normality. Furthermore, the mean values of inflammatory markers of controls (normal healthy volunteers) were compared with post-treatment mean values of inflammatory markers in treatment arm using independent t-test.
The proportion of depression among overt hypothyroidism before levothyroxine treatment and after treatment was compared using Chi-square test. The change in the inflammatory markers and depression scores and WHO QOL score was compared with the baseline and the control group. To find a correlation between inflammatory markers and depression prediction models were built using simple linear regression.
RESULTS
In this study, we included 33 patients, 28 female (85%) and 5 male patients (15%) with primary hypothyroidism, and 33 age-, sex-, and BMI-matched healthy controls. There was no significant difference between the cases and controls at the baseline except for the levels of TSH and anti-TPO antibody levels which were significantly elevated in the cases [Table 2].
Table 2.
Baseline characteristics: Group statistics (mean±standard deviation)
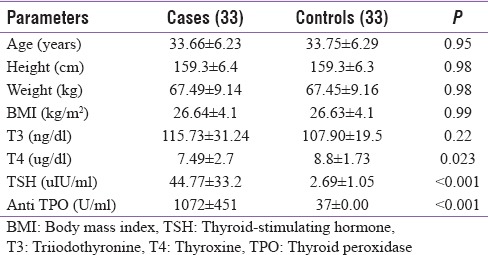
Table 3 shows the levels of inflammatory markers in cases pre- and post-treatment and controls. The markers are significantly elevated in cases compared to controls. After the treatment with levothyroxine for 6 months and achieving euthyroidism all the markers reduced significantly. Figure 1 shows the change in the profile of inflammatory markers.
Table 3.
Effect of treatment with levothyroxine on inflammatory markers (n=33)
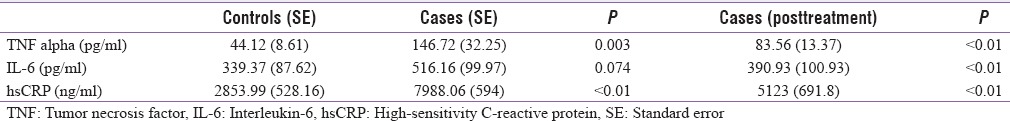
Figure 1.
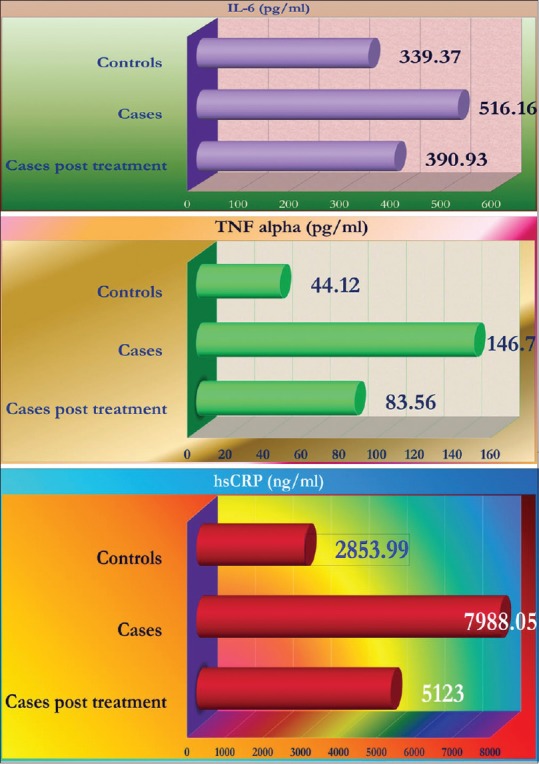
Inflammatory markers in cases and controls, before and after treatment
Table 4 and Figure 2 show the comparison of MADRS score and WHO quality of life scores in pre- and post-treatment groups. The MADRS score declined suggesting improvement in overall depressive symptoms whereas WHO QOL analysis reveals that all the domains of quality of life were positively impacted by the levothyroxine treatment.
Table 4.
