Abstract
Diagnostic delay is common in most respiratory diseases, particularly in bronchiectasis. However, sex bias in diagnostic delay has not been studied to date. Objective: Assessment of diagnostic delay in bronchiectasis by sex. Methods: The Spanish Historical Registry of Bronchiectasis recruited adults diagnosed with bronchiectasis from 2002 to 2011 in 36 centres in Spain. From a total of 2113 patients registered we studied 2099, of whom 1125 (53.6%) were women. Results: No differences were found for sex or age (61.0 ± 20.6, p = 0.88) or for localization of bronchiectasis (p = 0.31). Bronchiectasis of unknown aetiology and secondary to asthma, childhood infections and tuberculosis was more common in women (all ps < 0.05). More men than women were chronic obstructive pulmonary disease-related bronchiectasis and colonized by Haemophilus influenzae (p < 0.001 for both). Onset of symptoms was earlier in women. The diagnostic delay for women with bronchiectasis was 2.1 years more than for men (p = 0.001). Discussion: We recorded a substantial delay in the diagnosis of bronchiectasis. This delay was significantly longer in women than in men (>2 years). Independent factors associated with this sex bias were age at onset of symptoms, smoking history, daily expectoration and reduced lung function.
Keywords: Bronchiectasis, diagnostic delay, gender bias, gender gap, sex bias
Introduction
Bronchiectasis is a condition involving irreversible dilation of the bronchi and bronchioles as a consequence of the destruction of the elastic and muscular component of the bronchial wall. Bronchiectasis can be the outcome of many disorders that harm bronchial defence mechanisms and produce damage including alteration/imbalance in the mucociliary system, retention of secretions, and bacterial colonization. Bronchial colonization, in turn, results in release of inflammatory mediators that worsen ciliary motility and cause recurrent infections, thus closing the vicious circle that perpetuates the disease and leads to tissue damage.1
Data on the prevalence of bronchiectasis in the general population are scanty, with estimates varying from 25 to 272 cases per 100,000 individuals. Most of this variability is likely explained by age.2–5 However, prevalence is often underestimated for various reasons: the difficulty in differentiating between bronchiectasis and other chronic respiratory diseases, the fact that symptoms are progressive and unspecific during the early stages of the condition (bronchiectasis is clinically evident when the disease is exacerbated or very severe6), unawareness of the term bronchiectasis among the general population, and the absence/limitations of imaging studies in patients with chronic respiratory diseases. These factors often lead to delays in diagnosis.
Chronic conditions increase in number as the population ages. Moreover, the availability of HRCT has facilitated diagnosis. Therefore, the prevalence of bronchiectasis is expected to rise considerably in the future.
Diagnostic delay, or the time between onset of symptoms and clinical diagnosis, is common in many chronic diseases and particularly in respiratory conditions. Epidemiological studies in chronic obstructive pulmonary disease (COPD) have illustrated the high rate of underdiagnosis in mild to severe stages7; these findings led to strategies targeting an earlier diagnosis to minimize the social and healthcare impact of COPD.8 The only study on diagnostic delay in bronchiectasis to date concluded that the delay could be as long as 17 years.9 Despite the relevance of this delay, contributing factors have not been reported.
Sex bias occurs when, for whatever reason and in equal diagnostic opportunities, one of the sexes is systematically belatedly diagnosed with a medical event in comparison with the other one. Sex bias has been reported in the diagnosis of cystic fibrosis (CF) in the United States, with girls diagnosed 4 months later than boys.10 No publications to date have analysed sex bias in adults diagnosed with bronchiectasis.
The aim of our study was to determine clinical differences in the diagnosis of bronchiectasis by sex in order to quantify diagnostic delay and to assess sex bias and its determinants.
Methods
The Spanish Historical Registry of Bronchiectasis is an anonymous multicentre prospective registry that gathered information from 2002 to 2011 in adults with bronchiectasis who were diagnosed based on the findings in HRCT, CT, bronchography or chest X-ray and clinical presentation. All the included patients diagnosed using X-ray had CF. The study population was recruited during a stable phase of the disease from 36 centres in 11 Spanish Autonomous Communities. When the registry opened in 2002, there was no requirement to request clinical consent for inclusion in the registry. However, all patients were informed that they would not be identified and would remain anonymous. Informed consent was explained orally and obtained from all participants who were identified and followed up from respiratory departments, not from primary care.