Montgomery Asberg Depression Rating Scale and World Health Organization Quality of Life scale
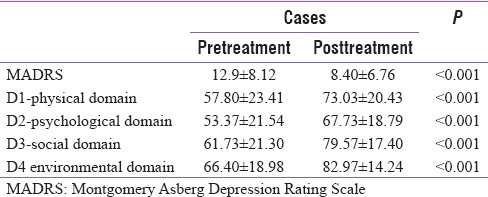
Figure 2.
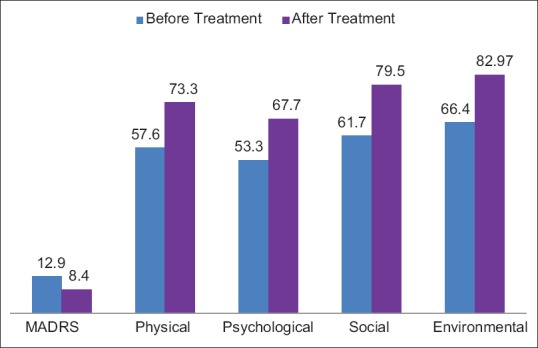
Montgomery Asberg Depression Rating Scale and World Health Organization Quality of Life scale - before and after treatment
Nineteen patients (57%) had mild to moderate depression (MADRS >11). After 6 months of treatment eight patients (42%) had remission of depression. With treatment, quality of life improved significantly in physical, psychological, social, and environmental domains (P < 0.05) [Table 4].
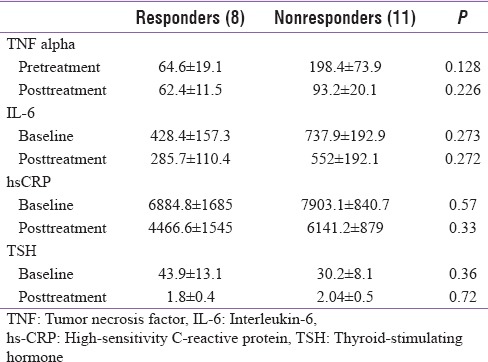
Nineteen patients of hypothyroidism had mild to moderate depression on MADRS. Patients who had remission of depression were grouped as responders and compared with nonresponders. The nonresponders had elevated levels of inflammatory markers compared to responders; however, this difference is not statistically significant due to a small number [Figures 4, 5 and Table 5].
Figure 4.
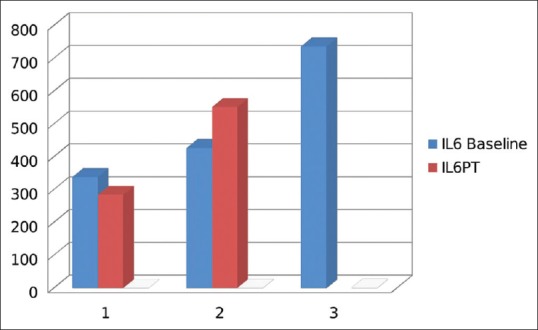
Comparison of inflammatory markers in responders and nonresponders: Interleukin-6
Figure 5.
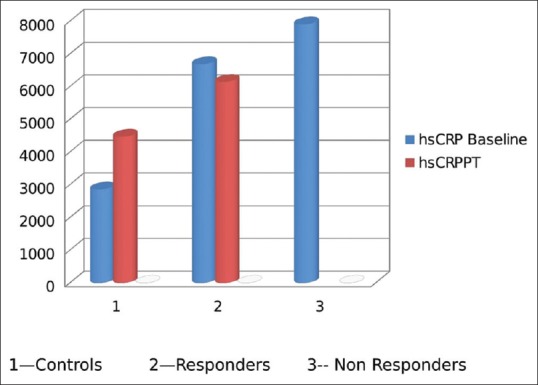
Comparison of inflammatory markers in responders and nonresponders: High-sensitivity C-reactive protein
Table 5.