Recruiting physicians were instructed to follow standardized clinical and diagnostic recommendations for the inclusion of patients and collection of their data. These recommendations were later included in Spanish Society of Pulmonology and Thoracic Surgery’s National Guidelines on Diagnosis and Treatment of Bronchiectasis, which were published in 200811 and mainly follow the British Thoracic Society guideline for non-CF bronchiectasis.12
Aetiology was classified as post-infection (tuberculosis and childhood infections), bronchial obstruction, primary and secondary immune defect (human immunodeficiency infection, haematological diseases and transplants), mucociliary clearance disorders (CF, primary ciliary dyskinesia and Young syndrome), aspiration and inhalation injury, congenital and airway abnormality, allergic bronchopulmonary aspergillosis, associated with other diseases (connective tissue diseases, asthma, COPD and inflammatory bowel disease), and idiopathic.
The complete physical examination included calculation of the body mass index (BMI), spirometry to evaluate forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) and radiography of the paranasal sinuses.
A microbiological analysis of sputum was performed at the first visit and then every 3–6 months depending on the symptoms to confirm chronic bronchial infection. Initially, Gram and auramine staining were conducted, and samples were subsequently cultured for mycobacteria and fungi in blood agar, MacConkey agar and chocolate agar as well as in other growth media. The patient was considered colonized when the same microorganism was isolated in three consecutive samples for a minimum of 1 month during a 6-month period.
Bronchiectasis was assessed using CT and classified depending on the location of the clinical presentation as follows: localized, bilateral (both lungs affected) and diffuse (≥4 lobes affected, with the lingula considered to be a separate lobe); and prevailing type (cylindrical or cystic). Pulmonary function tests were performed close in time to CT imaging.
The diagnostic delay in years was calculated as the difference between the date of onset of symptoms and the date of diagnosis. Age at onset was divided into three groups: <20 years, 21–40 years, and >40 years. Onset of symptoms was defined as persistent production of mucopurulent sputum or at least one episode of haemoptysis. Data were analysed for the individual patient and by subgroups (with/without CF).
Statistical analysis
Dara were preprocessed in Microsoft Excel and then imported into R 3.2.3. As this is a multicentre registry, study variables were first filtered before ranges, outliers and errors were determined. After discussion, some patients were excluded because of major errors in one or more key variables, for example, pulmonary function. Once data were filtered, continuous variables were tested for normality using the Kolmogorov–Smirnov test in order to continue the analysis with either the usual parametric tests, such as analysis of variance and the t test, or the non-parametric tests. Quantitative variables were expressed as mean ± standard deviation; qualitative variables were expressed as counts and percentage of the total. Qualitative variables were compared using the chi-square test.
Non-parametric tests were also used to analyse the variable diagnostic delay, namely, the Fligner-Killeen test for the analysis of the homogeneity of variances13 and the Wilcoxon–Mann–Whitney test for the comparison of populations by gender.
A linear multivariate model was also included, with diagnostic delay as the dependent variable and demographic and clinical variables that were significant in the bivariate analysis as the independent variables. The threshold for selection of variables was established using the Akaike information criterion and a stepwise algorithm. A p < 0.05 was deemed statistically significant.
Results
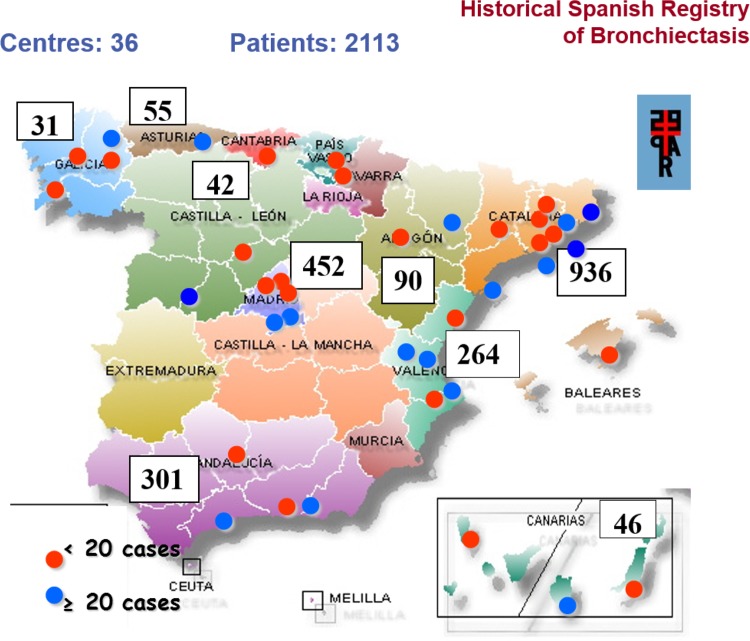
Of the 2113 patients recruited (Figure 1), 14 were excluded because of errors during data collection.
Figure 1.
Geographical distribution of centres participating in the Spanish Historic Registry of Bronchiectasis: number of patients with bronchiectasis enrolled by Autonomous Community.
Of the remaining 2099 patients (Figure 2), 1125 (53.6%) were women. There were no differences by sex in the following variables: age, bronchodilator test results, diagnostic test to determine bronchiectasis, location, haemoptysis, sinusitis, or chronic bronchial colonization with Pseudomonas aeruginosa (all p > 0.05; Table 1).
Figure 2.
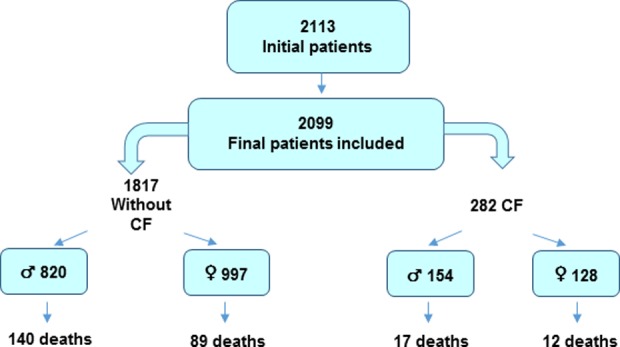
STROBE (Strengthening the Reporting of Observational studies in Epidemiology) flowchart of participation in the Spanish Historic Registry of Bronchiectasis.
Table 1.
Demographic and clinical characteristics of bronchiectasis patients.
| Mean ± SD/N (%) | Overall (n = 2099) | Men (n = 974) | Women (n = 1125) | p value |
|---|---|---|---|---|
| Age | 61.0 ± 20.6 | 60.9 ± 21.8 | 61.0 ± 19.6 | 0.88 |
| Smoking | <0.001 | |||
| Smoker | 206 (9.8) | 137 (14.1) | 69 (6.1) | |
| Former smoker | 512 (24.4) | 391 (40.2) | 121 (10.8) | |
| Non-smoker | 1378 (65.7) | 445 (45.7) | 933 (83.1) | |
| BMI | 24.3 ± 4.9 | 24.6 ± 4.5 | 24.1 ± 5.1 | 0.010 |
| FVC (%) | 70.0 ± 19.8 | 66.5 ± 19.7 | 73.1 ± 19.4 | <0.001 |
| FEV1 (%) | 64.8 ± 24.5 | 58.3 ± 24.5 | 70.5 ± 23.1 | <0.001 |
| FEV1/FVC | 68.6 ± 15.2 | 64.3 ± 16.1 | 72.5 ± 13.2 | <0.001 |
| SaO2 | 94.8 ± 4.0 | 94.2 ± 4.3 | 95.3 ± 3.5) | <0.001 |
| Bronchodilator test | 0.23 | |||
| Negative | 907 (46.0) | 426 (47.2) | 481 (45.0) | |
| Positive | 522 (26.5) | 222 (24.6) | 300 (28.0) | |
| Not performed | 543 (27.5) | 254 (28.2) | 289 (27.0) | |
| Diagnostic test for bronchiectasis | 0.20 | |||
| HRCT | 1716 (81.9) | 803 (82.4) | 913 (81.4) | |
| CT | 304 (14.5) | 129 (13.2) | 175 (15.6) | |
| Bronchography | 14 (0.7) | 7 (0.7) | 7 (0.6) | |
| Chest X-ray and clinical presentation | 62 (3.0) | 35 (3.6) | 27 (2.4) | |
| Location of bronchiectasis | 0.31 | |||
| Localized | 527 (25.2) | 258 (26.5) | 269 (24.0) | |
| Bilateral | 946 (45.2) | 424 (43.6) | 522 (46.5) | |
| Diffuse | 622 (29.7) | 291 (29.9) | 331 (29.5) | |
| Expectoration | 0.04 | |||
| No | 299 (14.2) | 119 (12.2) | 180 (16.0) | |
| Frequently | 631 (30.1) | 292 (30.0) | 339 (30.1) | |
| Daily | 1169 (55.7) | 563 (57.8) | 606 (53.9) | |
| Type of expectoration | 0.02 | |||
| White | 611 (29.1) | 298 (30.6) | 313 (27.8) | |
| White-yellow | 701 (33.4) | 315 (32.3) | 386 (34.3) | |
| Yellow-green | 489 (23.3) | 243 (24.9) | 246 (21.9) | |
| No | 298 (14.2) | 118 (12.1) | 180 (16.0) | |
| Haemoptysis | 0.69 | |||
| No | 1393 (66.4) | 656 (67.4) | 737 (65.6) | |
| Occasionally | 618 (29.5) | 280 (28.7) | 338 (30.1) | |
| Frequently | 86 (4.1) | 38 (3.9) | 48 (4.3) | |