Comparison of inflammatory markers in responders and nonresponders to levothyroxine therapy
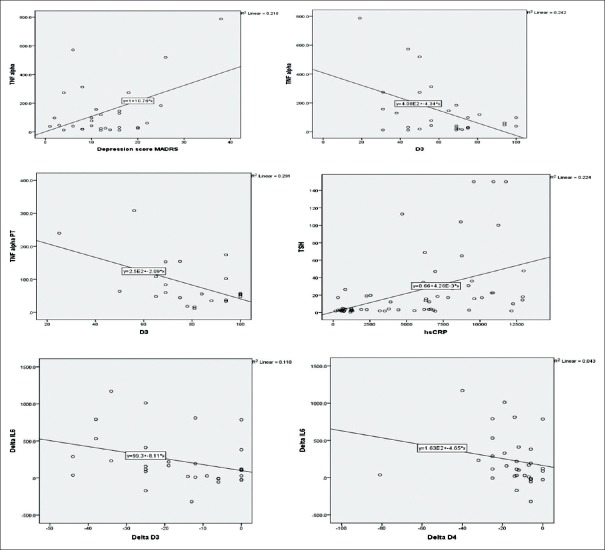
To find out the correlation between inflammatory markers and depression prediction models were built using simple linear regression. As shown in Figure 3, levels of TNF-α had positive correlation with baseline MADRS score and lower scores in the social domain of quality of life scores. The decline in TNF-α posttreatment correlated with improvement in score in social domain. Elevated TSH at baseline significantly correlates with elevated CRP. Change in the levels of IL 6 significantly correlated with improvement in social and environmental domains.
Figure 3.
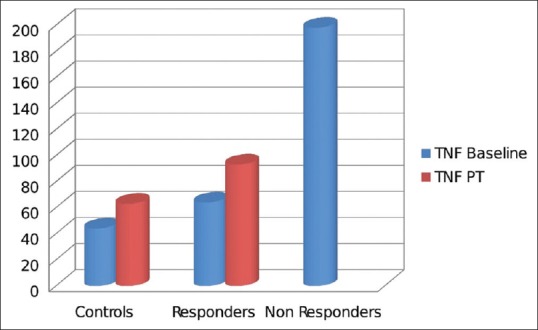
Comparison of inflammatory markers in responders and nonresponders: Tumor necrosis factor-alpha
Thus, there is a positive correlation of hypothyroidism with elevated inflammatory markers and higher MADRS scores. It negatively impacts the quality of life. Treatment with levothyroxine improves the functional scores which are most obvious in the social and environmental domains [Figure 6].
Figure 6.
Correlation of inflammatory markers with Montgomery Asberg Depression Rating Scale and World Health Organization Quality of Life
DISCUSSION
In this study, the prevalence of mild to moderate depression among patients with overt hypothyroidism was 57% at the baseline. After 6 months of treatment with levothyroxine and achieving euthyroidism, 42% of the cases had remission of depression.
The levels of inflammatory markers such as TNF-α, IL 6, hs-CRP were significantly elevated in cases of hypothyroidism compared to controls at baseline. Levothyroxine therapy resulted in significant decline in the levels of these inflammatory cytokines. However, the levels remained elevated as compared to healthy controls.
It was also observed that the levels of inflammatory markers were higher in those who did not have remission of depression, but the difference was not statistically significant, which could be due to limited sample size.
With treatment, the quality of life improved significantly in physical, psychological, social, and environmental domains (P < 0.05)
The relationship between thyroid dysfunction and psychiatric disorders has long been recognized.
Historically, it was described more than 200 years ago. Parry in 1825 reported an increased incidence of “nervous affectations” in thyroid disorders., Gull in 1873 showed the relation between myxedema and psychosis. It was confirmed in 1888 by the Committee of the Clinical Society. In 1949, Asher coined the term “myxedema madness” to describe the psychiatric status of subjects with hypothyroidism.
In last 100 years, this subject has been extensively evaluated and we have gained new insights in the association of these two conditions. The effects of hypothyroidism on disturbances in emotion and cognition are well known.