| Sinusitis | 0.12 | |||
| n (%) | 624 (29.7) | 273 (28) | 351 (31.2) | |
| Chronic bronchial colonization | 0.01 | |||
| Occasional | 51 (3.7) | 20 (3.2) | 31 (4.1) | |
| Yes | 807 (59.2) | 393 (63.8) | 414 (55.3) | |
| No | 247 (18.1) | 106 (17.2) | 141 (18.9) | |
| No expectoration | 259 (19.0) | 97 (15.7) | 162 (21.7) | |
| CBC Hi | <0.01 | |||
| n (%) | 234 (17.2) | 132 (21.4) | 102 (13.6) | |
| CBC Pa | 0.94 | |||
| n (%) | 500 (36.7) | 227 (36.9) | 273 (36.5) | |
| Deaths | <0.001 | |||
| n (%) | 258 (14.1) | 157 (18.6) | 101 (10.2) |
SD: standard deviation; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; SaO2: oxygen saturation; HRCT: high-resolution computed tomography; CBC: chronic bronchial colonization; Hi: Haemophilus influenzae; Pa: Pseudomonas aeruginosa.
Men were more frequently smokers or smoked more (p < 0.001), weighed more (p = 0.010), and expectorated yellow-green sputum more frequently than women do (p < 0.04). Women had better pulmonary function and oxygen saturation (SaO2) than men did (p < 0.001). Hence, men expectorated darker sputum, experienced haemoptysis, had chronic bronchial colonization with Haemophilus influenzae, and died more frequently compared to women (18.6% vs. 10.2%, p < 0.001).
Bronchiectasis of unknown aetiology was the most common type in both sexes (Table 2) and more frequent in women (p < 0.001). It was also diagnosed as secondary to asthma (p = 0.007), infections in childhood (p < 0.001), and tuberculosis (p = 0.02). COPD and CF were more frequent in men (p < 0.001). No differences by sex were observed for treatment (Table 3).
Table 2.
Aetiology of bronchiectasis.
| N (%) | Overall (n = 2099) | Men (n = 974) | Women (n = 1125) | p value |
|---|---|---|---|---|
| Idiopathic | ||||
| n (%) | 506 (24.1) | 189 (19.4) | 317 (28.2) | <0.001 |
| Tuberculosis | ||||
| n (%) | 386 (18.4) | 158 (16.2) | 228 (20.3) | 0.02 |
| Cystic fibrosis | ||||
| n (%) | 282 (13.4) | 154 (15.8) | 128 (11.4) | 0.004 |
| Primary immunodeficiency | ||||
| n (%) | 215 (10.2) | 114 (11.7) | 101 (9) | 0.05 |
| Infections in childhood | ||||
| n (%) | 179 (8.5) | 55 (5.6) | 124 (11) | <0.001 |
| COPD | ||||
| n (%) | 164 (7.8) | 147 (15.1) | 17 (1.5) | <0.001 |
| Asthma | ||||
| n (%) | 123 (5.9) | 42 (4.3) | 81 (7.2) | 0.007 |
| Primary ciliary dyskinesia or Young syndrome | ||||
| n (%) | 67 (3.2) | 35 (3.6) | 32 (2.8) | 0.39 |
| Aspiration and inhalation injury | ||||
| n (%) | 55 (2.6) | 24 (2.5) | 31 (2.8) | 0.78 |
| Other | ||||
| n (%) | 51 (2.4) | 22 (3.4) | 18 (1.6) | 0.012 |
| Connective tissue disease | ||||
| n (%) | 43 (2) | 14 (1.4) | 29 (2.6) | 0.09 |
| Acquired immunodeficiency | ||||
| n (%) | 28 (1.3) | 9 (0.9) | 19 (1.7) | 0.18 |
COPD: chronic obstructive pulmonary disease.