These abnormalities are generally reversible with adequate thyroid treatment.
In this study, hypothyroid patients frequently demonstrated features of depression, cognitive dysfunction, apathy, and there was significant impact of this condition on the quality of life.
These findings were consistent with earlier studies by Türemen et al., Ramazan et al., and Tuzcu et al.[14,15,16]
After treatment with levothyroxine and achievement of euthyroidism the levels of inflammatory markers decreased significantly. Similar observations were noted by Kebapcilar et al.[17]
Marfella et al. observed a reduction in CRP in levothyroxine treated patients, but CRP was still significantly increased compared to the control group. He further observed significantly lower plasma TNF-α, IL-6, and IL-18 levels in treated with subclinical hypothyroidism compared to controls.[18]
Guclu et al. demonstrated a significant decrease in serum IL-12, a statistically nonsignificant decrease in interferon-gamma (INF-γ) and no change in serum IL-2 or IL-4 in patients with hypothyroidism due to Hashimoto's thyroiditis that underwent 12 weeks of levothyroxine treatment.[19]
Similar findings were replicated in the recent study by Marchiori et al. in which proinflammatory and anti-inflammatory cytokines before and after thyroxin treatment were studied. There was a significant decrease in the levels of IL-1, IL-6, INF-γ, and TNF-α and with a significant increase in IL-10 (anti-inflammatory cytokine) with levothyroxine therapy.[20]
In the current study, the levels of TNF-α, IL-6, and hs-CRP all showed significant decline (P < 0.01) after achieving euthyroidism in 6 months with levothyroxine therapy. However, the levels in the patients remained elevated as compared to the normal control population.
MADRS score of 11 was used as a cutoff to define mild to moderate depression.[11] With this cutoff 19 patients (57%) were recognized to have depression. The WHO quality of life scale was used at baseline and after 6 months on follow-up. There was significant improvement in the quality of life, after levothyroxine treatment. After 6 months of treatment eight patients (42%) had remission of depression. The quality of life improved significantly in physical, psychological, social, and environmental domains (P < 0.05).
Our study has several strengths. We studied patients with autoimmune hypothyroidism who constitute a majority of cases in an outpatient setting. The patients did not have obvious symptoms of depression but on screening a large number of them had mild to moderate depression. Thus we could study the impact of disease in its subclinical stage and how it adversely impacted the quality of life, which often goes unnoticed.
We could also document objectively that patients with primary hypothyroidism have elevated levels of cytokines and with appropriate treatment these levels also come down significantly as does the depression score, which parallels the improvement in quality of life. The study could provide consistent scientific evidence for depressive disorders and thyroid dysfunction. These findings may give novel insights in the pathogenesis of depression in general and cognitive affection in particular in patients with hypothyroidism.
The limitations of our study are the number of patients and duration of follow-up.
The role of antidepressant therapy in nonresponders and assessment of its impact on further improvement in quality of life remains an area for future research.
CONCLUSIONS
Primary autoimmune hypothyroidism is an inflammatory state characterized by elevated cytokines. A possible role for thyroid autoimmunity and inflammatory markers in the pathogenesis of depression can be elucidated. It may be associated with depression and poor quality of life. Levothyroxine therapy leads to significant decline in the level of inflammatory cytokines.