Table 3.
Treatment.
| Overall (n = 2099) | Men (n = 974) | Women (n = 1125) | p value | |
|---|---|---|---|---|
| Oral antibiotics | ||||
| (n = 2013) | (n = 935) | (n = 1078) | 0.07 | |
| No | 1645 (81.7) | 776 (83.0) | 869 (80.6) | |
| Continuous | 77 (3.8) | 26 (2.8) | 51 (4.7) | |
| Cyclic | 291 (14.5) | 133 (14.2) | 158 (14.7) | |
| Inhaled antibiotics | ||||
| (n = 1954) | (n = 911) | (n = 1043) | 0.58 | |
| No | 1586 (81.2) | 731 (80.2) | 855 (82.0) | |
| Continuous | 273 (14.0) | 132 (14.5) | 141 (13.5) | |
| Periodic | 95 (4.9) | 48 (5.3) | 47 (4.5) | |
| Inhaled bronchodilators | ||||
| (n = 1986) | (n = 919) | (n = 1067) | 0.08 | |
| n (%) | 1519 (76.5) | 720 (78.3) | 799 (74.9) | |
| Inhaled corticosteroids | ||||
| (n = 1961) | (n = 909) | (n = 1052) | 0.55 | |
| n (%) | 1336 (68.1) | 626 (68.9) | 710 (67.5) |
The main analysis of diagnostic delay is presented in Table 4. Onset of symptoms was earlier in women (32.2 vs. 34.9 years, p = 0.015) and age at diagnosis was not significantly different between men and women (45.5 vs. 46.0, p = 0.61). The diagnostic delay in women was 2.1 years more than in men (p = 0.001). When individuals were analysed globally, with and without the subgroup of patients with CF, an effect of age on diagnostic delay was observed. Accordingly, women had a diagnostic delay of 5 years when onset was before age 20 years (21 vs. 16, p = 0.001). This result was sustained when individuals with CF were excluded (22.2 vs. 25.7, p = 0.03). On the contrary, diagnostic delay was 1.3 years longer in men than women when patients older than 40 years were taken into consideration. This result also held when CF was excluded (Table 4). When the analysis was repeated after excluding 163 patients with COPD, the difference in diagnostic delay between men and women was also sustained (11.4 vs. 13.3 years, p = 0.009).
Table 4.
Diagnostic delay, by gender and CF, according to age onset of symptoms.
| Mean ± SD | Overall (n = 2099) | Men (n = 974) | Women (n = 1125) | p value (t test) |
|---|---|---|---|---|
| Age at diagnosis | 45.7 ± 23.9 | 46.0 ± 25.1 | 45.5 ± 22.7 | 0.61 |
| Age at symptoms onset | 33.5 ± 25.1 | 34.9 ± 26.0 | 32.2 ± 24.3 | 0.01 |
| Diagnostic delay | 12.2 ± 15.5 | 11.1 ± 14.0 | 13.2 ± 16.6 | 0.001 |
| All | ||||
| <20 years at onset (n = 825) | 19 ± 19.19 | 16 ± 17.5 | 21 ± 20.13 | <0.001 |
| 20–40 years (n = 411) | 13.41 ± 14 | 13.9 ± 14.3 | 13.1 ± 13.8 | 0.51 |
| >40 years (n = 863) | 5.1 ± 6.5 | 5.7 ± 6.9 | 4.4 ± 6.1 | 0.003 |
| Only individuals with CF | (n = 282) | (n = 154) | (n = 128) | |
| <20 years at onset (n = 251) | 7.2 ± 12.4 | 6.23 ± 10.6 | 8.43 ± 14.5 | 0.18 |
| 20–40 years (n = 22) | 5.32 ± 7.3 | 2.22 ± 2.9 | 7.5 ± 8.7 | 0.06 |
| >40 years (n = 9) | 7.7 ± 8.91 | 8 ± 7.1 | 7.57 ± 9.9 | 0.95 |
| Only individuals without CF | (n = 1817) | (n = 820) | (n = 997) | |
| <20 years at onset (n = 574) | 24.34 ± 19.3 | 22.2 ± 18.2 | 25.7 ± 19.9 | 0.03 |
| 20–40 years (n = 389) | 13.9 ± 14.2 | 14.6 ± 14.4 | 13.4 ± 14 | 0.40 |
| >40 years (n = 854) | 5.04 ± 6.5 | 5.7 ± 6.9 | 4.4 ± 6 | 0.003 |
SD: standard deviation; CF: cystic fibrosis.
Finally, analysis of the independent effect of various demographic and clinical variables on explaining diagnostic delay in bronchiectasis (Table 5) revealed that the 2.1-year delay in women was sustained and even increased to 4.5 years in the multivariate linear model. The variables confirmed as independent factors accounting for this association were higher BMI, lower FVC, smoking, characteristics of sputum and early age at onset of symptoms (all with p < 0.05).
Table 5.
Multivariate linear regression model of factors associated with diagnostic delay.
| Dependent variable | |
| Diagnostic delay (years) | |
| Gender: Female | 4.5a (0.9) |
| Non-smoker | −5.1a (1.2) |
| Smoker | −5.3a (1.8) |
| BMI | 0.8a (0.1) |
| FVC | −0.1a (0.03) |
| SaO2 | −0.2 (0.1) |
| Type of expectoration: white or yellow | −3.9a (1.3) |
| Type of expectoration: yellow or green | −4.5a (1.4) |
| Type of expectoration: none | −0.04 (1.3) |
| Death | 3.6b (1.4) |
| Age at onset of symptoms | −0.3a (0.02) |
| Constant | 30.8b (13.0) |
BMI: body mass index; FVC: forced vital capacity; SaO2: oxygen saturation.
a p < 0.01.
b p < 0.05.
Discussion
We present the first results from the study cohort of the Spanish Historical Registry of Bronchiectasis, which comprises more than 2000 patients, with emphasis on differences by sex. We conclude that there is a significant diagnostic delay in bronchiectasis of 12 years and that this is considerably higher in women (>2 years). The independent variables responsible for this sex bias were age at onset of symptoms, smoking, daily expectoration and poor pulmonary function.
The delay in diagnosing bronchiectasis in women was also related to COPD, perhaps because of differences in the presentation of symptoms (more dyspnoea in women and more expectoration in men) as well as a predisposition among physicians to think that COPD disproportionately affects men who were called earlier for spirometry.14
The 4-month delay in diagnosing CF in girls compared with boys reported by Lai et al.10 in a series of patients in the United States (1986–1998) was associated with more functional and nutritional deterioration and earlier acquisition of P. aeruginosa in females. Implementation of neonatal screening revealed earlier diagnosis of CF that improved nutritional development and growth and thus minimized deterioration of pulmonary function. This success enabled interventions to be implemented earlier, before further irreversible pulmonary damage. It also enabled more aggressive treatment to be started at the first isolation of P. aeruginosa and the microorganism to be eradicated, thus modifying the natural history of the disease and extending survival.15
A recent 3-year (2006–2008) prospective study of 189 patients with bronchiectasis from the northeast of England revealed a diagnostic delay of 17 years for both idiopathic and non-idiopathic disease.9 However, this analysis did not report differences by sex or examined related factors.
Any delay in diagnosis of bronchiectasis holds up initiation of treatment that might delay or prevent disease progression. King et al.16 reported an average annual loss in FEV1 of 50 ml more than in the general population of the same age and sex, which is similar to that observed in other respiratory conditions, such as COPD. However, not all studies have reported this loss. Factors associated with accelerated loss of pulmonary function include systemic inflammation and number of severe exacerbations.17 However, the factor most likely associated with the loss of pulmonary function in bronchiectasis is colonization or chronic infection with P. aeruginosa, although no causal relationship has been established to date between these phenomena. Martínez-García et al.18 observed that patients who were chronically colonized by P. aeruginosa presented a 124-ml annual loss of FEV1 compared with only 30 ml in non-colonized patients. Therefore, it is important to eradicate this bacterium.11,12 Extrapolation of these data would indicate that a diagnostic delay of 2 years in women could produce a loss of up to 250 ml in FEV1 in patients infected by P. aeruginosa, thus potentially leading to frequent exacerbations.