Levothyroxine therapy significantly improves the MADRS scores. It significantly improves the quality of life. Physical, psychological, social, and environmental domains show significant positive impact of the therapy. A patient with mild to moderate depression should have thyroid functions evaluated as achieving euthyroidism improves the depression and quality of life. The impact of antidepressant therapy on nonresponders remains an area for future research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Larry Jameson J, Anthony Weetman A. Harrison's Principles of Internal Medicine. 18th ed. USA: McGraw Hill Publishers; 2012. Disorders of thyroid gland; p. 2918. [Google Scholar]
- 2.Cleare AJ, McGregor A, O'Keane V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin Endocrinol (Oxf) 1995;43:713–9. doi: 10.1111/j.1365-2265.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 3.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–22. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 4.Boswell EB, Anfinson TH, Nemeroff CB. Depression associated with endocrine disorders. In: Robertson MM, Katona CL, editors. Depression and Physical Illness. England: Wiley, Chichester; 1997. pp. 256–92. [Google Scholar]
- 5.Wolkowitz OM, Rothschild AJ. Psychoneuroendocrinology: The Scientific Basis of Clinical Practice. 1st ed. Washington, DC, USA: American Psychiatric; 2003. [Google Scholar]
- 6.Nemeroff CB, Simon JS, Haggerty JJ, Jr, Evans DL. Antithyroid antibodies in depressed patients. Am J Psychiatry. 1985;142:840–3. doi: 10.1176/ajp.142.7.840. [DOI] [PubMed] [Google Scholar]
- 7.Joffe RT. Antithyroid antibodies in major depression. Acta Psychiatr Scand. 1987;76:598–9. doi: 10.1111/j.1600-0447.1987.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 8.Haggerty JJ, Jr, Evans DL, Golden RN, Pedersen CA, Simon JS, Nemeroff CB, et al. The presence of antithyroid antibodies in patients with affective and nonaffective psychiatric disorders. Biol Psychiatry. 1990;27:51–60. doi: 10.1016/0006-3223(90)90019-x. [DOI] [PubMed] [Google Scholar]
- 9.Anisman H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 10.Maes M, Bosmans E, Meltzer HY, Scharpé S, Suy E. Department of psychiatry, university hospitals of Cleveland, OH 44106. Am J Psychiatry. 1993;150:1189–93. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 12.Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D, et al. The montgomery asberg and the hamilton ratings of depression: A comparison of measures. Eur Neuropsychopharmacol. 2006;16:601–11. doi: 10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Last accessed on 2017 Mar 03]. Available from: http://www.who.int/mental_health/media/en/76.pdf .
- 14.Türemen EE, Çetinarslan B, Şahin T, Cantürk Z, Tarkun İ. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocr J. 2011;58:349–54. doi: 10.1507/endocrj.k10e-333. [DOI] [PubMed] [Google Scholar]
- 15.Ramazan G, Akbey E, Sezer K. Insulin resistance and cardiovascular risk factors in patients with mild and severe subclinical hypothyroidism. The Endocrinologist. 2010;3:128–30. [Google Scholar]
- 16.Tuzcu A, Bahceci M, Gokalp D, Tuzun Y, Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive c-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J. 2005;52:89–94. doi: 10.1507/endocrj.52.89. [DOI] [PubMed] [Google Scholar]
- 17.Kebapcilar L, Akinci B, Bayraktar F, Comlekci A, Solak A, Demir T, et al. Subclinical hypothyroidism could be regarded as benign condition in oxidative stress but not in atherosclerosis. Eur Congr Endocrinol. 2006;11:232. [Google Scholar]
- 18.Marfella R, Ferraraccio F, Rizzo MR, Portoghese M, Barbieri M, Basilio C, et al. Innate immune activity in plaque of patients with untreated and L-thyroxine-treated subclinical hypothyroidism. J Clin Endocrinol Metab. 2011;96:1015–20. doi: 10.1210/jc.2010-1382. [DOI] [PubMed] [Google Scholar]
- 19.Guclu F, Ozmen B, Kirmaz C, Kafesciler SO, Degirmenci PB, Taneli F, et al. Down-regulation of the auto-aggressive processes in patients with hypothyroid Hashimoto's thyroiditis following substitutive treatment with L-thyroxine. Eur Cytokine Netw. 2009;20:27–32. doi: 10.1684/ecn.2009.0147. [DOI] [PubMed] [Google Scholar]
- 20.Marchiori RC, Pereira LA, Naujorks AA, Rovaris DL, Meinerz DF, Duarte MM, et al. Improvement of blood inflammatory marker levels in patients with hypothyroidism under levothyroxine treatment. BMC Endocr Disord. 2015;15:32. doi: 10.1186/s12902-015-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]