Kapur and Karadag19 explored the differences and similarities in paediatric patients with non-CF bronchiectasis and found that many children, despite having symptoms during their first year of life, were not diagnosed until much later, thus accumulating a diagnostic delay of 4–8 years, which is similar to that found in countries with different socioeconomic levels and healthcare facilities. In our series, we also observed a longer diagnostic delay in women who experienced onset of symptoms before age 20 years. This delay decreased as respiratory symptoms subsequently appeared, probably because women could have minimized their symptoms, as they would have to endure bronchiectasis from an early age, and may not have sought healthcare or could have been misdiagnosed for years.
Other clinical data that influenced diagnostic delay were symptoms, mainly expectoration, smoking status, and poor pulmonary function. Daily expectoration and yellow-green sputum were more frequent in men, probably because more men smoked and expectoration by women is less socially and culturally tolerated.20 Daily expectoration is a key symptom of bronchiectasis and an indicator of the diagnosis.11,12 Previous smoking and its consequences, such as lung cancer, oblige the clinician to request imaging studies earlier. Therefore, in this sense, men could again be diagnosed earlier than women.8 Likewise, better pulmonary function in women might also influence diagnostic delay. The superior functional data in women (FVC, FEV1, FEV1/FVC and SaO2) translated into a milder clinical presentation and less need to seek healthcare. However, this conclusion cannot be corroborated in the absence of quality-of-life questionnaires or number of medical visits, neither of which was available at the onset of this register. In addition, the fact that patients are often vague about the origin of their symptoms makes diagnosis challenging in the sense that it is difficult to define when and how symptoms become clinically significant.
Bronchiectasis complicates other respiratory diseases, increases hospital stay, requires costly treatments, deteriorates quality of life, and is associated with high health costs and substantial use of resources.21,22 Establishing an earlier diagnosis should enable initiation of treatment with reduced morbidity, fewer exacerbations, and delayed chronic bronchial colonization by P. aeruginosa. It should also reduce the cost burden of the disease. Symptoms should be recognized at the primary care level in order to refer the patient to a specialist as soon as possible and to start the path towards a differential diagnosis, since HRCT and other tests are more widely available nowadays.
Our study has both advantages and limitations. With more than 2000 cases of bronchiectasis, the sample size is sufficient for the main objective and for the sensitivity analysis. Furthermore, with a follow-up of over 10 years, it brings together a multicentre experience of 20,000 person-years. The internal quality control guaranteed the value and usefulness of the data set, making it amongst the largest available. As ours is a historical registry, it provides relevant information that will enable us to explore the natural history of bronchiectasis and compare our findings with those of subsequent series and series reported elsewhere. Our registry is limited by the fact that it was designed at the beginning of this century. Current practice requires HRCT or bronchography before inclusion in a bronchiectasis registry; hence, 3% of patients were diagnosed using thoracic radiography only. Likewise, follow-up variables that evaluate changes in the disease were not gathered prospectively. The same is true of data on exacerbations and hospitalizations, although mortality data were collected. Finally, baseline assessment of dyspnoea using the mMRC (modified Medical Research Council) or other scales limits the estimation of newly available multicomponent indices such as BSI (Bronchiectasis Severity Index)23 or FACED,24 although extrapolation of data can be explored.
Conclusions
This first analysis of the Spanish Historical Registry of Bronchiectasis enabled us to explore sex differences in diagnostic delay in bronchiectasis. We found a diagnostic delay of 12 years that was significantly higher in women (>2 years vs. men). Age at symptom onset, smoking habits, daily expectoration, and low pulmonary function were identified as independent explanatory factors of this sex bias and should be evaluated in depth, given the implications of a delay in diagnosing bronchiectasis.
Footnotes
Author contributions: Rosa Ma Girón accepts final responsibility for the material published in the article and had full access to all the study data. She also takes responsibility for the integrity of the data and the accuracy of the analysis. Rosa Ma Girón, Javier de Gracia Roldán, Casilda Olveira, Montserrat Vendrell, Miguel Ángel Martínez-García, David de la Rosa, Luis Máiz, Julio Ancochea, Luis Borderías, Eva Polverino, Eva Martínez-Moragón and Olga Rajas recruited patients and collected data. Rosa Girón, Liliana Vázquez and Joan B Soriano designed the study, interpreted data and revised the report. Liliana Vázquez and Joan B Soriano analysed and interpreted data. Rosa Girón, Liliana Vázquez, Julio Ancochea, Miguel Ángel Martínez-García and Joan B Soriano wrote and revised the report. All authors have given their final approval of the version to be published.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors declare that no funding was received for this study. The Spanish Historical Registry of Bronchiectasis is hosted on a web server managed by SEPAR, the Spanish Society of Pulmonology and Thoracic Surgery (Sociedad Española de Neumología y Cirugía Torácica, www.separ.es). Neither data collection nor data curation was funded.
References
- 1. Cole PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis Suppl 1986; 147: 6–15. [PubMed] [Google Scholar]
- 2. Bilton D, Jones AL. Bronchiectasis: epidemiology and causes. Eur Respir Mon 2011; 52: 1–10. [Google Scholar]
- 3. Zengli W. Bronchiectasis: still a problem. Chin Med J 2014; 127: 157–172. [PubMed] [Google Scholar]
- 4. Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005; 12: 205–209. [Google Scholar]
- 5. Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47(1): 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamarrón de Lucas E, Prados Sánchez C, Quirós Fernández S. Las bronquiectasias en el mundo. Epidemiología actual In: Martínez-García MA. (ed) Clínicas Respiratorias SEPAR: bronquiectasias. Barcelona: Ergon Creación, S.A, 2016, pp. 1–96. [Google Scholar]
- 7. Lamprecht B, Soriano JB, Studnicka M, et al. BOLD Collaborative Research Group, the EPI-SCAN Team, the PLATINO Team, and the PREPOCOL Study Group. Determinants of underdiagnosis of COPD in national and international surveys. Chest 2015; 148(4): 971–985. [DOI] [PubMed] [Google Scholar]
- 8. Ministerio de Sanidad y Política Social. Estrategia en EPOC del Sistema Nacional de Salud. Madrid: Imgraf Impresores, 2009. [Google Scholar]
- 9. Anwar G, MCDonnell M, Worthy S, et al. Phenotyping adults with non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Med 2013; 107: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 10. Lai HC, Kosorok MR, Laxova A, et al. Delayed diagnosis of US females with cystic fibrosis. Am J Epidemiol 2002; 156(2): 165–173. [DOI] [PubMed] [Google Scholar]
- 11. Vendrell M, De Gracia J, Olveira C, et al. Diagnóstico y tratamiento de las BQ. Arch Bronconeumol 2008; 44: 629–640. [DOI] [PubMed] [Google Scholar]
- 12. Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis Non-CF Guideline Group. British thoracic society guideline for non-CF bronchiectasis. Thorax 2010; 65(Suppl 1): i1–58. [DOI] [PubMed] [Google Scholar]
- 13. Conover WJ, Johnson ME, Johnson MM. A Comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 1981; 23: 351–361. [Google Scholar]
- 14. Camp PG, Goring SM. Gender and the diagnosis, management, and surveillance of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4(8): 686–691. [DOI] [PubMed] [Google Scholar]
- 15. Dijk FN, Mckay K, Barzi F, et al. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch Dis Child 2011; 96: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 16. King PT, Holdsworth SR, Freezer NJ, et al. Outcome in adult bronchiectasis. COPD. 2005; 2: 27–34. [DOI] [PubMed] [Google Scholar]
- 17. Ellis DA, Thornley PE, Wightman AJ, et al. Present outlook in bronchiectasis: clinical study and factors influencing prognosis. Thorax 1981; 36: 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martínez-García MA, Perpiña-Tordera M, Román-Sánchez P, et al. Factors associated with lung function decline in patients with non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 19. Kapur N, Karadag B. Differences and similarities in non-cystic fibrosis bronchiectasis between developing and affluent countries. Paediatr Respir Rev 2011; 12: 91–96. [DOI] [PubMed] [Google Scholar]
- 20. Morrissey BM, Harper RW. Bronchiectasis: sex and gender considerations. Clin Chest Med 2004; 25(2): 361–372. [DOI] [PubMed] [Google Scholar]
- 21. Roberts ME, Lowndes L, Milne DE, et al. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J 2011; 2: 129–136. [DOI] [PubMed] [Google Scholar]
- 22. Seitz AE, Oliver KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest 2010; 138: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43(5): 1357–1367. [DOI] [PubMed] [Google Scholar]